Abstract
Aims/background—CD44 is a widely distributed cell surface molecule which has numerous isoforms generated by alternative splicing. The diverse functions related to the CD44 variants (CD44v) have been reported in various physiological and pathological conditions. The pattern of expression of CD44v among meningioma subtypes was investigated to ascertain whether CD44 variants play a role in a variety of biological processes, such as epithelial differentiation and extracranial metastasis.
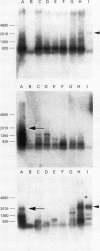
Methods—Twenty three meningiomas were studied immunohistochemically using novel antibodies directed against CD44 isoforms. Six of the 23 samples were analysed by reverse transcription polymerase chain reaction (RT-PCR), followed by Southern blotting with CD44v specific probes.
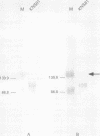
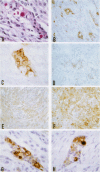
Results—In meningothelial, fibrous and anaplastic meningiomas, a standard form of CD44 was detected by RT-PCR and was homogeneously expressed in tumour cells when studied immunohistochemically. CD44v was not detected in these subtypes. In secretory meningiomas, however, CD44v isoforms were strongly expressed in the cell clusters that produce secretory granules and also accumulated in the granules. The population of tumour cells immunopositive for CD44v was similar to that which stained with antibodies directed against carcinoembryonic antigen, epithelial membrane antigen and ezrin. On RT-PCR with Southern blotting, only the secretory type showed high level expression of CD44v.
Conclusions—CD44v in meningiomas is expressed in relation to tumour cell differentiation towards the epithelial type.
Keywords: CD44
Keywords: meningioma
Keywords: cell differentiation
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi J., Kazumoto K., Uki J., Takeda F., Kurozumi M., Nakazato Y. Secretory meningioma: a case report with immunohistochemical and ultrastructural study. Noshuyo Byori. 1993;10(2):93–98. [PubMed] [Google Scholar]
- Alguacil-Garcia A., Pettigrew N. M., Sima A. A. Secretory meningioma. A distinct subtype of meningioma. Am J Surg Pathol. 1986 Feb;10(2):102–111. doi: 10.1097/00000478-198602000-00003. [DOI] [PubMed] [Google Scholar]
- Arai K., Iiai T., Nakayama M., Hasegawa K., Sato K., Ohtsuka K., Watanabe H., Hanyu T., Takahashi H. E., Abo T. Adhesion molecules on intermediate TCR cells. I. Unique expression of adhesion molecules, CD44+ L-selectin-, on intermediate TCR cells in the liver and the modulation of their adhesion by hyaluronic acid. Immunology. 1995 Jan;84(1):64–71. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Driessens M. H., Stroeken P. J., Rodriguez Erena N. F., van der Valk M. A., van Rijthoven E. A., Roos E. Targeted disruption of CD44 in MDAY-D2 lymphosarcoma cells has no effect on subcutaneous growth or metastatic capacity. J Cell Biol. 1995 Dec;131(6 Pt 2):1849–1855. doi: 10.1083/jcb.131.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejeckam G. C., Azadeh B., Hamad A. Secretory meningioma. Histopathology. 1992 Nov;21(5):475–477. doi: 10.1111/j.1365-2559.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Harn H. J., Lee H. S., Ho L. I., Lee W. H., Ding J. H. Selective expression of CD44 messenger RNA splice variants in four high grade human brain tumour cell lines. Biochem Mol Biol Int. 1994 Jul;33(4):743–749. [PubMed] [Google Scholar]
- Haynes B. F., Liao H. X., Patton K. L. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991 Sep;3(9):347–350. [PubMed] [Google Scholar]
- Jackson D. G., Schenker T., Waibel R., Bell J. I., Stahel R. A. Expression of alternatively spliced forms of the CD44 extracellular-matrix receptor on human lung carcinomas. Int J Cancer Suppl. 1994;8:110–115. doi: 10.1002/ijc.2910570724. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Iizuka R., Tateishi J. An amber mutation of prion protein in Gerstmann-Sträussler syndrome with mutant PrP plaques. Biochem Biophys Res Commun. 1993 Apr 30;192(2):525–531. doi: 10.1006/bbrc.1993.1447. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., Van Meir E., Gauthier T., Hamou M. F., de Tribolet N. Differential expression of the CD44 molecule in human brain tumours. Int J Cancer. 1992 Feb 20;50(4):572–577. doi: 10.1002/ijc.2910500414. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Meis J. M., Ordóez N. G., Bruner J. M. Meningiomas. An immunohistochemical study of 50 cases. Arch Pathol Lab Med. 1986 Oct;110(10):934–937. [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgami T., Kitamoto T., Weidmann A., Beyreuther K., Tateishi J. Alzheimer's amyloid precursor protein-positive degenerative neurites exist even within kuru plaques not specific to Alzheimer's disease. Am J Pathol. 1991 Dec;139(6):1245–1250. [PMC free article] [PubMed] [Google Scholar]
- Ohta M., Iwaki T., Kitamoto T., Takeshita I., Tateishi J., Fukui M. MIB1 staining index and scoring of histologic features in meningioma. Indicators for the prediction of biologic potential and postoperative management. Cancer. 1994 Dec 15;74(12):3176–3189. doi: 10.1002/1097-0142(19941215)74:12<3176::aid-cncr2820741217>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Ohta M., Kitamoto T., Iwaki T., Ohgami T., Fukui M., Tateishi J. Immunohistochemical distribution of amyloid precursor protein during normal rat development. Brain Res Dev Brain Res. 1993 Oct 15;75(2):151–161. doi: 10.1016/0165-3806(93)90019-7. [DOI] [PubMed] [Google Scholar]
- Penno M. B., August J. T., Baylin S. B., Mabry M., Linnoila R. I., Lee V. S., Croteau D., Yang X. L., Rosada C. Expression of CD44 in human lung tumors. Cancer Res. 1994 Mar 1;54(5):1381–1387. [PubMed] [Google Scholar]
- Ruiz P., Schwärzler C., Günthert U. CD44 isoforms during differentiation and development. Bioessays. 1995 Jan;17(1):17–24. doi: 10.1002/bies.950170106. [DOI] [PubMed] [Google Scholar]
- Schnitt S. J., Vogel H. Meningiomas. Diagnostic value of immunoperoxidase staining for epithelial membrane antigen. Am J Surg Pathol. 1986 Sep;10(9):640–649. doi: 10.1097/00000478-198609000-00006. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R. W., Iwaki T., Kitamoto T., Tateishi J. Hydrated autoclave pretreatment enhances tau immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest. 1991 May;64(5):693–702. [PubMed] [Google Scholar]
- Takeuchi K., Sato N., Kasahara H., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994 Jun;125(6):1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D., Bolodeoku J., Hatfill S. J., Sugino T., Woodman A. C., Yoshida K. The clinical significance of malfunction of the CD44 locus in malignancy. J Neurooncol. 1995 Dec;26(3):209–219. doi: 10.1007/BF01052624. [DOI] [PubMed] [Google Scholar]
- Terpe H. J., Stark H., Prehm P., Günthert U. CD44 variant isoforms are preferentially expressed in basal epithelial of non-malignant human fetal and adult tissues. Histochemistry. 1994 Feb;101(2):79–89. doi: 10.1007/BF00269353. [DOI] [PubMed] [Google Scholar]
- Toyama-Sorimachi N., Sorimachi H., Tobita Y., Kitamura F., Yagita H., Suzuki K., Miyasaka M. A novel ligand for CD44 is serglycin, a hematopoietic cell lineage-specific proteoglycan. Possible involvement in lymphoid cell adherence and activation. J Biol Chem. 1995 Mar 31;270(13):7437–7444. doi: 10.1074/jbc.270.13.7437. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Oishi K., Sato N., Sagara J., Kawai A., Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994 Jul;126(2):391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielenga V. J., Heider K. H., Offerhaus G. J., Adolf G. R., van den Berg F. M., Ponta H., Herrlich P., Pals S. T. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993 Oct 15;53(20):4754–4756. [PubMed] [Google Scholar]