Abstract
In spite of high-dose chemotherapy followed by autologous hematopoietic SCT multiple myeloma (MM) eventually recurs, highlighting the need for more effective treatment approaches. Patients received topotecan 3.5 mg/m2 intravenously on days −6 to −2, melphalan 70 mg/m2 intravenously on days −3 and −2 and CY 1 g/m2 intravenously on days −6, −5 and −4. Overall response rate (ORR) consisting of complete response and partial response (CR + PR, PFS, OS and toxicity are reported. Between August 2002 to March 2004, 60 patients (34 men and 26 women) with a median age of 61 years (range 45–72) were enrolled. Forty-one patients were treated for consolidation of first remission, while 19 patients had relapsed/refractory disease. ORR was 85% (CR 12%, very good PR 43% and PR 30%). Median time to neutrophil (ANC>0.5 × 109/L) and plt engraftment (>20 × 109/L) was 10 (range 7–12 days) and 9 days (range 6–79 days), respectively. A majority of the common adverse events were grade 1–3 mucositis/stomatitis (65%), grade 1 or 2 nausea (59%) and grade 1 or 2 diarrhea (41%). Median PFS was 18.5 months and median OS has yet not been reached. In conclusion, topotecan, melphalan and CY is a safe and active conditioning regimen for auto hematopoietic SCT in MM. The ORR and PFS were comparable to high-dose melphalan.
Keywords: topotecan, melphalan, CY, multiple myeloma, autologous stem cell transplant
Introduction
Approximately 20 000 new cases of multiple myeloma (MM) are diagnosed in the United States each year. In spite of the many recent developments in therapy, MM remains an incurable disease, with a 5-year survival rate of 34% according to the SEER database.1 At least two randomized trials have shown higher response rates and longer EFS and OS with high-dose chemotherapy and autologous hematopoietic SCT (auto HCT), when compared with conventional dose therapy.2,3 Two recently reported randomized trials have, however, failed to show similar benefits with the high-dose chemotherapy approach.4,5 A number of prognostic factors such as high ISS stage, certain chromosomal abnormalities (deletion 1p, t 4:14 and deletion 13q and high tumor burden have been identified as predictors of poor outcome.6–8 Achievement of complete response (CR) is considered an important predictor of durable response in treatment of MM.9,10 Therefore, the current goal in the management of MM is to identify strategies that can increase the CR rates and survival.
I.v. melphalan 200 mg/m2 is considered the standard conditioning regimen for auto HCT based on the randomized trial reported by Moreau et al.11 Melphalan 200 mg/m2 is associated with a CR rate of 22–44%, PFS of 18–30 months and an OS of approximately 5 years.2–5,11 There is clearly a need to improve these outcomes. Our hypothesis was that a combination of active anti-myeloma agents would improve the CR rate, a surrogate for longer PFS and OS. To improve the CR rate and survival, strategies such as novel combinations,12,13 tandem autologus transplants14 and maintenance therapy15 are being studied in phase II and III trials. As mentioned, the focus of our group in the last decade was to develop novel preparative regimens against myeloma. Topotecan, a semi-synthetic analog of camptothecin, prevents DNA replication in cancer cells by inhibiting the enzyme topoisomerase I and has shown activity against relapsed and refractory myeloma when used as a single agent and it has been studied in combination with CY.16,17 CY has been studied in MM and has been tolerated at doses as high as 4–7 g/m2 when used to collect peripheral blood hematopoietic stem cells.18 These two were combined with melphalan in a new preparative regimen (topotecan, melphalan and CY (TMC)). In this phase II trial, we evaluated the safety and efficacy of the TMC regimen in patients with MM.
Patients and methods
Patients
Patient in either first remission or refractory/relapsed MM were enrolled in the study. Patients were eligible if their physiological age was <70, Zubrod performance status <3, left ventricular ejection fraction >50%, lung diffusing capacity >50%, serum creatinine <133 μmol/L (<1.5 mg per 100 mL), serum bilirubin less than twice the upper limit of normal with no evidence of chronic or active hepatitis or cirrhosis and no active central nervous system disease. Patients provided written informed consent and were enrolled on a protocol approved by the institutional review board of the University of Texas—MD Anderson Cancer Center (ClinicalTrials.gov Identifier: NCT01039025).
Stem cell mobilization and collection
With a target dose of >4 × 106 CD34+ cells/kg, PBSCs were mobilized with either filgrastim alone or with chemotherapy and filgrastim. Apheresis was performed using the COBE Spectra Version 4.7 cell separator (COBE BCT, Lakewood, CO, USA). The CD34+ cell content of the collection product was measured immediately after apheresis. The cells were subsequently cryopreserved using programmed freezing.
Treatment and supportive care
CY 1 g/m2/day was given intravenously over 2 h on days −6, −5 and −4; melphalan 70 mg/m2/day was administered i.v. over 30 min on days −3 and −2 and topotecan was given i.v. at 3.5 mg/m2/day over 30 min for five total doses on days −6 to −2. For patients >20% over ideal body weight, all chemotherapy agents were dosed based on adjusted body surface area. Filgrastim at 5 mcg/kg rounded to the nearest vial size was given subcutaneously daily from the day of PBSC infusion until the ANC reached at least 1.5 × 109/L. Patients received prophylactic antibiotics according to the standard departmental guidelines. Blood products were transfused to maintain a hemoglobin level above 80 g/L and plts above 15 × 109/L.
Criteria for response
Patients were evaluated for response at 1, 3 and 6 months post transplantation. Responses were based on the International Myeloma Working Group Uniform Response Criteria.19 CR was defined as the absence of monoclonal protein in serum and urine by immunofixation, <5% plasma cells in the BM and disappearance of any soft tissue plasmacytoma. Stringent CR was defined as normal FLC ratio and absence of clonal cells in BM by immunohistochemistry and immunoflorescence. Very good partial response (VGPR) was defined as detectable serum and urine M-protein by immunofixation but not on electrophoresis or at least a 90% reduction in serum M-protein with a urine M-protein <100 mg per 24 h. Partial response (PR) was described as ≥50% reduction in serum M-protein and reduction of 24-h urinary M-protein by 90% or to <200 mg per 24 h. In non-secretory myeloma >50% decrease in the difference between kappa and lambda FLC levels denoted a PR. If the FLC levels were immeasurable at baseline, a >50% reduction in BM plasma cells was accepted as PR as long as the original BM contained at least 30% plasma cells. All PR required a >50% reduction in size of any soft tissue plasmacytomas. Stable disease was defined when the above criteria were not fulfilled. Relapse or progression required a 25% increase in the level of paraprotein from baseline or reappearance of a paraprotein after CR.
Statistical analysis
The primary objective of this phase II study was to evaluate the response rate to TMC. All analyses were performed using Statistica for Windows Release 6.1 (StatSoft, Tulsa, OK, USA). Secondary objectives were to assess the toxicity of TMC regimen, OS and PFS. Descriptive statistics were used to describe the basic features of patients. χ2-test was used to find any differences in response between various categories of the patients. The PFS was calculated from the date of transplantation until the time of known progression, relapse after CR, death or the date the patient was last known to be in remission. The OS was calculated from the date of transplantation until the time of death or the last follow-up date in the record. The curves of PFS and OS were plotted according to the method of Kaplan and Meier and various categories were compared using log-rank test. Toxicity of the TMC preparative regimen was reported according National Cancer Institute-Common Toxicity Criteria for Adverse Events version 3.
Results
Patient characteristics
From August 2002 to March 2004, 60 patients received TMC regimen in a phase II trial at MD Anderson Cancer Center. Baseline patient characteristics are summarized in Table 1. The median age was 61 years (45–72). According to Durie and Salmon criteria,20 11 patients had stage I, 16 had stage II and 33 had stage III disease at diagnosis. Fifteen patients (25%) had chromosomal abnormalities on conventional cytogenetic studies, out of which only five patients had well-known high-risk chromosomal abnormalities (del 13q, del 1p, t4;14, t14;16). Forty-one patients (68%) that received auto HCT were in first remission while 19 patients (32%) had refractory or relapsed disease. The median interval between diagnosis and auto HCT was 7 months (2–88) with 38 patients (63%) receiving auto HCT within 12 months of diagnosis. Forty-one patients (68%) had achieved at least a PR to previous induction or salvage therapy before auto HCT.
Table 1.
Patient characteristics
| Characteristics | Number of patients (%) |
|---|---|
| Age (year) | 61 (45–72) |
| Sex | |
| Male | 34 (57) |
| Female | 26 (43) |
| Durie Salmon | |
| I | 11 (18) |
| II | 16 (27) |
| III | 33 (55) |
| Race | |
| White | 39 (65) |
| African American | 12 (20) |
| Other | 9 (15) |
| Ig Subtype | |
| Non-secretory | 2 (3) |
| Light chain only | 4 (7) |
| IgG | 38 (63) |
| IgA | 15 (25) |
| IgD | 1 (2) |
| Light Chain | |
| Lambda | 25 (42) |
| Kappa | 35 (58) |
| Disease status | |
| First remission | 41 (68) |
| Refractory/relapsed | 19 (32) |
| Cytogenetics | |
| Normal | 45 (75) |
| Abnormal | 15 (25) |
| Interval between diagnosis and transplant (months) | 7 (2–88) |
| BM plasmacytosis (% of cells) | 8.5 (0–95) |
| β2 microglobulin level | |
| >3.5 mg/L | 23 (38) |
| <3.5 mg/L | 37 (62) |
| Median LDH | |
| <618 IU/L | 38 (63) |
| >618 IU/L | 22 (37) |
| Serum creatinine (μmol/L) | 80 (53–124) |
| Serum albumin | |
| >35 g/L | 51 (85) |
| <35 g/L | 9 (15) |
| Transplant within 12 months | 38 (63) |
| At least a PR before transplant | 41 (68) |
Abbreviation: PR = partial response.
Adverse events
Toxicity data are summarized in Table 2. A majority of the common non-hematological side effects were grade 1 or 2 nausea (58%), grade 1 or 2 diarrhea (42%) and mucositis/stomatitis (65%). Non-hematological grade 3 and 4 toxicities were observed in 22 patients (36%) that included infectious complications (2 patients), congestive heart failure exacerbation (2 patients), electrolyte abnormalities including hypocalcemia and hypokalemia (1 patient each), gastrointestinal bleeding and vomiting (1 patient each), and cardiac arrhythmia (1 patient) and mucositis/stomatitis (13 patients). Febrile neutropenia was reported in eight patients (18%). Hematological toxicities were high with this regimen as all patients developed grade 3 or 4 leukopenia and thrombocytopenia. The time to neutrophil (ANC >0.5 × 109/L), and plt engraftment (plt count >20 × 109/L) was 10 (range 7–11) and 9 days (6–79), respectively.
Table 2.
Adverse events secondary to topotecan, melphalan and CY regimen
| Toxicity grade 1 | Toxicity grade 2 | Toxicity grade 3 | Toxicity grade 4 | |
|---|---|---|---|---|
| Cardiovascular | Edema: 1 | Edema: 3 Venous thrombus: 1 |
Arrhythmia: 1 Mild heart failure: 1 |
Severe heart failure: 1 |
| General | Fever: 1 Lethargy: 6 |
0 | 0 | 0 |
| Gastrointestinal | Diarrhea: 22 Nausea: 25 Vomiting: 11 Mucositis/stomatitis: 8 |
Diarrhea: 3 Nausea: 10 Mucositis/stomatitis: 18 |
GI Hemorrhage: 1 Vomiting: 1 Mucositis/stomatitis: 13 |
|
| Infection | 3 | 3 | 2 | 0 |
| Metabolic | Hypokalemia: 1 | Hypokalemia: 1 | Hypokalemia: 2 Hypomagnesemia: 1 |
0 |
| Hematological | Leukopenia: 60 Thrombocytopenia: 60 |
Outcome
Transplant-related outcomes are shown in Table 3, while overall response rates (ORRs) in MM patients to the TMC regimen are shown in Table 4. Overall, 51 patients (ORR 85%; CR 12% + VGPR/PR 73%) achieved PR or better response. Two patients had stringent CR and five patients had CR with a total CR rate of 12%. In total, 26 patients (43%) had VGPR and 18 patients (30%) had PR. Eight patients (13%) had stable disease and one patient progressed within 3 months of auto HCT. The VGPR + CR were significantly better in patients undergoing auto HCT within 12 months of diagnosis (68 vs 32%, P=0.007) and patients who had achieved at least a PR to induction or salvage therapy before auto HCT (63 vs 31%, P=0.02). Old age, chromosomal abnormalities or disease status at transplant did not significantly affect the CR + VGPR.
Table 3.
Outcomes for topotecan, melphalan and CY regimen
| Outcome | Median (range) | |
|---|---|---|
| Plt >20 × 109/L post transplant | 9 (6–79) | |
| ANC >0.5 × 109/L post transplant | 10 (7–11) | |
| Mortality | 24 (40) | |
| First remission | 16/41 (39) | P 0.51 |
| Relapse-refractory | 8/19 (42) | |
| Progression | 40 (67) | |
| First remission | 29/41 (70) | P 0.384 |
| Relapse-refractory | 8/19 (57) | |
| 4-year OS | 66% | |
| 4-year PFS | 30% |
Table 4.
Response to topotecan, melphalan and CY regimen
| First remission |
Refractory/ relapsed |
Total N (%) | |
|---|---|---|---|
| sCR | 1 | 1 | 2 (3.3) |
| CR | 3 | 2 | 5 (8.3) |
| VGPR | 19 | 7 | 26 (43.3) |
| Partial response | 11 | 7 | 18 (30) |
| Stable disease | 6 | 2 | 8 (13) |
| Progressive disease | 1 | 0 | 1 (2) |
Abbreviations: CR = complete response; sCR = stringent complete response; VGPR = very good partial response.
Survival
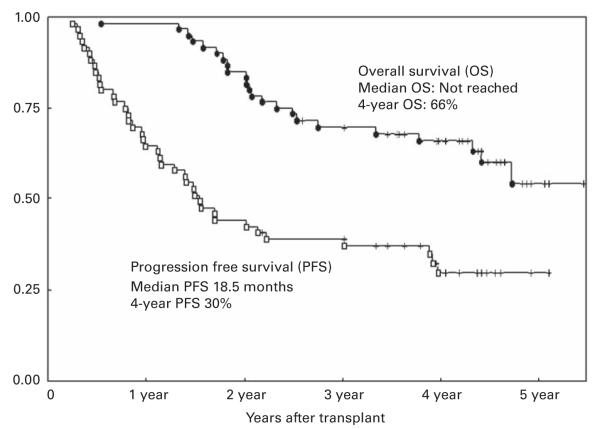
The median follow-up in surviving patients was 32 months (range 16–66). The median PFS for all patients was 18.2 months (range 3–66) and the median OS has not yet been reached. Kaplan–Meier estimates of 4-year PFS and OS were 30 and 66%, respectively (Figure 1). At the time of this analysis, 36 patients (60%) were still alive up to 5 years after auto HCT with 20 patients (33%) in remission. In contrast to response rates, there was no improvement in PFS in patients transplanted <12 months of diagnosis or in patients with at least a PR to induction or salvage therapy before transplant. The OS was shorter in patients who failed to at least achieve a PR before auto HCT and it was marginally significant (P=0.06). In contrast, there was no improvement in OS in patients with first remission vs relapsed disease, in those who were transplanted within 12 months of diagnosis, or in those with cytogenetic abnormalities at the time of diagnosis.
Figure 1.
Kaplan–Meier graph depicting probability of PFS and OS after TMC conditioning regimen followed by auto HCT in MM patients.
Discussion
Our results show that TMC is an active conditioning regimen for auto HCT in MM, with a response rate of 85%, median PFS of 18.5 months and estimated 4-year PFS and OS of 30 and 66%, respectively. The response was observed regardless of the disease status, that is, consolidation of first remission or refractory/relapsed disease. TMC regimen was safe and well tolerated with no treatment-related deaths within 100 days of auto HCT. A majority of the common adverse events were limited to grade 1–3 gastrointestinal toxicity, mostly stomatitis and mucositis, whereas febrile neutropenia was observed in 18% of patients. There was no adverse effect on engraftment, with the median time to plt and ANC recovery of 9 and 10 days, respectively. One potential disadvantage with TMC regimen was its administration over a period of 6 days compared with high-dose melphalan that is given over 1 or 2 days. This prolonged administration may increase the cost of treatment and the duration of hospitalization.
The primary end point of the study was response rate. ORR to TMC regimen was high (85%) with significantly better response observed in patients that received auto HCT early in the course of their therapy (<12 months) and in patients that responded to the previous induction or salvage chemotherapy before auto HCT. However, this better response did not translate into improved PFS and OS. This observation in part can be explained by the low CR rate (12%) observed in our study. Attainment of CR is a predictor of sustained response and long-term survival.9,10 It can be speculated that the low CR rate may be secondary to the lower dose of melphalan used in the TMC regimen. However, increasing the melphalan dose may increase the overall toxicity of this regimen. The ORR, PFS and OS were comparable to historical controls from our institution who received high-dose melphalan only.21 These comparisons, however, are arbitrary and a phase III randomized trial will be needed to determine if TMC regimen offers any potential advantage over high-dose melphalan alone. Patients with chromosomal abnormalities, β2 microglobulin >3, before autologous transplant had lower PFS and OS in our study but failed to reach statistical significance in part because of the small sample size available for these analyses.
Several multi-agent preparative regimens have been studied in MM in the last few years. Fenk et al.12 compared the combination of idarubicin i.v. at 42 mg/m2, CY i.v. at 120 mg/kg and melphalan i.v. at 200 mg/m2 (ICM) to i.v. melphalan 200 mg/m2 alone. The ICM regimen was associated with greater toxicity and 100-day treatment-related mortality, with no significant improvement in EFS or OS. Anagnostopoulos et al.21 used a combination of thiotepa 150 mg/m2 i.v., BU 1 mg/kg orally or 0.8 mg/kg i.v. every 6 h for 10 doses and CY 60 mg/kg i.v. for 2 days (thiotepa, BU, CY) as a conditioning regimen and compared the outcomes with high-dose melphalan alone. They failed to show any improvement in CR, PFS or OS with this more intensive regimen. In another report, Benson et al.22 compared the outcomes between patients receiving BCV regimen (14 oral BU doses at 1 mg/kg IBW every 6 h on days −8 to −6, etoposide 60 mg/kg i.v. 36 h continuous infusion on days −5 and −4, and CY 120 mg/kg i.v. on days −2 and −1) with high-dose melphalan only. Like the previous two studies, they did not see any improvement in response rates, PFS or OS with the BCV regimen.22 The TMC regimen, in comparison, was well tolerated but as observed with other more intensive regimens the CR rate, PFS or OS were not significantly different from the single-agent high-dose melphalan.
In summary, TMC conditioning regimen is safe and active in patients with first remission or relapsed myeloma. A phase III randomized trial comparing TMC with high-dose melphalan alone will be needed to determine any potential benefit with this regimen. With the availability of highly active anti-myeloma agents and approaches like tandem autologous transplantation and maintenance therapy such a trial may not be feasible.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER cancer statistics review, 1975–2006. National Cancer Institute; Bethesda: 2008. Available from http://seer.cancer.gov/csr/1975_2006/ based on November 2008 SEER data submission, posted to the SEER website, 2009. [Google Scholar]
- 2.Attal M, Harousseau JL, Stoppa AM, Sotto KL, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–936. doi: 10.1200/JCO.2005.04.5807. [DOI] [PubMed] [Google Scholar]
- 5.Blade J, Rosinol L, Sureda A, Ribera JM, Diaz-Mediavilla J, Garcia-Larana J, et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 6.Conte LG, Figueroa MG, Lois VV, Cabrera CME, Leon RA, Garcia LH, et al. Prognostic value of the new international staging system in multiple myeloma. Comparison with Durie-Salmon staging system. Rev Med Chil. 2008;136:7–12. [PubMed] [Google Scholar]
- 7.Qazilbash MH, Saliba RM, Ahmed B, Parikh G, Mendoza F, Ashraf N, et al. Deletion of the short arm of chromosome 1 (del 1p) is a strong predictor of poor outcome in myeloma patients undergoing an autotransplant. Biol Blood Marrow Transplant. 2007;13:1066–1072. doi: 10.1016/j.bbmt.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Qazilbash MH, Saliba RM, Aleman A, Lei X, Weber D, Carrasco A, et al. Risk factors for relapse after complete remission with high-dose therapy for multiple myeloma. Leuk Lymphoma. 2006;47:1360–1364. doi: 10.1080/10428190500520806. [DOI] [PubMed] [Google Scholar]
- 9.Alexanian R, Weber D, Giralt S, Dimopoulos M, Delasalle K, Smith T, et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant. 2001;27:1037–1043. doi: 10.1038/sj.bmt.1703035. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Delasalle K, Feng L, Thomas S, Giralt S, Qazilbash M, et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 2010;45:498–504. doi: 10.1038/bmt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 12.Fenk R, Schneider P, Kropff M, Huenerlituerkoglu AN, Steidl U, Aul C, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–594. doi: 10.1111/j.1365-2141.2005.05641.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimoni A, Smith TL, Aleman A, Weber D, Dimopoulos M, Anderlini P, et al. Thiotepa, busulfan, cyclophosphamide (TBC) and autologous hematopoietic transplantation: an intensive regimen for the treatment of multiple myeloma. Bone Marrow Transplant. 2001;27:821–828. doi: 10.1038/sj.bmt.1703007. [DOI] [PubMed] [Google Scholar]
- 14.Lahuerta JJ, Grande C, Martinez-Lopez J, De La Serna J, Toscano R, Ortiz MC, et al. Tandem transplants with different high-dose regimens improve the complete remission rates in multiple myeloma. Results of a Grupo Espanol de Sindromes Linfoproliferativos/Trasplante Autologo de Medula Osea phase II trial. Br J Haematol. 2003;120:296–303. doi: 10.1046/j.1365-2141.2003.04067.x. [DOI] [PubMed] [Google Scholar]
- 15.Spencer A, Prince HM, Roberts AW, Prosser IW, Bradstock KF, Coyle L, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 16.Kraut EH, Crowley JJ, Wade JL, Laufman LR, Alsina M, Taylor SA, et al. Evaluation of topotecan in resistant and relapsing multiple myeloma: a Southwest Oncology Group study. J Clin Oncol. 1998;16:589–592. doi: 10.1200/JCO.1998.16.2.589. [DOI] [PubMed] [Google Scholar]
- 17.Kraut EH, Young D, Farag S, James AG, Solove RJ. Phase II study of topotecan and cyclophosphamide in patients with relapsed and refractory multiple myeloma. Leuk Res. 2005;29:1233–1234. doi: 10.1016/j.leukres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A, et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27:837–842. doi: 10.1038/sj.bmt.1702879. [DOI] [PubMed] [Google Scholar]
- 19.Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 20.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostopoulos A, Aleman A, Ayers G, Donato M, Champlin R, Weber D, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100:2607–2612. doi: 10.1002/cncr.20294. [DOI] [PubMed] [Google Scholar]
- 22.Benson DM, Jr, Elder PJ, Lin TS, Blum W, Penza S, Avalos B, et al. High-dose melphalan versus busulfan, cyclophosphamide, and etoposide as preparative regimens for autologous stem cell transplantation in patients with multiple myeloma. Leuk Res. 2007;31:1069–1075. doi: 10.1016/j.leukres.2006.09.021. [DOI] [PubMed] [Google Scholar]