Abstract
The highly conserved eukaryotic process of macroautophagy (autophagy) is a non-specific bulk-degradation program critical for maintaining proper cellular homeostasis, and for clearing aged and damaged organelles. This decision is inextricably dependent upon prevailing metabolic demands and energy requirements of the cell. Soluble monomeric decorin functions as a natural tumor repressor that antagonizes a variety of receptor tyrosine kinases. Recently, we discovered that decorin induces endothelial cell autophagy, downstream of VEGFR2. This process was wholly dependent upon Peg3, a decorin-inducible genomically imprinted tumor suppressor gene. However, the signaling cascades responsible have remained elusive. In this report we discovered that Vps34, a class III phosphoinositide kinase, is an upstream kinase required for Peg3 induction. Moreover, decorin triggered differential formation of Vps34/Beclin 1 complexes with concomitant dissolution of inhibitive Bcl-2/Beclin 1 complexes. Further, decorin inhibited anti-autophagic signaling via suppression of Akt/mTOR/p70S6K activity with the concurrent activation of pro-autophagic AMPK-mediated signaling cascades. Mechanistically, AMPK is downstream of VEGFR2 and inhibition of AMPK signaling abrogated decorin-evoked autophagy. Collectively, these findings hint at the complexity of the underlying molecular relays necessary for decorin-evoked endothelial cell autophagy and reveal important therapeutic targets for augmenting autophagy and combatting tumor angiogenesis.
Keywords: Decorin, IR-A, Signaling, Proliferation
1. Introduction
The role of matrix constituents in regulating diverse intracellular processes has emerged as a key regulator of tumorigenic growth (Iozzo and Sanderson, 2011; Karamanos and Tzanakakis, 2012). In particular, the SLRP class remains as the iconoclastic gene family for potently restraining tumorigenesis (Iozzo and Sanderson, 2011; Iozzo et al., 2011b). Initially characterized as a critical regulator of collagen fibrillogenesis and a modulator of growth factor activity (Iozzo and Cohen, 1993; Reed and Iozzo, 2002), decorin has matured into a truly multifaceted signaling molecule derived from the matrix (Schaefer and Iozzo, 2008; Merline et al., 2012). For example, decorin is elevated in diabetes and patients with septicemia (Merline et al., 2009, 2011), and plays a role in modulating the biophysical properties of tendons and ligaments (Häkkinen et al., 2000; Robinson et al., 2005; Dunkman et al., 2013), in collagen fibrillogenesis (Ferdous et al., 2007; Rühland et al., 2007; Zhang et al., 2009; Reese et al., 2013), myogenesis (Brandan and Gutierrez, 2013), angiogenesis (Grant et al., 2002; Schönherr et al., 2004; Järveläinen et al., 2006), bone and skin homeostasis (Ameye and Young, 2002; Nikitovic et al., 2012), Wnt signaling (Ichii et al., 2012), and vertebrate convergent extension (Zoeller et al., 2009). Moreover, a rapidly expanding body of evidence implicates a complex biological interplay between decorin and the Toll-like family of receptors for innate immunomodulation (Frey et al., 2013).
However, the most well-established and therapeutically-compelling property of decorin is functioning as a soluble tumor repressor for inhibiting cancer growth, migration, and angiogenesis (Reed et al., 2002; Schaefer and Iozzo, 2008; Buraschi et al., 2010; Iozzo and Schaefer, 2010; Neill et al., 2012a, 2012b; Schaefer and Iozzo, 2012). Indeed mutant animals deficient in both decorin and the tumor suppressor p53 die prematurely of aggressive T-cell lymphomas (Iozzo et al., 1999a) and decorin-null mice have a propensity to develop intestinal neoplasia especially when fed a high-fat western diet (Bi et al., 2008, 2012). Our current working model envisages a direct binding of monomeric soluble decorin (Goldoni et al., 2004) to various RTKs typically over-expressed by the tumor parenchyma (Neill et al., 2012b). Decorin binding triggers transient activation, receptor dimerization, internalization and consequent degradation of the target RTK (Iozzo et al., 1999b; Goldoni and Iozzo, 2008). Decorin attenuates RTK downstream signaling of EGFR (Iozzo et al., 1999b), ErbB2 (Santra et al., 2000), ErbB4 (Minor et al., 2011), Met (Goldoni et al., 2009), PDGFR (Baghy et al., 2013), and IGF-IR (Iozzo et al., 2011a). The only signaling system decorin activity differs involves the IGF/IGF-IR pathway whereby decorin neither perturbs receptor stability nor promotes its internalization (Morrione et al., 2013). Importantly, decorin downregulates potent oncogenes (Myc, β-catenin, and HIF-1α) and concurrently induces antiangiogenic effectors, resulting in a suppression of angiogenesis (Buraschi et al., 2010; Neill et al., 2012a, 2013a). Further, an immunomodulatory role for decorin in cancer has been found (Merline et al., 2011), and decorin might also play a role in the progression of both human and mouse hepatocellular carcinomas (Baghy et al., 2013; Duncan, 2013; Horváth et al., 2013). Thus, decorin could function as a pan-RTK inhibitor thereby earning the title of “guardian from the matrix” (Neill et al., 2012b).
Despite the wealth of knowledge concerning decorin and the inhibition of tumorigenesis in vitro (Buraschi et al., 2010) and in vivo via systemic and adenovirus-based decorin gene therapy (Reed et al., 2005; Goldoni et al., 2008), it was discovered that decorin exclusively modulates gene networks within the tumor microenvironment (Buraschi et al., 2012). Unexpectedly, during a pre-clinical screen searching for decorin-regulated genes, a novel microarray was employed capable of high-resolution transcriptome profiling of both human tumor mRNAs (i.e. the triple negative breast carcinoma) and the stroma (derived from Mus musculus) on the same chip (Buraschi et al., 2012). We discovered that decorin differentially regulates a small subset of stromal-specific genes (Buraschi et al., 2012). Among these is the genomically-imprinted tumor suppressor gene Peg3 (Feng et al., 2008). Human PEG3 is silenced in a number of cancers by either biallelic loss of heterozygosity or promoter hypermethylation (Maegawa et al., 2001; Dowdy et al., 2005; Feng et al., 2008; Jiang et al., 2010).
Delving into the mechanism underlying decorin-mediated regulation of Peg3 and utilizing endothelial cells as a proxy for the tumor microenvironment, we serendipitously discovered that Peg3 co-localizes with Beclin 1 and LC3, critical factors orchestrating autophagy (Buraschi et al., 2013). We further found that decorin evokes autophagy by transcriptionally upregulating autophagic effectors such as BECN1 and MAPLC3A (Buraschi et al., 2013). Autophagic induction within macrovascular and microvascular endothelial cells critically relied on Peg3, as RNAi-mediated depletion of Peg3 abrogated both decorin as well as responses elicited from traditional autophagic stimuli, such as rapamycin and nutrient deprivation (Buraschi et al., 2013). In essence, Peg3 functions as a master regulator of autophagy and this signaling is regulated upstream by the major endothelial cell receptor, VEGFR2 (Buraschi et al., 2013). Intriguingly, decorin evokes Peg3 induction via direct, high-affinity binding interactions with specific VEGFR2 epitopes. It is known that leucine-rich repeat (LRR) 7 on the concave surface of decorin is involved in mediating decorin binding EGFR and ErbB4 (Goldoni et al., 2004). Further, it has been shown that LRR5 contains binding determinants for directing decorin-VEGFR2 interactions (Khan et al., 2011). Thus, decorin might interact with VEGFR2 via binding sites located within LRR5-7. Therefore, we postulate that decorin quells endothelial cell-directed angiogenesis via autophagy induction, in a Peg3-dependent manner (Neill et al., 2013b).
In spite of these advances, we currently do not know the various biomolecular relays decorin utilizes for signal transduction and activation of the autophagic machinery. However, an emerging participant, AMPK is proving vital (Kim et al., 2011). AMPK exists as a heterotrimeric (e.g. α, β, and γ subunits) signaling complex that functions as an energy sensor and favors homeostatic balance for the maintenance of cellular energy networks (Liang and Milson, 2013). Importantly, the α subunit functions as the catalytic center for AMPK activity (Liang and Milson, 2013). AMPK achieves this balance by blocking de novo protein synthesis (via PI3K/Akt/mTOR inhibition), inducing cell cycle arrest (via p27Kip1), and enhancing glucose transport and fatty acid oxidation (Kuhajda, 2008). Intriguingly, cell cycle arrest is a hallmark of decorin and mediated by p21WAF1 and p27Kip1 induction (Santra et al., 1997; Schönherr et al., 2001; Xaus et al., 2001). However, for autophagic induction, AMPK affects two key signaling pathways (Kim et al., 2011). First, AMPK prevents mTORC1-mediated phosphorylation and disassembly of the tri-molecular initiator complex that is required for assembling Vps34/Beclin 1 complexes for isolation membrane formation. Second, AMPK directly phosphorylates and activates ULK1, and thereby promotes downstream signaling (Kim et al., 2011).
Our primary goal was the deconstruction of the signaling pathways emanating from VEGFR2 that are ultimately responsible. We present novel data that directly interrogate and identify critical and necessary upstream effectors. Collectively, we have elucidated novel signaling roles, required by decorin, for both Vps34 and AMPK in transducing a signal to the autophagic machinery (Peg3, Beclin 1, and LC3) for orchestrating decorin-evoked endothelial cell autophagy.
2. Results
2.1. Vps34 is required for decorin evoked Peg3 induction in endothelial cells
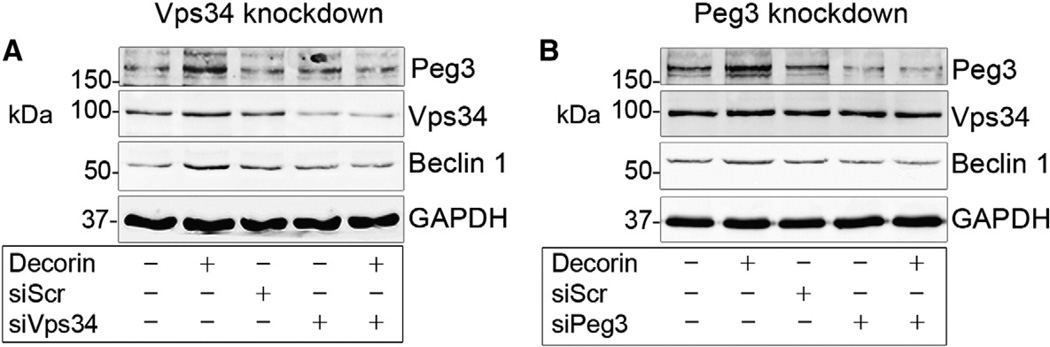
The anti-oncogenic class III PI3K Vps34, has been implicated as a critical upstream regulator of autophagy and appears to be required for autophagic initiation (Funderburk et al., 2010). Therefore, we investigated the potential contributions of Vps34 in relaying signals for decorin-evoked endothelial cell autophagy. As such, we depleted endogenous Vps34 (siVps34) via an RNAi approach in HUVEC. After verifying transient Vps34 knockdown (Fig. 1A) relative to appropriate scrambled siRNA controls (siScr), we observed that loss of Vps34 wholly abrogated decorin evoked induction of Peg3 and Beclin 1, when compared with decorin alone (Fig. 1A). Importantly, performing the reciprocal experiment where Peg3 was effectively silenced (Fig. 1B), we noted no change in Vps34 or Beclin 1 (Fig. 1B).
Fig. 1.
Vps34 is required for decorin evoked Peg3 induction in endothelial cells. [A] HUVEC were transfected with either scrambled siRNA (siScr, 20 pM) or Vps34-targeting siRNA (siVps34, 1 nM) and challenged with 200 nM decorin for 6 h in full HUVEC culture medium (please refer to Section 4.1 for additional details). Representative immunoblot analyses confirmed efficacy of Vps34 depletion. [B] HUVEC were transfected with siPeg3 (1 nM) and challenged with 200 nM decorin for 6 h relative to siScr (20 pM) transfected controls. Immunoblot confirmed RNAi-mediated silencing of Peg3 in HUVEC. GAPDH served as a positive loading control. Experiment was repeated at least four independent times with comparable results.
These data indicate that Vps34 functions upstream of the Peg3/Beclin 1 complex (Buraschi et al., 2013) as loss of Peg3 did not abrogate Peg3 or Beclin 1 induction, via decorin, in a manner similar to loss of Vps34. Therefore, Vps34 functions as an upstream kinase that relays signals permissive for decorin-evoked stimulation of pertinent autophagic markers.
2.2. Decorin stimulates formation of a Vps34/Beclin 1 complex while simultaneously inhibiting formation of the Bcl-2/Beclin 1 complex
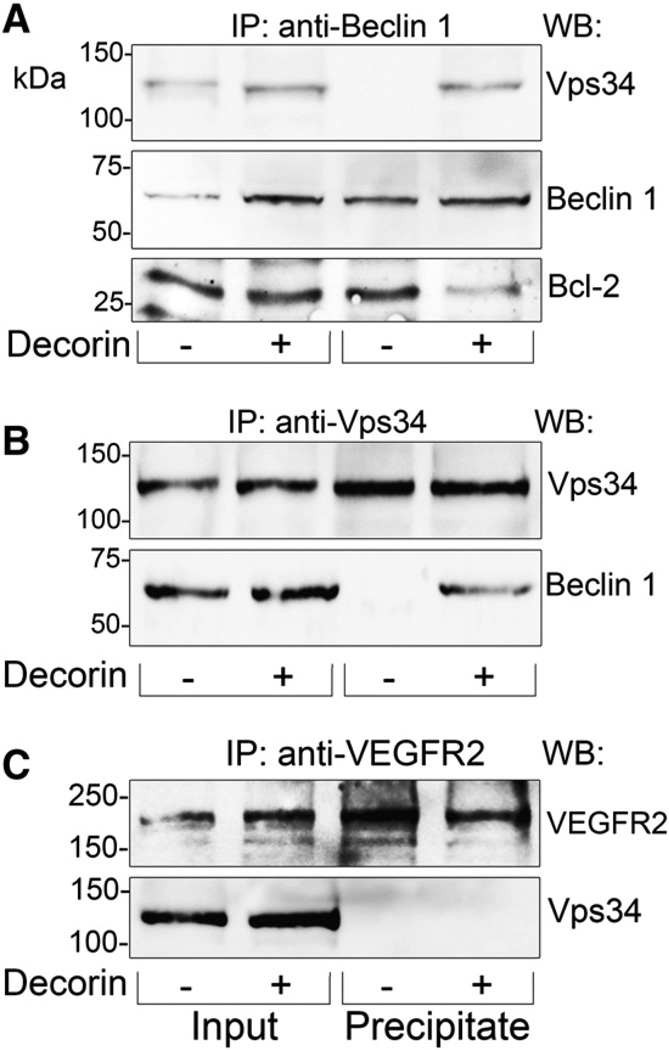
Next, we explored potential upstream regulatory mechanisms by which decorin induces autophagosome formation in endothelial cells. Under basal conditions, where energy homeostasis is not compromised, Beclin 1 is rendered inactive at the mitochondrial membrane by Bcl-2 via direct interactions with the Beclin 1 BH3 domain (Rubinsztein et al., 2012). However when metabolic homeostasis is disrupted (e.g. rapamycin, nutrient deprivation, decorin), autophagic induction occurs via disengagement of Beclin 1 fromBcl-2 and re-association with Vps34, thereby forming an active and competent Vps34/Beclin 1 signalome (Rubinsztein et al., 2012). Thus, we hypothesized that decorin could enhance association of Beclin 1 with Vps34 concurrent with attenuating Bcl-2 binding for autophagic activation. Utilizing co-immunoprecipitation using antibodies directed toward either Beclin 1 (Fig. 2A) or Vps34 (Fig. 2B), we found that decorin promoted a physical association of Vps34 with Beclin 1. Intriguingly, this interaction was concomitant with the dissociation of Bcl-2 from Beclin 1 (Fig. 2A, bottom panel). Mechanistically, the dissociation of Bcl-2 from Beclin 1 followed by an increase in binding of Vps34 with Beclin 1 manifests as autophagic initiation.
Fig. 2.
Decorin stimulates formation of a Vps34/Beclin 1 complex while simultaneously inhibiting formation of the Bcl-2/Beclin 1 complex. [A–C] Representative co-immunoprecipitation experiments performed in HUVEC with Beclin 1 [A], Vps34 [B], or VEGFR2 [C] immunoprecipitates following stimulation with 200 nM decorin for 6 h. All immunoprecipitates were executed with 5 µg of appropriate primary antibody. Notably, for Beclin 1 immunodetection [A,B], membranes were incubated with a mouse anti-rabbit light chain antibody following primary antibody incubation that effectively switches the antibody isotype from rabbit to mouse and thereby precludes detection of the heavy IgG chain in this region from immunoprecipitates. All experiments were repeated at least three independent times with similar results.
Previously, we have shown that competent VEGFR2 signaling is required for decorin evoked autophagy as pharmacological inhibition with SU5416 or genetic depletion with targeting RNAi prevented autophagy marker induction (Neill et al., 2013b). Thus we investigated whether a functional pool of Vps34 could directly associate with VEGFR2 and thereby downstream of VEGFR2 tyrosine kinase activity following decorin stimulation. The results of several experiments showed that there was no detectable association of VEGFR2 with Vps34 (Fig. 2C).
Collectively, these data indicate a combinatorial interaction of Beclin 1 with Vps34 with simultaneous loss of binding to Bcl-2. These findings elucidate the early upstream signaling events necessary for autophagy. Surprisingly, Vps34 did not bind VEGFR2, therefore an intermediate kinase acting between VEGFR2 and Vps34 relays the initial impetus for autophagic induction.
2.3. Vps34mobilizes from plasma membrane and co-localizes with Beclin 1 in a decorin-dependent manner
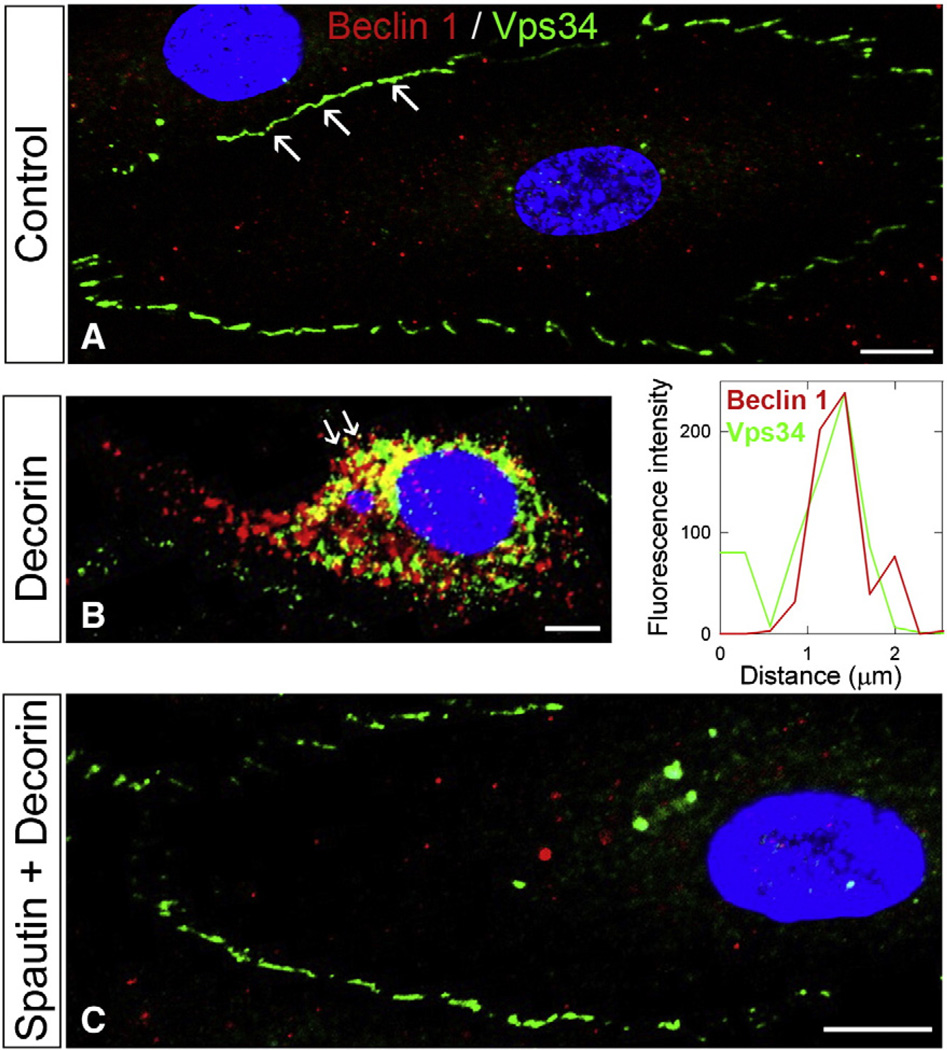
To further corroborate the above-described co-immunoprecipitation studies of Vps34 and Beclin 1, we employed confocal laser scanning microscopy while under the influence of decorin. Under prevailing metabolic conditions, Vps34 localized at discrete patches along the plasma membrane (white arrows, Fig. 3A) while Beclin 1 retained a cytoplasmically diffuse staining pattern (Fig. 3A). However, following a 6-h stimulation with decorin, Vps34 mobilized from the plasma membrane and displayed strong co-localization with Beclin 1 (Fig. 3B, left panel). To reinforce co-localization, we used line scanning, a semi-quantitative technique (Goyal et al., 2012) which permits an unbiased assessment of differentially-labeled pixels (e.g. fluorophores) for co-localization studies. Thus, upon decorin treatment, Vps34 (green) and Beclin 1 (red) co-localized significantly, as shown by the calculated line scanning signatures (Fig. 3B, right panel), reinforcing our biochemical findings (cfr. Fig. 2B). In contrast, recruitment of Vps34 into Beclin 1-positive puncta was blocked with the specific autophagy inhibitor spautin-1, which suppresses USP10 activity (Liu et al., 2011) following decorin (Fig. 3C). Notably, Vps34 retained plasma membrane staining, whereas total Beclin 1 levels were slightly decreased relative to control (Fig. 3C), consistent with the proposed mechanism of action for spautin-1 (Liu et al., 2011). Therefore, our biochemical and confocal analyses indicate an association between Vps34 and Beclin 1 under the influence of decorin. This was efficiently blocked with a small molecule inhibitor that promotes degradation of Vps34-containing complexes.
Fig. 3.
Vps34 mobilizes from plasma membrane and co-localizes with Beclin 1 in a decorin-dependent manner. [A] Confocal laser scanning microscopy evaluating Vps34 and Beclin-1 distribution in HUVEC under basal conditions [B, left panel] Decorin (200 nM, 6 h) stimulated HUVEC depicting redistribution and co-localization of Vps34 with Beclin 1 as shown in the accompanying line scanning profile [B, right panel]. [C] HUVEC were pre-treated with Spautin-1 (10 µM, 30 min) then challenged with decorin (200 nM, 6 h) before evaluation of Vps34 and Beclin-1. Nuclei were stained with DAPI. Images were collected with the same gain, intensity, and exposure. Further, images are representative of multiple independent experiments (n = 4–5). Scale bar: ~6 µm.
2.4. Decorin attenuates activation of the anti-autophagic Akt/mTOR/p70S6K pathway
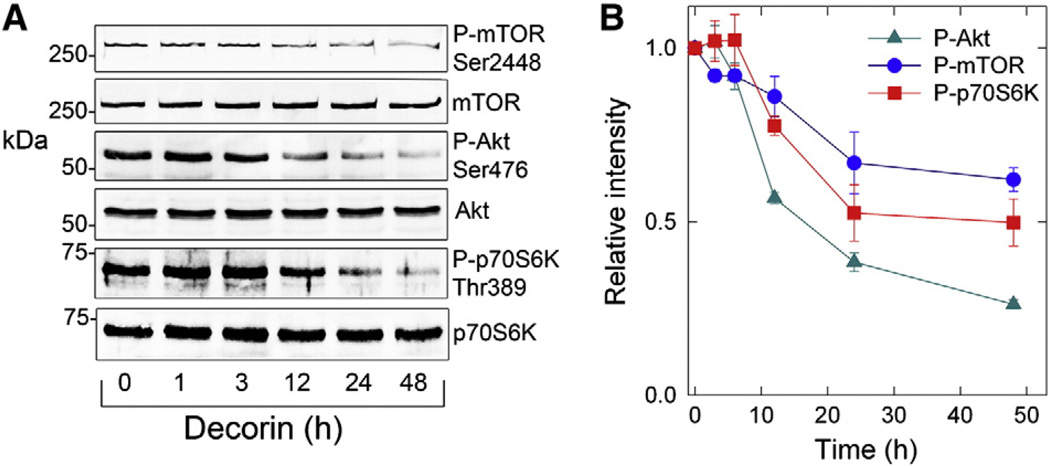
Previous studies have demonstrated an anti-autophagic role mediated by the Akt/mTOR/p70S6K signaling axis (Rubinsztein et al., 2012). Based on the positive and differential regulation of Vps34 and Beclin 1, we postulated that components of the Akt/mTOR/p70S6K pathway would be attenuated in response to the pro-autophagic properties afforded by soluble decorin. To this end, we performed time-course experiments using HUVEC under nutrient-enriched conditions as above ± equimolar amounts (200 nM) of soluble decorin. We found a time-dependent decrease in the phosphorylated species of Akt, mTOR, and p70S6K (Fig. 4A), with Akt showing the most severe inhibitory phenotype beginning at 12 h (Fig. 4A,B) relative to total protein species. Moreover, decorin exerted a protracted attenuation of P-Akt, P-mTOR, and P-p70S6K at 24 h and lasting until 48 h (Fig. 4B), without affecting total protein levels.
Fig. 4.
Decorin suppresses activation of the anti-autophagic Akt/mTOR/p70S6K pathway. [A] Representative immunoblots depicting P-mTOR (Ser2448), mTOR; P-Akt (S476), Akt; and P-p70S6K (Thr389), p70S6K following treatment with decorin (200 nM) at the indicated time points. [B] Corresponding quantification of phosphorylated Akt, mTOR, and p70S6K as reported in [A] following normalization of the phospho-signal to total Akt, mTOR, and p70S6K, respectively. All experiments have been repeated several times (n = 4–5) with similar kinetics concerning phosphorylation of target proteins in HUVEC while under the influence of decorin.
2.5. Decorin promotes activation of a pro-autophagic pathway via AMPKα phosphorylation
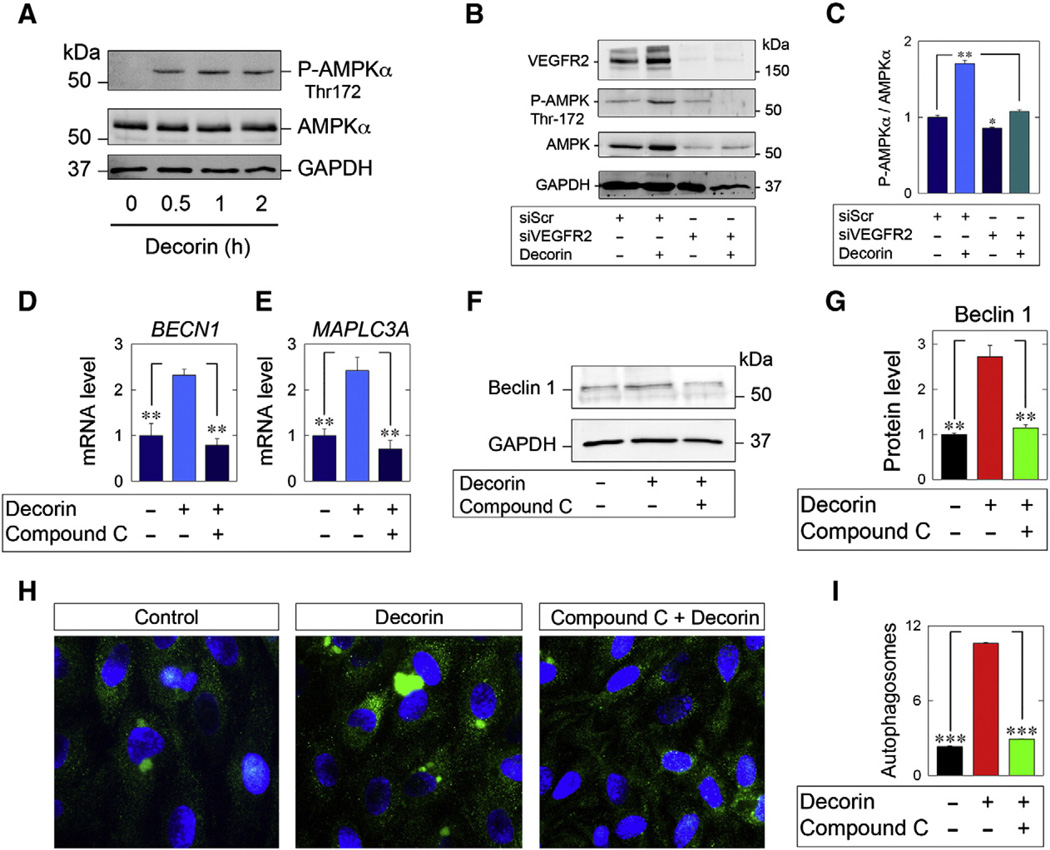
As we have shown above, decorin attenuates a potent anti-autophagic pathway, presumably downstream of VEGFR2. Autophagic induction is regulated via the positive regulatory function of the master energy sensing kinase, AMPK with ULK1 (Kuhajda, 2008; Lee et al., 2010; Kim et al., 2011). AMPK is a heterotrimeric complex comprised of α, β, and γ subunits (Alers et al., 2012). The latter subunits function as regulatory modules that interrogate cellular levels of AMP (Kuhajda, 2008). The catalytic effector activity of AMPK is mediated and contained wholly within the α subunit (Alers et al., 2012). Phosphorylation of the T-loop (at residue Thr172) by the upstream activation complex STRAD1/MO25 in response to a perturbed energy imbalance serves as a major impetus for autophagy (Kuhajda, 2008; Kim et al., 2011). Thus, we hypothesized that decorin would attenuate autophagy by evoking phosphorylation of AMPK at Thr172. We discovered for the first time that decorin evoked a rapid and time-dependent phosphorylation of AMPKα at Thr172 in as little as 30 min and this induction was maintained for up to 2 h in HUVEC cultured in nutrient-enriched (glucose containing-full bovine serum) with no apparent changes in total AMPKα levels (Fig. 5A).
Fig. 5.
Decorin promotes activation of a pro-autophagic pathway via AMPKα phosphorylation. [A] Representative immunoblot interrogating P-AMPKα (Thr172) in response to decorin (200 nM) at the indicated time points [B,C] Representative immunoblots depicting RNAi-mediated silencing of VEGFR2 (siVEGFR2, 80pM) relative to siScr control (20 pM) in HUVEC stimulated with 200 nM decorin for 1 h [B] and corresponding quantification [C]. [D,E] Gene expression analysis via qPCR of BECN1 [D] and MAPLC3A [E] following pre-treatment with Compound C (30 µM, 30 min) followed by a challenge with decorin (200 nM, 4 h). Data were normalized to the endogenous housekeeping gene, ACTB. [F,G] Representative immunoblots of Beclin 1 [F] following pre-incubation with Compound C (30 µM, 30 min) followed by decorin (200 nM, 4 h) and quantified [G] after normalization to GAPDH. [H] Immunofluorescence imaging of LC3-positive autophagosome formation ensuing treatment with either decorin alone (200 nM, 3 h) or in combination with Compound C (30 µM, 30 min pre-treatment) in HUVEC. All images were procured with the same gain, intensity, and exposure. Nuclei were visualized with DAPI. [I] Quantification of the number of autophagosomes per cell in HUVEC treated with decorin only (200 nM, 3 h) or pre-treated with Compound C (30 µM, 30 min). Fold changes ± SEM have been reported for gene expression analyses [D,E] and were calculated in accordance with the ΔΔCt method (please consult Section 4.6 for more information). All experiments reported were repeated at least three independent times. ***p < 0.001; **p < 0.01.
As shown previously, VEGFR2 is entirely dependent for decorin evoked endothelial cell autophagy (Buraschi et al., 2013). We thus interrogated if AMPK is under VEGFR2 control. Canonical AMPK activation occurs via STRAD1/MO25α (Liang and Milson, 2013). Surprisingly, depletion of VEGFR2 abrogated decorin-evoked activation of AMPKα at Thr172 (Fig. 5B,C). Furthermore, knockdown of VEGFR2 alone decreased basal AMPKα activity (Fig. 5B,C). These data reveal that decorin exerts a non-canonical activation of AMPK via VEGFR2 and this receptor is required for maintaining basal AMPK activity and activation.
Next we evaluated the relevance of AMPK signaling for decorin-mediated autophagy in endothelial cells. As reported previously, decorin transcriptionally induces BECN1 and MAPLC3A expression in a Peg3-dependent manner (Buraschi et al., 2013). As such, utilizing an established AMPKαinhibitor, Compound C (Kim et al., 2011), we discovered that blocking AMPK signaling completed prevented induction of BECN1 (Fig. 5D) and MAPLC3A (Fig. 5E) in a decorin-dependent manner. Moreover, evaluation of Beclin 1 protein showed the same response pattern insofar as inhibition via Compound C precluded decorin from inducing Beclin 1 protein (Fig. 5F). Indeed, levels of Beclin 1 in the presence of Compound C resembled control Beclin 1 levels (Fig. 5G).
Decorin promotes the formation of LC3 positive autophagosomes (Buraschi et al., 2013), a key biological manifestation of mature autophagy. Importantly, pre-treatment with Compound C prevented decorin stimulated autophagosome formation relative to decorin alone (Fig. 5H). The total number of autophagosomes formed while under the influence of Compound C mirrored control conditions (Fig. 5I).
Collectively, our data indicate a critical role for AMPK in transducing a pro-autophagic signal in response to decorin for positive ATG marker expression and formation of LC3 positive autophagosomes in endothelial cells.
3. Discussion
Autophagy is a tightly regulated process that results in non-specific degradation of cellular organelles that have been compromised or have surpassed a functionally viable threshold (Mizushima et al., 2010). The physiological importance of proper autophagic induction and maintenance is underscored in various pathologies where regulatory circuits are malfunctioning as manifest in certain forms of Parkinsons, cachexia, angiogenesis, and cancer (Liang et al., 1999; Levine and Kroemer, 2008; Dagda et al., 2009; Du et al., 2012; Choi et al., 2013). Autophagic initiation is regulated by a dichotomous system that functions as a molecular switch, integrating information derived from ambient metabolic and energy demands for maintaining cellular integrity and homeostasis (Funderburk et al., 2010). Two critical pathways are involved for regulating autophagy, the anti-autophagic PI3K/Akt/mTOR/p70S6K signaling arm and the pro-autophagic AMPK/ULK1/Vps34/Beclin 1 initiation cascade (Kim et al., 2011).
Interrogating the function of decorin-inducible Peg3, we serendipitously discovered that decorin induces endothelial cell autophagy downstream of VEGFR2 in a strict Peg3-dependent manner (Buraschi et al., 2013). The requirement of Peg3 for autophagy gene induction (BECN1 and MAPLC3A) and LC3-positive autophagosome formation by a variety of stimuli, both canonical (nutrient deprivation, rapamycin) and matrix derived (e.g. soluble decorin), permitted classification of Peg3 as a master autophagic regulator (Neill et al., 2013b). Autophagic induction mediated by decorin was identical between decorin proteoglycan and decorin protein core, therefore suggesting a dispensable role for the single glycosaminoglycan chain (Buraschi et al., 2013). However, the function of the glycosaminoglycan chain is context dependent and is seemingly necessary for immunomodulatory functions orchestrated by decorin in differentially regulating anti- and pro-inflammatory signals downstream of TLR2/4 (Merline et al., 2011).
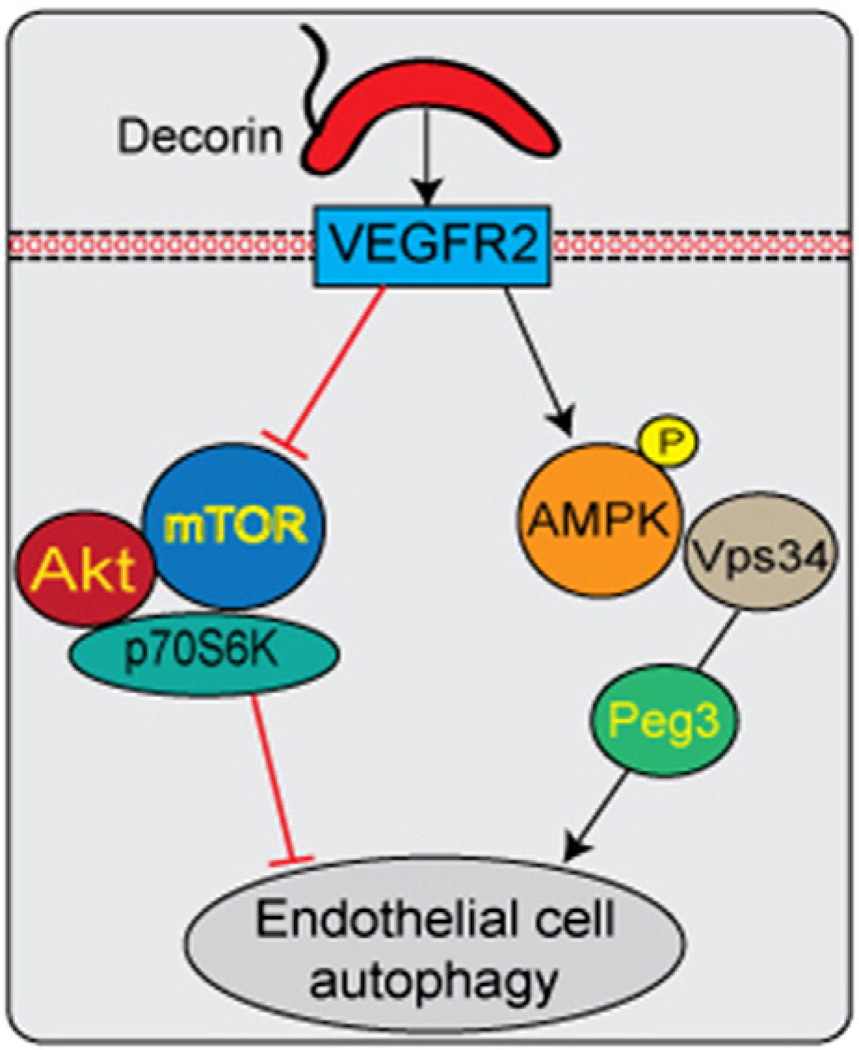
In the current study we have identified components of the upstream signaling apparatus responsible for activating autophagy in endothelial cells. According to our current model (Fig. 6), we have organized the signaling hierarchy insofar as Vps34 functions upstream of Peg3 as depletion of Vps34 prevents decorin-evoked induction of autophagy. Importantly, reciprocal known-down of Peg3 has no effect on Vps34. These results are intriguing as Vps34 is considered a potential tumor suppressor (Funderburk et al., 2010) that may phosphorylate Peg3, either directly or indirectly as part of a supramolecular complex responsible for autophagic initiation. As autophagic processes are intricately tied with metabolic inputs and energy sensors (see below), Peg3 might play a critical over-arching role in modulating cellular metabolism by integrating external stimuli. This is apparent, as mice carrying inactivation mutations of Peg3 have increased body fat (Curley et al., 2005).
Fig. 6.
Working model depicting the proposed mechanism through which decorin induces endothelial cell autophagy via differential modulation of anti- and pro-autophagic signaling cascades downstream of VEGFR2. Decorin functions as a soluble pro-autophagic signal via binding cell surface localized VEGFR2 at the endothelial cell membrane for potent and sustained activation of AMPKα. Concurrently, decorin attenuates the anti-autophagic arm (Akt/mTOR/p70S6K). Moreover, decorin promotes the differential assembly of Vps34/Beclin 1 positive complexes by overriding the putatively repressive association of Beclin 1with Bcl-2. Collectively, modulation of these signaling cascades provides mechanistic insights into decorin evoked endothelial cell autophagy.
Beclin 1 activation is an important rate-limiting step in forming the isolation membrane (Levine and Kroemer, 2008; Lee et al., 2009). Indeed, Beclin 1 is sequestered and rendered inactive by the antiapoptotic protein Bcl-2 via direct interaction with the BH3 domain of Beclin 1 at the outer mitochondrial membrane (Pattingre et al., 2005; Pattingre and Levine, 2006). Upon autophagic stimuli, Bcl-2 releases Beclin 1 and associates with Vps34 for positive autophagic signaling (Pattingre and Levine, 2006; Funderburk et al., 2010). This combinatorial switch is recapitulated in the presence of decorin insofar as Beclin 1 bound Vps34 in higher abundance and with a corresponding decrease in Bcl-2/Beclin 1 complexes. As Beclin 1 binds Peg3 (Buraschi et al., 2013), it remains plausible that Peg3 competes for Beclin 1, perhaps via an interaction with the BH3 domain of Beclin 1, and thus disengaging inhibitory Bcl-2/Beclin 1 complexes. Moreover, Bcl-2 over-expression is associated with aggressive tumorigenesis and a concomitant decrease in autophagy (Pattingre and Levine, 2006). As decorin ameliorates the pro-tumorigenic properties of the surrounding tumor microenvironment, differential binding of Vps34 with Beclin 1 for upstream autophagic initiation might underlie this transition away from a nurturing, tumorigenic state.
Despite the importance of VEGFR2 and Vps34 in decorin mediated induction of autophagy, no discernible association of VEGFR2 with Vps34 was found. It is possible that an unbeknownst intermediate kinase functions between VEGFR2 and Vps34. Discovery of this kinase would prove critical for understanding the full contribution of VEGFR2 in autophagic activation.
Co-localization of Beclin 1/Vps34 complexes confirmed our combinatorial association in the presence of decorin. Strikingly, Vps34 under basal conditions exhibits a some-what linear plasma membrane localization that becomes rapidly mobilized into Beclin 1 positive puncta within the cytoplasm. This association was wholly blocked utilizing the USP10 inhibitor Spautin 1. Blocking USP10 activity prevents de-ubiquitination of Beclin 1 and permits accumulation. As ubiqutin signaling serves as an early harbinger of autophagy (Dagda et al., 2009), decorin might augment the USP10 activity (or generally modulate ubiquitin signaling in favor of autophagy) for increased Beclin 1 or Peg3 stability.
As mentioned above, autophagic induction relies on metabolic inputs and energy sensors as information for inhibition or activation, with the PI3K/Akt/mTOR/p70S6K and AMPK/ULK1 pathways serving as the molecular switches for each decision, respectively (Alers et al., 2012). The former pathway is an intrinsically staunch inhibitor of autophagic processes, as mTOR signaling promotes protein synthesis via p70S6K and other anabolic processes for continued plasticity, growth, and proliferation (Neill et al., 2013b). In contrast, autophagy is a strictly catabolic process that functions as a scavenger for recycling and shunting metabolites into vital homeostatic pathways for survival during times of metabolic duress (Neill et al., 2013b). Therefore, decorin promotes a protracted attenuation of active Akt/mTOR/p70S6K components, downstream of VEGFR2 (Fig. 6). The failure of pro-anabolicm TOR signaling will significantly stymie further protein synthesis and might affect overall cellular proteostasis. Compromised proteostatic integrity can interfere with overall cellular function, such as executing angiogenesis, by increasing autophagic flux and re-aligning cellular energy networks.
Concurrent with inhibiting a potent anti-autophagic pathway, decorin stimulates AMPK, a master energy sensing kinase (Liang and Milson, 2013) and upstream regulator of autophagy (Alers et al., 2012). Decorin requires AMPK activity, downstream of VEGFR2, for autophagic gene induction and autophagosome formation (Fig. 6). This is the first time, based on our knowledge, of an RTK regulating AMPK phosphorylation and function. Importantly, AMPK directly binds and phosphorylates ULK1 for autophagy activation (Lee et al., 2010; Kim et al., 2011). Moreover, mTORC1 mediated phosphorylation of AMPK prevents formation of AMPK/ULK1 complexes and thereby abrogates autophagy (Kim et al., 2011). This inhibitory process should be avoided as decorin attenuates mTOR signaling, thereby resulting in AMPK/ULK1 complexes for autophagic stimulation. Indeed, the AMPK/ULK1/FIP200/Atg13L complex is crucially important in forming Vps34/Beclin 1 complexes (Alers et al., 2012). Further, in concert with DAPK, active AMPK complexes directly phosphorylate Beclin 1, and perhaps Peg3, for positive regulation (Liang and Milson, 2013). Thus, it is possible that decorin utilizes this complex for Vps34/Beclin 1/Peg3 complex formation.
Taken together, our data underscore the intricate complexities of decorin, a soluble pro-autophagic stimulator, in tipping the fine balance in favor of endothelial cell autophagy (Fig. 6). The dependence of VEGFR2 in regulating autophagy via AMPK activation is novel reveals new regulatory paradigms in controlling autophagy initiation and maintenance. The molecular targets discovered herein represent crucial therapeutic targets exploitable for enhanced autophagic activity for combating tumor angiogenesis.
4. Experimental procedures
4.1. Cells and reagents
Human umbilical vein endothelial cells (HUVEC) were obtained from LifeLine Cell Technology and grown in Basal Media supplemented with VascuLife EnGS LifeFactors Kit (LifeLine Cell Technology) with cells being utilized within the first five passages. The rabbit polyclonal antibodies detecting human PEG3were purchased from Santa Cruz Biotechnology. The rabbit polyclonal antibody directed against Beclin 1 came from Abcam. Rabbit polyclonal LC3-I/II was from Sigma-Aldrich. The mouse monoclonal antibodies detecting Beclin 1 were purchased from Novus Biosciences. The monoclonal rabbit antibodies against human VEGFR2, Phospho-VEGFR2 (detecting Tyr1175), Akt, Phospho-Akt (at residue Thr308), mTOR, Phospho-mTOR (at residue Ser2448), p70S6K, Phospho-p70S6K (at residue Thr389), PI3 kinase class III (Vps34), Phospho-AMPKα (at residue Thr172), AMPKα mouse anti-rabbit light chain IgG, and GAPDH were procured from Cell Signaling. Mouse anti- β actin was from Sigma-Alrich. Secondary antibodies conjugated with HRP (both donkey anti–rabbit and sheep anti–mouse) were purchased from Millipore, Inc. Goat anti-mouse and anti-rabbit (AlexaFluor 488) and goat anti-mouse (AlexaFluor 594) antibodies were purchased from Invitrogen. Compound C was obtained from Sigma Aldrich. Spautin-1 was procured from EMD Millipore. The protein A-Sepharose magnetic beads were obtained from GE Healthcare. SuperSignal West Pico chemiluminescence substrate was purchased from Thermo Fisher Scientific. RNAi targeting human PEG3 and corresponding control siRNA (siScrambled, denoted as siScr) was purchased from Santa Cruz Biotechnology; whereas siRNA targeting VEGFR2 and Vps34 hailed from Ambion (Life Technologies). Human recombinant decorin was expressed and purified as described previously (Buraschi et al., 2012, 2013).
4.2. RNAi-mediated depletion of target genes
Transient silencing of Vps34 and Peg3 via RNAi was utilized in HUVECs as described elsewhere (Neill et al., 2012a; Buraschi et al., 2013), via a cocktail of three validated RNA oligos. Briefly, six-well plates seeded with ~2 × 105 HUVEC and incubated overnight at 37 °C until the plate reached ~70% confluency. Targeting siRNA (final concentration of 1 nM) or non-targeting (siScr) siRNA (final concentration of 20 pM) was mixed with transfection media and Lipofectamine RNAiMAX (Invitrogen). After incubation at an ambient temperature (~25 °C), the ribonucleic acid/cationic complexes were applied directly to the cells. Following a 48 h transfection, the cells were treated and lysed as per the parameters of the experimental conditions. Immunoblotting for Vps34 and Peg3 was performed as verification for evaluating the efficacy of RNAi-silencing in HUVEC. Presentation of target gene silencing has been included where necessary.
4.3. Immunoblotting and co-immunoprecipitation (Co-IP)
HUVECs were treated as necessary for the given analysis and lysed in modified RIPA buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA/EGTA/sodium vanadate, 10 mM β-glycerophosphate, and protease inhibitors: 1 mM phenylmethanesulfonyl fluoride and 10 µg/ml leupeptin/tosylphenylalanyl chloromethyl ketone/aprotinin each) for 20–25 min on ice. The insoluble material was removed by centrifugation at 14,000 ×g prior to resolution via SDS-polyacrylamide gel electrophoresis (SDS-PAGE). For co-immunoprecipitation studies, Protein A-Sepharose magnetic beads were absorbed with antibodies for 4 h at 4 °C, and precleared cell lysates were applied to the beads for 18 h at 4 °C with end-over-end mixing. After extensive washing in TBS supplemented with protease inhibitors, the beads were amply boiled in reducing buffer, and supernatants were separated SDS-PAGE. Resolved proteins were then transferred to nitrocellulose membranes (Bio-Rad), probed with the indicated antibodies, and developed with the enhanced chemiluminescence technique. Resulting chemiluminescent signatures were detected via an ImageQuant LAS-4000 (GE Healthcare) visualization platform, as described previously (Buraschi et al., 2013).
4.4. Immunofuorescence and confocal laser microscopy
HUVECs, grown upon 0.2% gelatin-coated four-chamber slides (Nunc), were exposed to decorin protein core (200 nM) for 6 h. Cells were subsequently washed with PBS and fixed for 30 min in 4% paraformaldehyde at 4 °C. Cells were blocked in PBS/1% BSA, incubated with various antibodies for 1 h, washed in PBS, and incubated for 1 h with the appropriate secondary antibodies (e.g. goat anti-mouse IgG AlexaFluor 488 and/or goat anti-rabbit IgG AlexaFluor 594). Nuclei were stained and visualized with DAPI (Vector Laboratories). Fluorescence images were acquired with the 63×, 1.3 oil-immersion objective, using a Zeiss LSM-780 confocal laser-scanning microscope equipped with the Zeiss Zen (LSM-780) software. The merged images represent single optical slices (<0.8 µm), collected with the pinhole set to 1 Airy Unit (AU) for the red channel and adjusted to give the same optical section thickness in the green and blue channels. Images were acquired in single confocal planes to determine colocalization precisely using Zeiss LSM-780 software, with filters set at 488/594 nm for dual-channel imaging, respectively. Immunofluorescence imaging was perfumed with a Leica DM5500B microscope programed with the Leica Application Suite, Advanced Fluorescence v1.8 from Leica Microsystems, Inc. All resulting immunofluorescence images were analyzed using ImageJ software (National Institutes of Health) and Adobe Photoshop CS5.1 (Adobe Systems). Line scanning was performed as described previously (Goyal et al., 2012; Buraschi et al., 2013).
4.5. RNA isolation and HUVEC complementary DNA (cDNA) library synthesis
Briefly, sub-confluent (>90%) six-well plates seeded with ~2× 105√of HUVEC's (importantly, HUVECs were used within the first five passages) and variably treated (as required per the experimental parameter) in 2% serum supplemented HUVEC media. Cells were lysed in 1 ml of TRIzol Reagent (Life Technologies) for total RNA extraction (phase-ethanol based extraction). For gene expression analyses, approximately 2 µg of total RNA was utilized and annealed with oligo dT18–20 primers and complementary DNA (cDNA) was synthesized with a SuperScript Reverse Transcriptase III kit (SSRT III, Life Technologies).
4.6. Gene expression analysis via quantitative real-time PCR (qPCR)
Gene expression analysis by quantitative real-time polymerase chain reaction (qPCR) was executed for several autophagy gene markers including BECN1, and MAPLC3A. Following cDNA synthesis, PCR amplicons representing target genes and the endogenous housekeeping gene, ACTB, were amplified in quadruplicate, independent reactions with the Brilliant SYBR Green Master Mix II reagent (Agilent Technologies, Cedar Creek, TX). All samples were then run on the Roche LightCycler 480-II Real Time PCR platform (Roche, Basel, Switzerland) and cycle number (Ct) was recorded for each independent reaction. Messenger RNA (mRNA) fold change determinations were made utilizing the Comparative Ct method. Delta Ct (ΔCt) values represent normalized gene expression levels to ACTB. Delta Delta Ct (ΔΔCt) values were calculated and represent the experimental cDNA minus the corresponding gene levels (ΔCt values) of the calibrator sample (i.e., control). Fold change determinations were calculated using the double ΔCt (ΔΔCt) Method ± SEM.
Acknowledgments
We thank all the members of the Iozzo's laboratory for their valuable input during the course of these studies. This work was supported in part by the National Institutes of Health Grants RO1 CA39481, RO1 CA47282 and RO1 CA164462 (R.V.I.), and by grants from the German Research Council SFB 815, project A5, SFB 1039, project B2, and Excellence Cluster ECCPS (L.S.). T. Neill was supported by the NIH training grant T32 AA07463.
Abbreviations
- SLRP
small leucine-rich proteoglycan
- RTK
receptor tyrosine kinase
- HUVEC
human umbilical vein endothelial cells
- Peg3
paternally expressed gene 3
- AMPK
5′ adenosine monophosphate-activated protein kinase
- mTOR
mammalian target of rapamycin
- Vps34
class III phosphoinositide kinase
- ULK1
serine/threonine-protein kinase ULK1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: crosstalk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers–Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Baghy K, Horváth Z, Regõs E, Kiss K, Schaff Z, Iozzo RV, Kovalszky I. Decorin interferes with platelet-derived growth factor receptor signaling in experimental hepatocarcinogenesis. FEBS J. 2013;280:2150–2164. doi: 10.1111/febs.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Tong C, Dokendorff A, Banroft L, Gallagher L, Guzman-Hartman G, Iozzo RV, Augenlicht LH, Yang W. Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation. Carcinogenesis. 2008;29:1435–1440. doi: 10.1093/carcin/bgn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Pohl NM, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–330. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandan E, Gutierrez J. Role of skeletal muscle proteoglycans during myogenesis. Matrix Biol. 2013;32:289–297. doi: 10.1016/j.matbio.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and downregulates β-catenin and Myc levels. J. Biol. Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraschi S, Neill T, Owens RT, Iniguez LA, Purkins G, Vadigepalli R, Evans B, Schaefer L, Peiper SC, Wang Z, Iozzo RV. Decorin protein core affects the global gene expression profile of the tumor microenvironment in a triple-negative orthotopic breast carcinoma xenograft model. PLoS ONE. 2012;7:e45559. doi: 10.1371/journal.pone.0045559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, Schaefer L, Torres AT, Iozzo RV. Decorin causes autophagy in endothelial cells via Peg3. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. N. Engl. J. Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- Curley JP, Pinnock SB, Dickson SL, Thresher R, Miyoshi N, Surani MA, Keverne EB. Increased body fat in mice with a targeted mutation of the paternally expressed imprinted gene Peg3. FASEB J. 2005;19:1302–1322. doi: 10.1096/fj.04-3216fje. [DOI] [PubMed] [Google Scholar]
- Dagda R, Cherra SJI, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J. Biol. Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy SC, Gostout BS, Shridhar V, Wu X, Smith DI, Podratz KC, Jiang S-W. Biallelic methylation and silencing of paternally expressed gene 3 (PEG3) in gynecologic cancer cell lines. Gynecol. Oncol. 2005;99:126–134. doi: 10.1016/j.ygyno.2005.05.036. [DOI] [PubMed] [Google Scholar]
- Du J, Teng R-J, Guan T, Eis A, Kaul S, Konduri GG, Shi Y. Role of autophagy in angiogenesis in aortic endothelial cells. Am. J. Physiol. Cell Physiol. 2012;302:C383–C391. doi: 10.1152/ajpcell.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MB. Extracellular matrix transcriptome dynamics in hepatocellular carcinoma. Matrix Biol. 2013;32:393–398. doi: 10.1016/j.matbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Marquez RT, Lu Z, Liu J, Lu KH, Issa J-PJ, Fishman DM, Yu Y, Bast RC. Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer. 2008;112:1489–1502. doi: 10.1002/cncr.23323. [DOI] [PubMed] [Google Scholar]
- Ferdous Z, Wei VM, Iozzo RV, Höök M, Grande-Allen KJ. Decorin-transforming growth factor-β interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J. Biol. Chem. 2007;282:35887–35898. doi: 10.1074/jbc.M705180200. [DOI] [PubMed] [Google Scholar]
- Frey T, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013;280:2165–2179. doi: 10.1111/febs.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex — at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldoni S, Iozzo RV. Tumor microenvironment: modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int. J. Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J. Biol. Chem. 2004;279:6606–6612. doi: 10.1074/jbc.M310342200. [DOI] [PubMed] [Google Scholar]
- Goldoni S, Seidler DG, Heath J, Fassan M, Baffa R, Thakur ML, Owens RA, McQuillan DJ, Iozzo RV. An anti-metastatic role for decorin in breast cancer. Am. J. Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J. Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Poluzzi C, Willis AC, Smythies J, Shellard A, Neill T, Iozzo RV. Endorepellin affects angiogenesis by antagonizing diverse VEGFR2-evoked signaling pathways: transcriptional repression of HIF-1α and VEGFA and concurrent inhibition of NFAT1 activation. J. Biol. Chem. 2012;287:43543–43556. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- Häkkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Iozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab. Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- Horváth Z, Kovalszky I, Fullár A, Kiss K, Schaff Z, Iozzo RV, Baghy K. Decorin deficiency promotes hepatic carcinogenesis. Matrix Biol. 2013;33 doi: 10.1016/j.matbio.2013.11.004. http://dx.doi.org/10.1016/j.matbio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichii M, Frank MB, Iozzo RV, Kincade PW. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119:1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Cohen I. Altered proteoglycan gene expression and the tumor stroma. Cell. Mol. Life Sci. 1993;49:447–455. doi: 10.1007/BF01923588. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Schaefer L. Proteoglycans in health and disease: novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germline mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 1999a;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Moscatello D, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J. Biol. Chem. 1999b;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Buraschi S, Genua M, Xu S-Q, Solomides CC, Peiper SC, Gomella LG, Owens RT, Morrione A. Decorin antagonizes IGF receptor I (IGF-IR) function by interfering with IGF-IR activity and attenuating downstream signaling. J. Biol. Chem. 2011a;286:34712–34721. doi: 10.1074/jbc.M111.262766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, Goldoni S, Berendsen A, Young MF. Small leucine-rich proteoglycans. In: Mecham RP, editor. Extracellular Matrix: An Overview. Springer; 2011b. pp. 197–231. [Google Scholar]
- Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage H, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yu Y, Yang HW, Agar NYR, Frado L, Johnson MD. The imprinted gene PEG3 inhibits Wnt signaling and regulates glioma growth. J. Biol. Chem. 2010;285:8472–8480. doi: 10.1074/jbc.M109.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanos NK, Tzanakakis GN. Glycosaminoglycans: from “cellular glue” to novel therapeutical agents. Curr. Opin. Pharmacol. 2012;12:220–222. doi: 10.1016/j.coph.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Khan GA, Girish GV, Lala N, DiGuglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Mol. Endocrinol. 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phopshorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int. J. Obes. 2008;32:536–541. doi: 10.1038/ijo.2008.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Shi J, Jeoung NH, Kim MS, Zabolotny JM, Lee SW, White MF, Wei L, Kim B-K. Targeted disruption of ROCK1 causes insulin resistance in vivo. J. Biol. Chem. 2009;284:11776–11780. doi: 10.1074/jbc.C900014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Park S, Takahashi Y, Wang H-G. The association of AMPK with ULK1 regulates autophagy. PLoS ONE. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Milson C. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norber HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Li Y, Hao Y, Choi A, Ke H, Ma D, Yuan J. Beclin 1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa S, Yoshioka H, Itaba N, Kubota N, Nishihara S, Shirayoshi Y, Nanba E, Oshimura M. Epigenetic silencing of PEG3 gene expression in human glioma cell lines. Mol. Carcinog. 2001;31:1–9. doi: 10.1002/mc.1034. [DOI] [PubMed] [Google Scholar]
- Merline R, Lazaroski S, Babelova A, Tsalastra-Greul W, Pfeilschifter J, Schluter KD, Gunther A, Iozzo RV, Schaefer RM, Schaefer L. Decorin deficiency in diabetic mice: aggravation of nephropathy due to overexpression of profibrotic factors, enhanced apoptosis and mononuclear cell infiltration. J. Physiol. Pharmacol. 2009;60(Suppl. 4):5–13. [PMC free article] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci. Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline R, Nastase MV, Iozzo RV, Schaefer L. Small leucine-rich proteoglycans: multifunctional signaling effectors. In: Karamanos N, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin: Walter de Gruytier Gmbh and Co.; 2012. pp. 185–196. [Google Scholar]
- Minor KH, Bournat JC, Toscano N, Giger RJ, Davies SJA. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011;134:1140–1155. doi: 10.1093/brain/awq304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrione A, Neill T, Iozzo RV. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013;280:2138–2149. doi: 10.1111/febs.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Painter H, Buraschi S, Owens RT, Lisanti MP, Schaefer L, Iozzo RV. Decorin antagonizes the angiogenic network. Concurrent inhibition of Met, hipoxia inducible factor-1α and vascular endothelial growth factor A and induction of thrombospondin-1 and TIMP3. J. Biol. Chem. 2012a;287:5492–5506. doi: 10.1074/jbc.M111.283499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Schaefer L, Iozzo RV. Decorin, a guardian from the matrix. Am. J. Pathol. 2012b;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Jones HR, Crane-Smith Z, Owens RT, Schaefer L, Iozzo RV. Decorin evokes rapid secretion of thrombospondin-1 in basal breast carcinoma cells via inhibition of Ras homolog gene family, member A/Rho-associated coiled-coil containing protein kinase 1. FEBS J. 2013a;280:2353–2368. doi: 10.1111/febs.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill T, Torres AT, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy. 2013b;9:1626–1628. doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- Nikitovic D, Aggelidakis J, Young MF, Iozzo RV, Karamanos NK, Tzanakakis GN. The biology of small leucine-rich proteoglycans in bone pathophysiology. J. Biol. Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Levine B. Bcl-2 inhibition of autophagy: a new route to cancer? Cancer Res. 2006;66:2885–2888. doi: 10.1158/0008-5472.CAN-05-4412. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- Reed CC, Gauldie J, Iozzo RV. Suppression of tumorigenicity by adenovirus-mediated gene transfer of decorin. Oncogene. 2002;21:3688–3695. doi: 10.1038/sj.onc.1205470. [DOI] [PubMed] [Google Scholar]
- Reed CC, Waterhouse A, Kirby S, Kay P, Owens RA, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- Reese SP, Underwood CJ, Weiss JA. Effects of decorin proteoglycan on fibrillogenesis, ultrastructure, and mechanics of type I collagen gels. Matrix Biol. 2013;32:414–423. doi: 10.1016/j.matbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J. Biomech. Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat. Rev. Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühland C, Schönherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274:4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- Santra M, Mann DM, Mercer EW, Skorski T, Calabretta B, Iozzo RV. Ectopic expression of decorin protein core causes a generalized growth suppression in neoplastic cells of various histogenetic origin and requires endogenous p21, an inhibitor of cyclin-dependent kinases. J. Clin. Invest. 1997;100:149–157. doi: 10.1172/JCI119507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra M, Eichstetter I, Iozzo RV. An anti-oncogenic role for decorin: downregulation of ErbB2 leads to growth suppression and cytodifferentiation of mammary carcinoma cells. J. Biol. Chem. 2000;275:35153–35161. doi: 10.1074/jbc.M006821200. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: from genetics to signal transduction. J. Biol. Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L, Iozzo RV. Small leucine-rich proteoglycans, at the crossroad of cancer growth and inflammation. Curr. Opin. Genet. Dev. 2012;22:56–57. doi: 10.1016/j.gde.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Levkau B, Schaefer L, Kresse H, Walsh K. Decorin-mediated signal transduction in endothelial cells. Involvement of Akt/protein kinase B in upregulation of p21WAF1/CIP1 but not p27KIP1. J. Biol. Chem. 2001;276:40687–40692. doi: 10.1074/jbc.M105426200. [DOI] [PubMed] [Google Scholar]
- Schönherr E, Sunderkotter C, Schaefer L, Thanos S, Grässel S, Oldberg Å, Iozzo RV, Young MF, Kresse H. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J. Vasc. Res. 2004;41:499–508. doi: 10.1159/000081806. [DOI] [PubMed] [Google Scholar]
- Xaus J, Comalada M, Cardó M, Valledor AF, Celada A. Decorin inhibits macrophage colony-stimulating factor proliferation of macrophages and enhances cell survival through induction of p27Kip1 and p21Waf1. Blood. 2001;98:2124–2133. doi: 10.1182/blood.v98.7.2124. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J. Biol. Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller JJ, Pimtong W, Corby H, Goldoni S, Iozzo AE, Owens RT, Ho S-Y, Iozzo RV. A central role for decorin during vertebrate convergent extension. J. Biol. Chem. 2009;284:11728–11737. doi: 10.1074/jbc.M808991200. [DOI] [PMC free article] [PubMed] [Google Scholar]