Abstract
Objective
Accumulation of reactive oxygen species (ROS) and remodeling of the microstructure of the cusp characterize aortic valve sclerosis, the early phase of calcific aortic valve disease. These events are associated with activation of valvular interstitial cells (VICs) toward an osteogenic-like phenotype. Because ROS cause DNA damage and transcriptional activation we investigated the relationship between ROS, DNA damage response, and transdifferentiation of VICs.
Methods and Results
Human aortic valve cusps and patient-matched VICs were collected from 39 patients both with and without calcific aortic valve disease. VICs were exposed to hydrogen peroxide (0.1–1 mmol/L) after cell transduction with extracellular superoxide dismutase/catalase adenoviruses and characterized for DNA-damage response, osteogenic transdifferentiation, and calcification. ROS induce relocalization of phosphorylated γH2AX, MRE11, and XRCC1 proteins with expression of osteogenic signaling molecule RUNX2 via AKT. We report a sustained activation of γH2AX in aortic valve sclerosis-derived VICs suggesting their impaired ability to repair DNA damage. Adenovirus superoxide dismutase/catalase transduction decreases ROS-induced DNA damage and VIC transdifferentiation in aortic valve sclerosis-derived cells. Finally, adenoviral transduction with catalase reverts ROS-mediated calcification and cellular transdifferentiation.
Conclusion
We conclude that the ROS-induced DNA damage response is dysfunctional in early asymptomatic stages of calcific aortic valve disease. We unveiled an association among ROS, DNA-damage response, and cellular transdifferentiation, reversible by antioxidant enzymes delivery.
Keywords: aortic valve sclerosis, DNA-damage response, reactive oxygen species, valvular interstitial cells
Calcific aortic valve disease (CAVD) is an active multifactorial process more common with increasing age, although not an inevitable consequence of aging.1–4 Over the last decade, several clinical trials have been performed to halt the progression of CAVD. Early enthusiastic findings, documenting a reduction in the progression of the disorder, have been questioned by later randomized studies, which show substantial equivalence between treatments and placebo.5–10 It has been proposed that the therapy may have been initiated too late in the course of the disease to be effective.4,11,12 Initial asymptomatic phases of CAVD include mild thickening of the cusps, without affecting the mechanical proprieties of the valve, a condition called aortic valve sclerosis (AVSc). Advanced stages are associated with impaired leaflets motion, resistance to blood flow, and biomineralization (aortic valve stenosis–AVS).2,4,13 The prevalence of AVSc has been estimated at 25% to 30% in patients >65 years of age and up to 40% in those >75 years of age.14 These patients are largely asymptomatic, and challenging to identify, because of the variable and qualitative nature of AVSc description by echocardiographic evaluation.12,14,15 Once AVSc is detectable, there is an increased risk of cardiovascular events from the expected event-free survival.16 At the onset of early symptoms (stenosis), survival curve deviates even more from expected, with a dramatic decline in case of severe symptomatic AVS. Despite its high prevalence, little is known about the early stages of development of aortic valve disease and the initiating pathogenetic mechanisms determining the thickening of the cusps, the activation of valvular interstitial cells (VICs), and their transdifferentiation into osteoblastic-like cells. In addition, asymptomatic AVSc tissues are generally not available to investigators because these valves are not surgically replaced until moderate to severe stenosis occurs.
At a microstructural level CAVD is characterized by extensive remodeling of the cusps with biomineralization of the fibrosa layer and by VICs adopting an osteogenic-like phenotype with expression of markers, such as osteopontin (OPN), osteonectin, and transcription factors, runt-related transcription factor 2 (Runx2) and muscle segment homeobox 2 (MSX2).4,15,17–19 Our previous work suggested that matricellular proteins, such as OPN, could be used for the identification of asymptomatic patients even before calcium deposition is detectable by echocardiography.15 However, the cellular mechanisms responsible for the early transdifferentiation of VICs remain unidentified. Recent studies support the concept that reactive oxygen species (ROS), likely produced by inflammatory infiltrates,20–24 play an important role in the development of the early cellular and extracellular changes associated with AVSc. Evidence of increased oxidative stress has been shown in experimental mouse models of valve stenosis, suggesting that the oxidant environment is not merely the consequence of increased cusp stress associated with calcification.1,13,25–27 ROS have been also implicated in pro-osteogenic and profibrotic signaling cascades, exogenous ROS accelerates calcification of vascular smooth muscle cells in vitro.25–27 In this regard, transcription factors, such as Runx2 and MSX2, have been shown to directly contribute to vascular calcification.28–31 Furthermore, antioxidant enzymes, such as superoxide dismutases (SOD) and catalase (CAT), are down-regulated in calcified region of human aortic valves.26
Several studies indicate that ROS can cause DNA strand breaks and base modifications.32–34 Elaborate cellular repair and genome surveillance mechanisms counteract genomic damage induced by ROS. The DNA-damage response (DDR) process is manifested cytologically by the formation of DNA-repair foci.35–38 These subnuclear structures are formed by the recruitment and accumulation of DNA-repair factors at sites of DNA damage.36,37,39,40 DNA damage results in rapid phosphorylation of γH2AX by PI3K-like kinases, including ataxia telangiectasia mutated, ATM-Rad3-related protein, and DNA-dependent protein kinase regulatory kinases of DDR.35,37 Within the repair complex, γH2AX is required for checkpoint-mediated cell cycle arrest and DNA repair after single and double-stranded DNA breaks. This very early event in the DDR is required for recruitment of a subsequent multitude of proteins, including MDC1, XRCC1, RAD50, MRE11, 53BP1, and BRCA1.37,41 In a recent study, it has been proposed that increased transcript level of poly(ADP-ribose) polymerase-1 in human tricuspid compared with bicuspid aortic valves correlates with stenosis severity.42 The study showed transcriptional activation of the DNA damage nick sensor protein poly(ADP-ribose) polymerase-1 in stenotic valves and in VICs. In addition, DNA strand breaks and poly(ADP-ribose) polymerase-1 promote recruiting of homologous recombination factors, such as ATM, ATR, and other DDR kinases.
Here, we explore the intriguing hypothesis that ROS act on the valve cusp microstructure through the activation of the DDR mechanism, inducing VICs to adopt an osteogenic phenotype typical of advanced disease phases. Oxidative stress was analyzed in explanted aortic valve tissues by immunostaining for nitro-tyrosine, a product of tyrosine nitration mediated by reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide.25 We provide evidence that ROS promote extensive oxidative DNA damage on VICs isolated from patients at different stages of CAVD. We analyzed the subnuclear relocalization of γH2AX, MRE11, and XRCC1 proteins in repair foci. Furthermore, for the first time using AVSc-derived VICs, we tested the ability of ROS to induce the expression of early osteogenic markers such as RUNX2 and MSX2 and in vitro calcification. Finally, using adenoviral transduction of SOD3 and CAT, we tested the ability of these enzymes to protect VIC from DNA damage and early phenotypic alteration.
Patients and Methods
Patient Population
Subject enrollment for the present study was performed at the Perelman School of Medicine of the University of Pennsylvania based on comprehensive echocardiographic assessment, and aortic valves were assigned calcium scores of 1 to 4. (Complete patient enrollment and demographics details are provided in Table I and Methods in the online-only Data Supplement).
Antioxidant Enzymes Expression and Activity
RNA isolation was performed on frozen tissue using the RNA Extraction Kit for Fibrotic Tissue (Qiagen, Valencia, CA) according to the manufacturer’s instruction. Real-time polymerase chain reaction was performed by SYBR green chemistry using the 7500 Fast PCR protocol (Applied Biosystems). The relative quantification of the transcripts was determined using the ddCT method calculated using the SDS software version 1.4.0. Gene expression level was standardized to actinB, and fold changes were calculated using aortic valve calcification (AVC) tissues as basal. The complete list of primer used for real-time analysis is provided in Table II in the online-only Data Supplement. SOD and CAT activity assay kits (Biovision, CA) were used to test the activity of these enzymes in the tissue of AVC, AVSc, and AVS patients.
Histological Dot Blot and Western Blotting Analysis
Protein expression was analyzed using whole tissue extract from explanted aortic valve or whole cells extract from isolated VICs using standard protocol as described in the online-only Data Supplement.
Isolation of Human Aortic VICs
Isolation of aortic VICs was performed using a modification of the method previously described.43 All the experiments were performed with cultured cells between the second and fifth passages. Cells were grown in Advanced DMEM supplemented with 10% fetal bovine serum, L-glutamine, Pen/Strep and Fungicide at 37°C, and 5% CO2.
Hydrogen Peroxide Treatment of Human-derived VICs
To induce DNA damage, human VICs isolated from AVC, AVSc, and AVS patients were treated with 1 mmol/L hydrogen peroxide (H2O2) in PBS for 1 hour at 37°C. Cells were either fixed or harvested 15 minutes, 4 hours, or 24 hours after treatment. To test cellular transdifferentiation, VICs were collected at 1, 4, and 7 days after being exposed to 1 mmol/L H2O2 in PBS for 1 hour or after 15 days of 0.1 mmol/L H2O2 exposure in osteogenic media with media changed every 3 days. The effect of oxidative stress on the expression of bone-related markers was determined by real time polymerase chain reaction and Western blotting.
Immunofluorescence Analysis
VICs were cultured on glass coverslips and treated with H2O2. After fixation, immunofluorescence was performed using confocal microscopy as described in the online-only Data Supplement.
Adenoviral Transduction
Replication-defective, type 5 Ad-SOD3, Ad-CAT, and Ad-enhanced green fluorescent protein constructs under the cytomegalovirus promoter for cell transduction with human extracellular SOD, mouse CAT, and enhanced green fluorescent protein were purchased from Vector BioLabs and Penn Vector Core of the University of Pennsylvania, respectively. Transduction was performed as described in Materials in the online-only Data Supplement.
DNA Damage and Apoptosis
Comet assay and TUNEL assay were performed following manufacturer’s instruction using VICs exposed with 1 mmol/L H2O2 in PBS for 1 hour, as described in Materials in the online-only Data Supplement.
Statistical Analysis
The data were analyzed using SPSS software (version 15; SPSS). Continuous variables were expressed as mean ± SE of mean. Comparisons of continuous variables between groups were performed with the Student t test or nonparametric (Mann–Whitney U test) tests as appropriate, depending on normal distribution. A value of P<0.05 was considered to be statistically significant. Comparisons between >2 groups were performed using Kruskal–Wallis test, with post hoc pair wise Mann–Whitney tests using the Bonferroni correction to determine significance of difference between individual groups.
Results
Oxidative Stress Accumulation Is Associated With Reduced Antioxidant Enzymes Expression and Increased DNA Damage in the Early Stage of CAVD
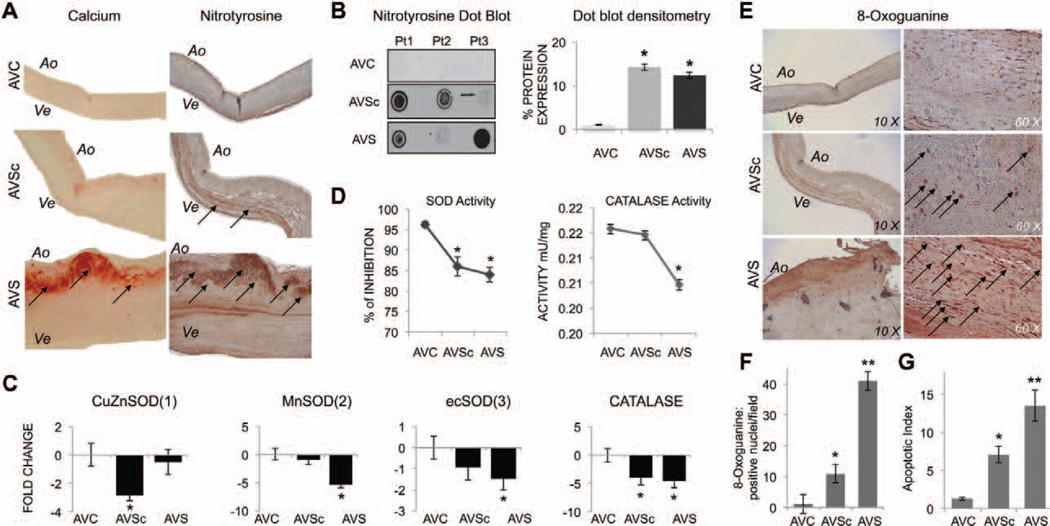
In control (transplant derived) aortic valve tissue (AVC), nitro-tyrosine staining is barely detectable and evenly distributed throughout the tissue (Figure 1A and 1B). In contrast, aortic valve tissue from patients with pathological dysfunctions of the valve (both aortic sclerosis and severe aortic stenosis) showed accumulation of nitrotyrosine, with side-specific accumulation in the fibrosa layers and accumulation of oxidative injuries around the calcified regions in AVS patients. Dot blot analysis, performed using whole cell extracts, from AVC-, AVSc- and AVS-derived tissues (n=6/group) confirmed that peroxynitrite levels were higher in calcified-stenotic (13.2±0.3 fold) and noncalcified sclerotic aortic valve tissues (14.9±0.5 fold) when compared with controls (Figure 1A and 1B; P<0.05). We then analyzed the expression of copper zinc SOD (SOD1), manganese SOD (SOD2), extracellular SOD (SOD3), and CAT (Figure 1C and 1D). mRNA analysis showed a differential downregulation of SODs expression linked to different phase of the disease with SOD1 significantly downregulated in the AVSc specimens, SOD2 downregulated in the severe stages of the disease, and SOD3 generally down-regulated in both pathological stages of CAVD (Figure 1C). In accordance, SOD total activity was reduced in sclerotic and stenotic patients, whereas CAT activity was mostly reduced in AVS patients (P<0.05; Figure 1D). Aortic valve tissues from controls also showed weaker immunoreactivity for oxidative DNA-damage marker 8-oxo-dG, whereas increased immunoreactivity of 8-oxo-dG was found in sclerotic tissues. Stenotic tissue showed strong 8-oxo-dG staining throughout all the 3 layers with highest immunoreactivity around calcifying foci (black arrows; Figure 1E and 1F). Severe DNA damage is often associated with increased rate of apoptosis in vitro. Accordingly, the analysis of native tissues demonstrated an increase in apoptosis between ex vivo control and sclerotic tissues with a significant increase in explanted, severely calcified, stenotic valves (Figure 1G).
Figure 1.
Oxidative stress accumulation is associated with reduced antioxidant enzymes expression and increased DNA damage in the early stage of calcific aortic valve disease. A, Representative images of Alizarin red stained calcifications (calcium) and nitrotyrosine staining in aortic valve tissue from AVC (control), AVSc, and AVS patients. Magnification, 10×. B, Dot blot for nitrotyrosine and relative densitometry using whole tissue extract from AVC, AVSc, and AVS tissues. Images are representative of dot blot performed in n=6 tissue/group. C, Bar graphs show fold change gene expression for superoxide dismutase 1, 2, 3 (SOD1, 2, 3) and catalase (CAT) in aortic valve tissues. Real Time polymerase chain reaction data were normalized against actinB gene expression and AVC tissue was used as basal. D, SOD and CAT activity measured in aortic valve tissue (n=3/group). *represents P<0.05 and refers to values toward AVC. E, Representative images of 8-oxo-guanine staining in aortic valve tissue from AVC, AVSc, and AVS patients. Magnification, 10× to 60×. F, Quantification of 8-oxo-guanine staining: positive nuclei were counted in 4 different field/tissue section and averaged (n=3 tissues/group analyzed). G, Quantification of apoptotic cells detected by TUNEL assay performed on tissues section. *represents P<0.05 and refers to values toward AVC. **represents P<0.05 and refers to values toward AVSc and AVC. Ao indicates aortic; AVC, aortic valve calcification; AVS, aortic valve stenosis; AVSc, aortic valve sclerosis; Pt1, 2, 3, patient number 1, 2, 3; and Ve, ventricularis.
Impaired DDR in Noncalcified, Asymptomatic, Patients With AVSc
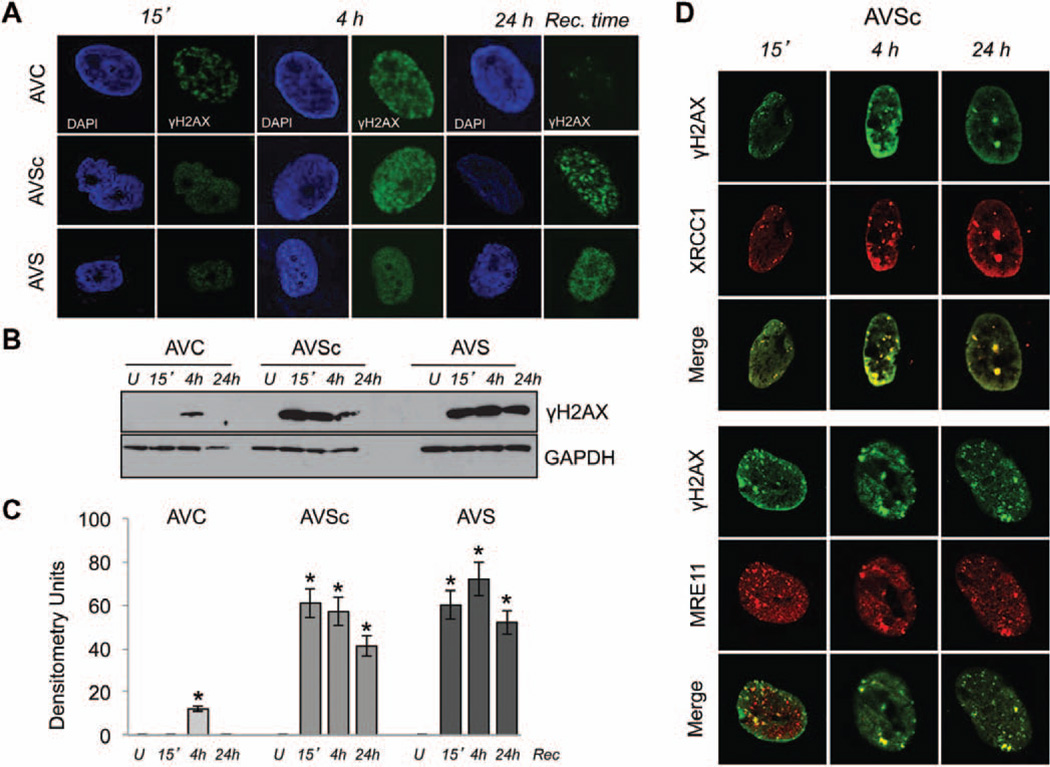
VICs were then isolated from control, AVSc, and AVS valves as previously reported.43 To examine how human VICs responded to oxidative DNA damage, cells were exposed to H2O2 (from 0.1–1 mmol/L), and early (15 minutes–4 hours) and late (24 hours) DDR was tested. As shown in Figure 2A (left panels), 15 minutes after H2O2 treatment phosphorylated H2AX (γH2AX) begin to accumulate in the nuclei of control, sclerotic, and stenotic VICs. After 4 hours of recovery all 3 cellular population showed γH2AX immunoreactivity with no differences in the nuclear localization (middle panel). Twenty-four hours after treatment, control cells showed the presence of very few repair foci, whereas sclerotic and stenotic VICs showed higher number of γH2AX foci. Western blotting analysis on total protein extracts confirm an impaired DDR in AVSc and AVS interstitial cells (Figure 2B and 2C) with sustained activation of phosphorylated H2AX (Figure 2A–2C). H2AX phosphorylation and subnuclear relocalization is a very early event of the DNA-damage response system but could also be the result of alternative events of chromatin remodeling.44,45 To show that the presence and subnuclear relocalization of γH2AX foci is a direct representation of oxidative-induced DDR mechanisms, and not because of chromatin remodeling, we tested the subnuclear colocalization of additional DDR enzymes, such as MRE11 and XRCC1. Figure 2D shows a colocalization of these proteins with γH2AX foci. Consistently, VICs derived from controls show a similar response to that of γH2AX (Figure I in the online-only Data Supplement).
Figure 2.
Impaired DNA damage response (DDR) in noncalcified, asymptomatic, patients with aortic valve sclerosis. A, Representative immunofluorescence images of γH2AX (green) in the nuclei of human valvular interstitial cells (VICs) 15 minutes (15′), 4 hours (4h), and 24 hours (24h) after exposure to H2O2. The nuclei are visualized by 4′,6-diamidino-2-phenylindole staining. Magnification, 100×. B, C, Western blotting and relative densitometry showing γH2AX expression using whole cell extract from VICs 15′, 4h, and 24h after exposure to H2O2. GAPDH was used as loading control. U indicates untreated cells. *P<0.05. D, Immunofluorescence images showing colocalization of γH2AX (green) and XRCC1 (red) or γH2AX (green) and Mre11 (red) 24 hours after exposure to H2O2 in VICs isolated from AVSc patients. Magnification, 100×. AVC indicates aortic valve calcification; AVS, aortic valve stenosis; and AVSc, aortic valve sclerosis.
Oxidative Stress Results in Unresolved DNA Damage in AVSc VICs
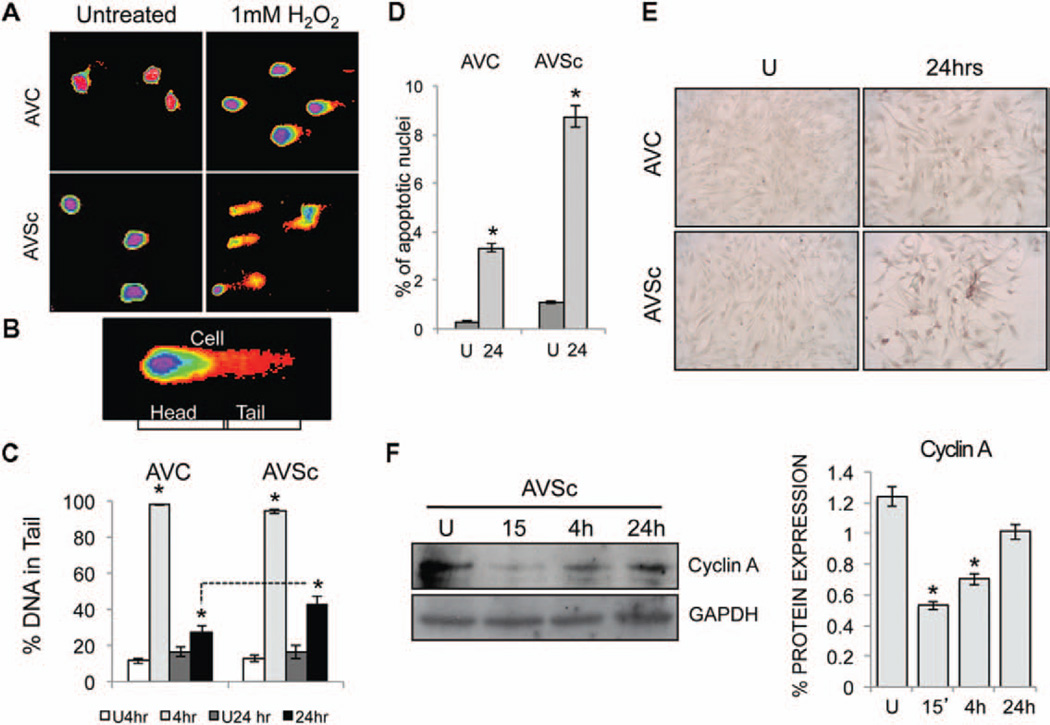
To test whether the sustained activation of γH2AX foci is the result of a greater accumulation of DNA damage in AVSc-derived VIC or a delay in the repair mechanism, we tested the level of DNA fragmentation and apoptosis after 1 mmol/L H2O2 treatment. Single cell electrophoresis (Comet Assay) on isolated VICs from controls and AVSc patients suggest a similar degree of DNA damage after oxidative stress exposure and 4 hours of recovery. At a later time point (24 hours of recovery), the damage in controls VICs is significantly lower than in sclerotic cells (Figure 3A–3C). To confirm this observation, we noticed an increase in TUNEL-positive staining in 1 mmol/L H2O2-treated VICs from sclerotic patients when compared with controls (Figure 3D and 3E). Finally, cell cycle arrest and recovery of H2O2-treated cells were confirmed by the reduction of cyclin A expression, 15 minutes after H2O2 treatments and recovery at later time-points (Figure 3F).
Figure 3.
Oxidative stress results in unresolved DNA damage in aortic valve sclerosis valvular interstitial cells (VICs). A, Representative pattern for comet assay to show DNA damage induced by 1 mmol/L H2O2 in AVC and AVSc-derived VICs. B, Single cell representing head and tail length. C, DNA in comet tail (mean±SE) calculated using CometScore software. *P<0.05. D, Percentage of apoptotic nuclei detected by TUNEL assay. Positive nuclei were counted in 4 different field/slide and averaged. E, TUNEL assay representative images. Magnification, 20×. F, Cyclin A expression detected by Western blotting and relative densitometry in VICs isolated from AVSc patients. Whole cell extract was made at different recovery time (15 minutes, 4 and 24 hours) after treatment with H2O2. *P<0.05. GAPDH was used as loading control. AVC indicates aortic valve calcification; AVSc, aortic valve sclerosis; and U, untreated VICs.
Oxidative Stress Modulates AVSc-derived VIC Transdifferentiation via AKT Signaling Pathway
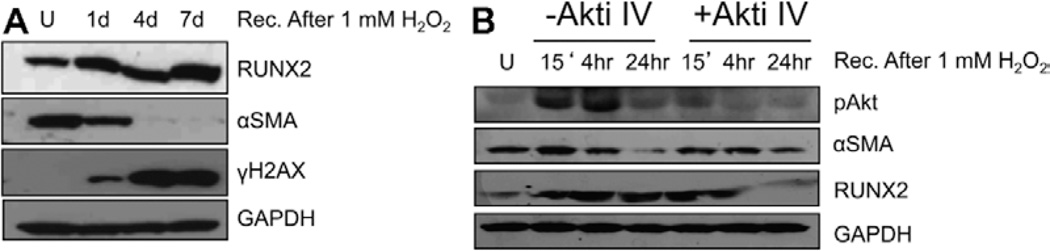
To directly test the impact of ROS on VIC transdifferentiation, AVSc-derived cells were tested for markers of osteogenic-like transdifferentiation and DNA damage up to 7 days after 1 mmol/L H2O2 exposure. Figure 4A shows accumulation of markers of early osteogenic transdifferentiation, such as RUNX2, with concurrent reduction of myofibroblast marker α-smooth muscle actin. Accordingly with previous results, we also noticed a sustained activation of phosphorylated H2AX in AVSc VICs. Because RUNX2 has been reported to be modulated by AKT signaling, we tested the effect of AKT inhibitor IV on AVSc-derived cells induced to osteogenic transdifferentiation via H2O2 treatment. AVSc-derived VICs were pretreated with 0.1 µmol/L of AKT inhibitor IV, followed by H2O2 (1 mmol/L for 1 hour) and recovery. Figure 4B shows that H2O2 -mediated RUNX2 accumulation at 24 hours of recovery is mediated by the activation of phospho-AKT signaling. Interestingly, H2O2-induced α-smooth muscle actin reduction at 24 hours is concomitant with the decrease in phospho-AKT activation.
Figure 4.
Oxidative stress modulates aortic valve sclerosis (AVSc)-derived valvular interstitial cell (VIC) transdifferentiation via AKT signaling pathway. A, Western blotting showing the expression of runt-related transcription factor 2 (RUNX2), alpha smooth muscle actin (αSMA), and γH2AX using whole cell lysate from AVSc-derived VICs treated with 1 mmol/L H2O2 and recovered at different time (1d, 1 day; 4d, 4 days; 7d, 7 days). GAPDH was used as loading control. B, pAkt, RUNX2, αSMA expression 15′, 4 hours, 24 hours, after treatment with 1 mmol/L H2O2 in the presence and absence of AKT inhibitor IV (AKTi IV). U indicates untreated VICs.
Adenoviral Transduction of Antioxidant Enzymes (SOD3 and CAT) Rescues VICs From an Impaired DDR and Reduces the Expression of Early Markers of VICs Activation
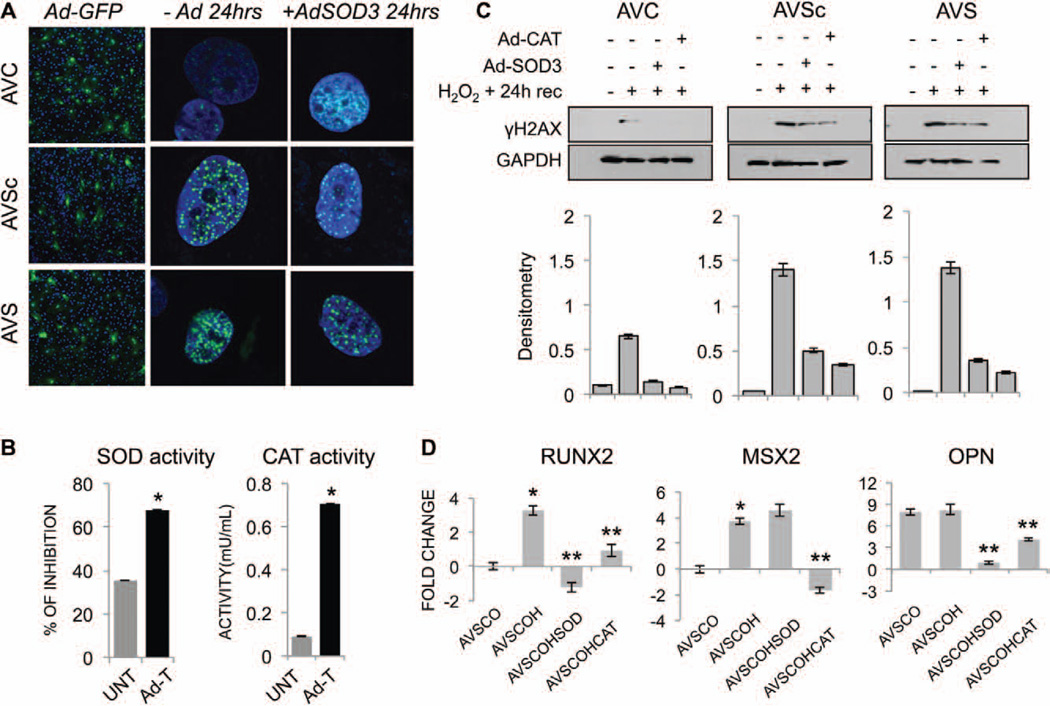
Because AVSc and AVS VICs showed an impaired oxidative DDR, we tested whether adenoviral transduction of antioxidant enzymes (SOD3 and CAT) could rescue VICs. VICs from 8 control, 8 AVSc, and 8 AVS patients were isolated and exposed to 1 mmol/L H2O2 in the presence or absence of adenovirally transduced SOD3 and CAT. DDR was assayed by visualizing H2AX phosphorylation at 24 hours of recovery. GFP-adenoviral delivery was used to determine transduction efficiency (Figure 5A, left panel). To confirm the efficiency of the adenoviral transduction, SOD3 and CAT activities were tested with results showing increased enzymatic activity up to 7 days after transduction (Figure 5B). Immunofluorescence (Figure 5A) and Western blotting on total protein extracts (Figure 5C) revealed that Ad-SOD3 and Ad-CAT are able to rescue VICs from an impaired DDR (decrease in the expression of γH2AX). We then tested the relationship among oxidative stress, DDR, and VICs transdifferentiation toward an osteogenic phenotype. AVSc-derived VICs were treated with 100 µmol/L H2O2 in osteogenic media for 15 days to determine the effect of oxidative stress on RUNX2, MSX2, and OPN expression. As shown in Figure 5D, ROS induce RUNX2, and MSX2 upregulation by 3.2- and 3.1-fold, respectively (P<0.05). These results are consistent with an early stage of osteogenic transdifferentiation because both these markers have been reported to be initiator of osteogenic activation.28,31 In accordance with our previous publications,15,46–48 OPN is already elevated in AVSc VICs when compared with controls. Adenoviral delivery of SOD3 and CAT partially reduced RUNX2, MSX2, and OPN, suggesting a functional correlation between the DDR and early events of VICs transdifferentiation toward an osteogenic phenotype (Figure 5D).
Figure 5.
Adenoviral transduction of antioxidant enzymes (SOD and CAT) rescues VICs from an impaired DNA damage response and reduces the expression of early markers of VICs activation. A, Immunofluorescence staining for γH2AX expression in human VICs isolated from AVC, AVSc, AVS patients 24 hours after treatment with H2O2 in the presence (SOD3) or absence (−Ad) of adenovirus carrying the isoform 3 of superoxide dismutase enzyme. Magnification, 100×. Ad-GFP was used to test transduction efficiency in all 3 cell types. Magnification, 10×. B, SOD and CAT activity in VICs transduced (Ad-T) or not (UNT) with the respective adenovirus. Assays were performed 7 days after transduction. *P<0.05 C, Western blotting and relative densitometry showing γH2AX expression in whole cell extract from AVC, AVSc, AVS VICs 24 hours after treatment with H2O2 in the presence or absence of adenovirus carrying either SOD3 or CAT enzyme. D, Gene expression for runt-related transcription factor 2 (RUNX2), muscle segment homeobox 2 (MSX2), and osteopontin (OPN) in VICs isolated from AVSc patients cultured in osteogenic media (O) and treated with 100 µmol/L H2O2 (OH) for 15 days in the presence or absence of adenovirus carrying either SOD3 (AVSCOHSOD) or catalase (AVSCOHCAT) enzyme. VICs from AVC patients cultured in OS media alone were used to calculate basal level of gene expression. Data were normalized against actinB and represented as fold change ± dCt SE. *represents P<0.05 and refers to values toward AVSCO. **represents P<0.05 and refers to values toward AVSCOH. AVC indicates aortic valve calcification; AVS, aortic valve stenosis; AVSc, aortic valve sclerosis; CAT, catalase; SOD, superoxide dismutase; and VIC, valvular interstitial cell.
Adenoviral Delivery of CAT Reduces H2O2-Mediated In Vitro Calcification and Osteogenic-like Transdifferentiation of VICs
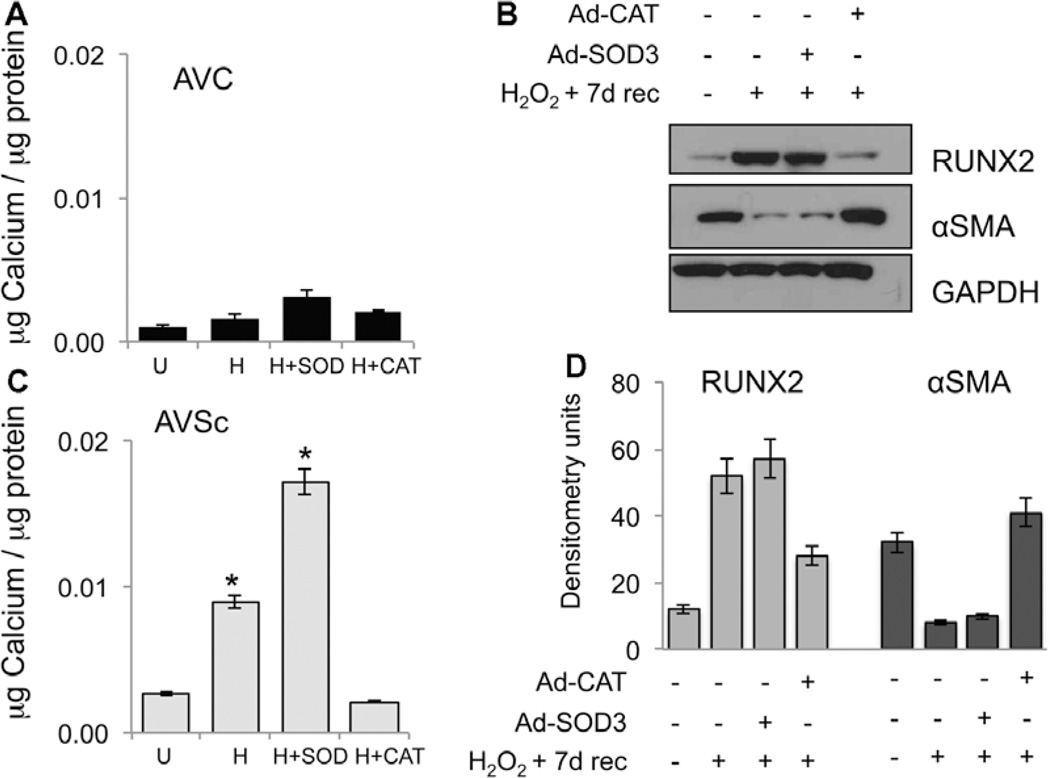
Finally, we investigated the impact of oxidative stress on in vitro calcification of AVSc-derived VIC (Figure 6). Our experiments show that although control-derived VIC are mostly unresponsive to in vitro calcification 7 days after being exposed to 1 mmol/L H2O2, the same treatment resulted in a significant calcium accumulation in AVSc VICs (Figure 6A and 6B). In vitro calcification was reduced to basal level when cells were transduced with Ad-CAT (Figure 6B), whereas Ad-SOD3 is not able to revert H2O2-induced in vitro calcification. Finally, accordingly with the in vitro calcification results, Ad-CAT, but not Ad-SOD3, reduces the expression of osteogenic markers RUNX2 and reverts H2O2-induced α-smooth muscle actin downregulation (Figure 6C).
Figure 6.
Adenoviral delivery of catalase reduces H2O2-mediated in vitro calcification of valvular interstitial cells (VICs). A and B, Calcium estimation in VICs 7 days after treatment with 1 mmol/L H2O2 in the presence or absence of Ad-SOD or Ad-CAT. U indicates untreated VICs. C and D, Western blotting and relative densitometry showing the expression of runt-related transcription factor 2 (RUNX2) and alpha smooth muscle actin (αSMA) using whole cell lysates of aortic valve sclerosis (AVSc)-derived VICs, 7 days after treatment with 1 mmol/L H2O2 in the presence or absence of Ad-SOD or Ad-CAT. GAPDH was used as loading control. *P<0.05. AVC indicates aortic valve calcification; CAT, catalase; and SOD, superoxide dismutase.
Discussion
Elevated levels of oxidative DNA damage have been reported in a number of diseases, but their presence may simply be epiphenomenal rather than pathogenic. A direct mechanism has been postulated to describe the role of oxidative DNA damage in the development of cardiovascular diseases, with increasing evidence of a direct role of the cascade oxidative stress-DNA damage-diseases in experimental model of atherosclerosis.34,49 It is now understood that aortic valve stenosis is the end-stage of a disease that progresses from microscopic early changes to aortic sclerosis and then, in a subset of patients, to severe biomineralization.50 To impact the progression from sclerosis to stenosis, we need to understand the earliest stages of disease so that we can measure the effects of targeted therapy on the microscopic processes in the valve cusps.12,51–53 Despite our best efforts, progress to understand, diagnose, and treat CAVD have been hindered by our inability to quantify in vivo the dynamic molecular events associated with early calcific changes in the valves.2,4,50 Despite its high prevalence, little is known about the developmental stages and pathogenetic mechanisms of aortic sclerosis. Our study provides several new insights into the early pathogenesis and the progression of AVSc.
First, the lack of oxidant defense in disease may lead to increased ROS and subsequent DNA damage (Figure 1). The oxidative stress typical of the very early events of valvular and vascular dysfunctions is mainly created by the production of ROS.20–24 Several studies indicate that ROS can cause DNA strand breaks and base modifications.32–34 Oxidative DNA damage resulting from free radical attack remains, however, a poorly examined field in CAVD. Our analysis shows, for the first time, accumulation of oxidative stress along with increased oxidative DNA damage in surgically resected tissues from patients with different degree of AV dysfunction.
Because DNA damage cannot be tolerated by the cell if left unrepaired, elaborate cellular repair and genome surveillance mechanisms counteract genomic damage induced by ROS. The DDR is manifested cytologically by the formation of DNA-repair foci.35–38 These structures can be seen as an affinity platform for a substantial number of proteins, allowing for the local subnuclear concentration of these factors.35,54 We therefore investigated the cellular response to DNA damage and its link to osteogenic phenotype in VICs. DNA damage results in rapid phosphorylation of H2AX by PI3K-like kinases, including ataxia telangiectasia mutated, ATM-Rad3-related protein, and DNA-dependent protein kinase, regulatory kinases of DDR,35,37 and cell cycle arrest. γH2AX is required for checkpoint-mediated cell cycle arrest and DNA repair after double-stranded DNA breaks. We show that oxidative stress induces a rapid subnuclear accumulation of γH2AX, MRE11, and XRCC1 proteins in human isolated VICs. AVSc-and AVS-derived VICs show a sustained phosphorylation of H2AX, suggesting an altered organization of the DDR after ROS treatments. Single gel electrophoresis and TUNEL assays suggest that the sustained activation of γH2AX, MRE11, and XRCC1 proteins could be the results of an impaired mechanisms of repair rather than a greater accumulation of DNA damage in AVSc isolated VIC (Figures 2 and 3). As a note, the incomplete colocalization between MRE11 and γH2AX foci at 15 minutes could be a result of the formation of the MNR (MRE11, Rad 50, and NSB1) complex and it is consistent with previous reports.35,37,49
The molecular cascade generated by the DDR proteins may have a number of effects on the valvular cellular populations. Here we show that ROS induces the expression of early markers of osteogenic transdifferentiation, such as RUNX2, via AKT signaling. AKT signaling has been implicated in the in vitro differentiation of skeletal cells, such as chondrocytes, osteoblast, myoblast, and adipocytes. Mice lacking of Akt1 and Akt2 show delayed bone development, suggesting an important role of AKT signaling in the differentiation of bone cells. Furthermore it has been shown that H2O2-induced activation of AKT signaling regulates RUNX2 expression and calcification in vascular smooth muscle cells.55 ROS-mediated VICs activation can be reversed by adenoviral transduction of SOD3 and CAT. In our experiments, in accordance with previous results,55 oxidative mediated-DNA damage results in VIC activation toward an osteogenic-like phenotype via AKT-RUNX2 signaling pathway reverted by antioxidant enzymes delivery (Figures 4 and 5). Finally, in vitro calcification assays show a direct link among ROS, VIC activation, and biomineralization of AVSc-derived VIC when compared with controls (Figure 6). In AVSc-derived VIC exposure to 1 mmol/L H2O2 resulted in parallel accumulation of RUNX2 and calcium, with this effect reverted by adenoviral transduction of antioxidant enzyme CAT. The ineffectiveness of SOD3 in contrasting high dose of H2O2, in this experiment, is in agreement with the specificities of the enzymes56: CAT acting specifically on H2O2 is capable of neutralizing it effectively within a broad range of H2O2 concentrations. On the contrary, SOD3 may be able to prevent toxic effects developing at low H2O2 concentrations by rapidly neutralizing superoxide anion formed presumably through Fenton chemistry, but may not be able to protect cells against toxicity caused by H2O2 itself when the latter is applied at relatively large amounts. Thus, as the concentration of H2O2 increases, the cell viability may not be effectively supported by SOD3 alone.
Despite the strong evidence that ROS are involved in CAVD, oral antioxidant treatments in atherosclerosis and restenosis, with the exception of probucol, have been unsuccessful.57–59 Although this may be explained by a number of factors, such as the use of inefficient antioxidants or suboptimal dosing, it may be that in select cardiovascular problems, a more direct approach of targeted delivery of specific antioxidant genes to the site of injury may prove to be more effective. Furthermore, stable and nontoxic antioxidant mimetics have been developed to overcome endogenous delivery limitations, such as short circulating half-life and poor delivery, and may be ideal candidate to further investigate the data presented in this article.
It is concluded that the DDR correlates with the transcriptional activation events involved in the pathological phenotype observed in human isolated cells from AVSc patients. Furthermore, the ROS-induced DNA response is dysfunctional in early asymptomatic stages of CAVD. In addition, it was demonstrated that there is an association among ROS, DNA-damage response, and cellular transdifferentiation reversible by antioxidant enzymes delivery. A better understanding of mechanisms that lead to increases in oxidative stress in CAVD, and indeed in many other cardiovascular diseases, may lead to more effective antioxidant prevention or treatments of this largely understudied patient population.
Supplementary Material
Acknowledgments
Sources of Funding
This project was partially supported by award number RC1HL100035 from the National Heart, Lung, and Blood Institute, National Institutes of Health (G. Ferrari), by the Harrison Memorial Fund of the University of Pennsylvania School of Medicine (G. Ferrari). This research was also supported by the Victor Musso Foundation (J.B. Grau), by the Kibel Foundation (R.J. Levy), and by the William J. Rashkind Endowment of the Children’s Hospital of Philadelphia.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.300177/-/DC1.
Disclosures
None.
References
- 1.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz CE, Otto CM. Aortic stenosis: clinical aspects of diagnosis and management, with 10 illustrative case reports from a 25-year experience. Medicine (Baltimore) 2010;89:349–379. doi: 10.1097/MD.0b013e3181fe5648. [DOI] [PubMed] [Google Scholar]
- 3.Beckmann E, Grau JB, Sainger R, Poggio P, Ferrari G. Insights into the use of biomarkers in calcific aortic valve disease. J Heart Valve Dis. 2010;19:441–452. [PMC free article] [PubMed] [Google Scholar]
- 4.Otto CM. Calcific aortic valve disease: new concepts. Semin Thorac Cardiovasc Surg. 2010;22:276–284. doi: 10.1053/j.semtcvs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Parolari A, Loardi C, Mussoni L, Cavallotti L, Camera M, Biglioli P, Tremoli E, Alamanni F. Nonrheumatic calcific aortic stenosis: an overview from basic science to pharmacological prevention. Eur J Cardiothorac Surg. 2009;35:493–504. doi: 10.1016/j.ejcts.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 8.Benton JA, Kern HB, Leinwand LA, Mariner PD, Anseth KS. Statins block calcific nodule formation of valvular interstitial cells by inhibiting alpha-smooth muscle actin expression. Arterioscler Thromb Vasc Biol. 2009;29:1950–1957. doi: 10.1161/ATVBAHA.109.195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossebø AB, Pedersen TR, Boman K, et al. SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 10.Rossi R, Lidonnici MR, Soza S, Biamonti G, Montecucco A. The dispersal of replication proteins after Etoposide treatment requires the cooperation of Nbs1 with the ataxia telangiectasia Rad3-related/Chk1 pathway. Cancer Res. 2006;66:1675–1683. doi: 10.1158/0008-5472.CAN-05-2741. [DOI] [PubMed] [Google Scholar]
- 11.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 13.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharacholou SM, Karon BL, Shub C, Pellikka PA. Aortic valve sclerosis and clinical outcomes: moving toward a definition. Am J Med. 2011;124:103–110. doi: 10.1016/j.amjmed.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Grau JB, Poggio P, Sainger R, Vernick WJ, Seefried WF, Branchetti E, Field BC, Bavaria JE, Acker MA, Ferrari G. Analysis of osteopontin levels for the identification of asymptomatic patients with calcific aortic valve disease. Ann Thorac Surg. 2012;93:79–86. doi: 10.1016/j.athoracsur.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 17.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol. 1996;12:231–236. [PubMed] [Google Scholar]
- 18.Gwanmesia P, Ziegler H, Eurich R, Barth M, Kamiya H, Karck M, Lichtenberg A, Akhyari P. Opposite effects of transforming growth factor-β1 and vascular endothelial growth factor on the degeneration of aortic valvular interstitial cell are modified by the extracellular matrix protein fibronectin: implications for heart valve engineering. Tissue Eng Part A. 2010;16:3737–3746. doi: 10.1089/ten.TEA.2010.0304. [DOI] [PubMed] [Google Scholar]
- 19.Wada T, McKee MD, Steitz S, Giachelli CM. Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 20.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 21.Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R. Novel aspects of oxidative stress in cardiovascular diseases. Circ J. 2009;73:201–207. doi: 10.1253/circj.cj-08-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breyne J, Juthier F, Corseaux D, Marechaux S, Zawadzki C, Jeanpierre E, Ung A, Ennezat PV, Susen S, Van Belle E, Le Marec H, Vincentelli A, Le Tourneau T, Jude B. Atherosclerotic-like process in aortic stenosis: activation of the tissue factor-thrombin pathway and potential role through osteopontin alteration. Atherosclerosis. 2010;213:369–376. doi: 10.1016/j.atherosclerosis.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 24.Yip CY, Simmons CA. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc Pathol. 2011;20:177–182. doi: 10.1016/j.carpath.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 26.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madamanchi NR, Hakim ZS, Runge MS. Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005;3:254–267. doi: 10.1111/j.1538-7836.2004.01085.x. [DOI] [PubMed] [Google Scholar]
- 28.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 29.Lai CF, Shao JS, Behrmann A, Krchma K, Cheng SL, Towler DA. TNFR1-activated reactive oxidative species signals up-regulate osteogenic Msx2 programs in aortic myofibroblasts. Endocrinology. 2012;153:3897–3910. doi: 10.1210/en.2012-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283:20505–20522. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sierra OL, Towler DA. Runx2 trans-activation mediated by the MSX2-interacting nuclear target requires homeodomain interacting protein kinase-3. Mol Endocrinol. 2010;24:1478–1497. doi: 10.1210/me.2010-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 33.Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- 34.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 2001;88:733–739. doi: 10.1161/hh0701.088684. [DOI] [PubMed] [Google Scholar]
- 35.Bekker-Jensen S, Mailand N. Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair (Amst) 2010;9:1219–1228. doi: 10.1016/j.dnarep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Bennett BT, Bewersdorf J, Knight KL. Immunofluorescence imaging of DNA damage response proteins: optimizing protocols for super-resolution microscopy. Methods. 2009;48:63–71. doi: 10.1016/j.ymeth.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misteli T, Soutoglou E. The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat Rev Mol Cell Biol. 2009;10:243–254. doi: 10.1038/nrm2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montecucco A, Rossi R, Ferrari G, Scovassi AI, Prosperi E, Biamonti G. Etoposide induces the dispersal of DNA ligase I from replication factories. Mol Biol Cell. 2001;12:2109–2118. doi: 10.1091/mbc.12.7.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas C, Falck J, Bartkova J, Bartek J, Lukas J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat Cell Biol. 2003;5:255–260. doi: 10.1038/ncb945. [DOI] [PubMed] [Google Scholar]
- 40.Lukas C, Bartek J, Lukas J. Imaging of protein movement induced by chromosomal breakage: tiny ‘local’ lesions pose great ‘global’ challenges. Chromosoma. 2005;114:146–154. doi: 10.1007/s00412-005-0011-y. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt E, Paquet C, Beauchemin M, Bertrand R. DNA-damage response network at the crossroads of cell-cycle checkpoints, cellular senescence and apoptosis. J Zhejiang Univ Sci B. 2007;8:377–397. doi: 10.1631/jzus.2007.B0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy E, Caidahl K, Franco-Cereceda A, Back M. Increased transcript level of poly(ADP-ribose) polymerase (PARP-1) in human tricuspid compared with bicuspid aortic valves correlates with the stenosis severity. Biochem Biophys Res Commun. 2012;420:671–675. doi: 10.1016/j.bbrc.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 43.Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, Acker MA, Gorman RC, Gorman JH, III, Hargrove CW, III, Bavaria JE, Ferrari G. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. 2012;227:2595–2604. doi: 10.1002/jcp.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dundr M, Misteli T. Biogenesis of nuclear bodies. Cold Spring Harb Perspect Biol. 2010;2:a000711. doi: 10.1101/cshperspect.a000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budde PP, Misteli T. Cell biology beyond the cell. J Cell Biol. 2010;190:7. doi: 10.1083/jcb.201006126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sainger R, Grau JB, Poggio P, Branchetti E, Bavaria JE, Gorman JH, III, Gorman RC, Ferrari G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers. 2012;17:111–118. doi: 10.3109/1354750X.2011.642407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrari G, Sainger R, Beckmann E, Keller G, Yu PJ, Monti MC, Galloway AC, Weiss RL, Vernick W, Grau JB. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010;163:12–17. doi: 10.1016/j.jss.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138:196–199. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 49.Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 2006;71:259–268. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Aikawa E, Otto CM. Look more closely at the valve: imaging calcific aortic valve disease. Circulation. 2012;125:9–11. doi: 10.1161/CIRCULATIONAHA.111.073452. [DOI] [PubMed] [Google Scholar]
- 51.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, Kappetein AP, Stepinska J, Kaden JJ, Naber CK, Acarturk E, Gohlke-Bärwolf C. ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J. 2012;33:822–828. doi: 10.1093/eurheartj/ehr061. 828a, 828b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgartner H, Otto CM. Aortic stenosis severity: do we need a new concept? J Am Coll Cardiol. 2009;54:1012–1013. doi: 10.1016/j.jacc.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 54.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batinić-Haberle I, Rebouças JS, Spasojević I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levonen AL, Vähäkangas E, Koponen JK, Ylä-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation. 2008;117:2142–2150. doi: 10.1161/CIRCULATIONAHA.107.718585. [DOI] [PubMed] [Google Scholar]
- 58.Hurttila H, Koponen JK, Kansanen E, Jyrkkänen HK, Kivelä A, Kylätie R, Ylä-Herttuala S, Levonen AL. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther. 2008;15:1271–1279. doi: 10.1038/gt.2008.75. [DOI] [PubMed] [Google Scholar]
- 59.Tardif JC, Cöté G, Lespérance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337:365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.