Abstract
Purpose/Objectives
To distinguish relationships among subjective and objective characteristics of sleep, mood, and quality of life (QOL) in patients receiving treatment for lung cancer.
Design
Descriptive, correlational study.
Setting
Two ambulatory oncology clinics.
Sample
35 patients with lung cancer.
Methods
The following instruments were used to measure the variables of interest: Pittsburgh Sleep Quality Index (PSQI), Hospital Anxiety and Depression Scale, Functional Assessment of Cancer Treatment–Lung (FACT-L), a sleep diary, and a motionlogger actigraph.
Main Research Variables
Sleep, mood, and QOL.
Findings
Significant differences were found between sleep diary and actigraph measures of sleep efficiency (p = 0.002), sleep latency (p = 0.014), sleep duration (p < 0.001), and wake after sleep onset (p < 0.001). Poor sleepers (PSQI score greater than 5) were significantly different from good sleepers (PSQI score of 5 or lower) on sleep diary measures of sleep efficiency and sleep latency and the FACT-L lung cancer symptom subscale, but not on mood or actigraphy sleep measures.
Conclusions
Although patients with lung cancer may report an overall acceptable sleep quality when assessed by a single question, those same patients may still have markedly increased sleep latencies or reduced total sleep time. The findings indicate the complexity of sleep disturbances in patients with lung cancer. Lung cancer symptoms had a stronger association with sleep than mood. Research using prospective methods will help to elucidate their clinical significance.
Implications for Nursing
Patients receiving treatment for lung cancer are at an increased risk for sleep disturbances and would benefit from routine sleep assessment and management. In addition, assessment and management of common symptoms may improve sleep and, ultimately, QOL.
Knowledge Translation
A high frequency of sleep disturbances in patients receiving treatment for lung cancer was evident, and poor sleepers had lower QOL. Sleep disturbances may be more related to lung cancer symptoms than anxiety or depression. Improving lung cancer symptoms such as dyspnea may improve sleep.
Lung cancer is the second most common form of cancer but the leading cause of cancer death in both men and women (Siegel, Naishadham, & Jemal, 2013). In 2013, an estimated 228,190 individuals will be diagnosed with lung cancer and 159,480 will die from the disease (American Cancer Society [ACS], 2013). Although advances in knowledge about cancer biology and improvements in early diagnosis and treatment have increased the opportunities for long-term survival, the prognosis remains poor with a five-year survival rate of only 16% (ACS, 2013). As such, the impact of the disease and treatment on patients’ symptoms and quality of life (QOL) requires exploration.
The vast majority of patients with lung cancer are diagnosed with advanced disease, a high burden of symptoms (i.e., dyspnea, hemoptysis, cough, and chest pain), poorer QOL, and shortened survival (Buchanan, Milroy, Baker, Thompson, & Levack, 2009; Hopwood & Stephens, 1995, 2000; Siegel et al., 2013; Steinberg et al., 2009). Research has demonstrated that early integration of symptom management leads to meaningful improvements in QOL, mood, and survival in patients with lung cancer (Temel et al., 2010). However, few studies have focused on sleep as a factor influencing symptom burden and QOL.
Individuals with lung cancer are reported as having the highest or second highest level of sleep disturbances relative to other cancers and noncancer controls, and so may be a particularly at-risk population for sleep problems (Davidson, MacLean, Brundage, & Schulze, 2002; Palesh et al., 2010). A Canadian study using well-established diagnostic criteria and involving 982 outpatients revealed that those with lung cancer (n = 114) had a higher prevalence of sleep problems, including excessive daytime sleepiness, sleeping more than usual, severe fatigue, and using sleeping pills more often than patients with other solid tumors (Davidson et al., 2002). Only patients with breast cancer had a higher prevalence of insomnia than patients with lung cancer (Davidson et al., 2002). The authors found that patients with lung cancer were diagnosed and treated earlier than other patients with cancer. The median time from diagnosis for all cancers was 34 months, whereas the median time from diagnosis for patients with lung cancer was only 11 months. This suggests that earlier treatment interferes with sleep (Davidson et al., 2002). Palesh et al. (2010) used the Hamilton Depression Inventory, which included six questions assessing frequency and duration of sleep, to study 823 patients undergoing chemotherapy, and found that patients with lung cancer (n = 120) had the highest prevalence of insomnia syndrome when compared to patients with breast, gynecologic, hematologic, or alimentary tract cancers. Insomnia syndrome is defined as difficulty falling asleep, difficulty staying asleep (waking up in the middle of the night), and/or early morning awakenings for at least three days a week for two weeks, with each episode lasting at least 30 minutes. Patients with insomnia syndrome had significantly more fatigue and depression than patients without insomnia syndrome (Palesh et al., 2010). These previous studies provide evidence for the increased prevalence of self-reported sleep disturbances in patients during treatment for lung cancer.
Disturbances of mood have an impact on sleep disturbances in patients with lung cancer. Previous estimates have suggested that 15%–40% of individuals with lung cancer have a clinically significant level of anxiety, meeting the criteria for referral (Greer, Pirl, Park, Lynch, & Temel, 2008; Savard, Villa, Ivers, Simard, & Morin, 2009), and 21%–44% are depressed (Hopwood & Stephens, 2000; Turner, Muers, Haward, & Mulley, 2007). Sleep disturbances are included in two of the six categories for anxiety disorders in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000), suggesting that sleep disturbance and anxiety are clinically linked. Sleep requires diminished cortical arousal, whereas anxiety manifests with heightened cortical and peripheral arousal (Stein & Mellman, 2005). A review of sleep electroencephalogram (EEG) studies found that sleep homeostasis was impaired and slow wave sleep activity was reduced in depression (Armitage, 2007). Silberfarb, Hauri, Oxman, and Schnurr (1993) screened for depression and anxiety and reported that patients with lung cancer scored within normal limits, but slept as poorly as insomniacs. Only 21% of people with cancer who complained of insomnia had concurrent psychopathology (Ginsburg, Quirt, Ginsburg, & MacKillop, 1995)—a rate similar to overlap rates seen in the general older adult population (Foley et al., 1995). Still, some of the ascribed causes of severe insomnia were related to anxiety, depression, and guilt (Ginsburg et al., 1995).
Sleep disturbances in patients with lung cancer have significant adverse effects on symptoms and QOL (Degner & Sloan, 1995; Gooneratne et al., 2007). Pain was significantly worse in patients with lung cancer with insomnia compared to patients without insomnia (Rumble, Keefe, Edinger, Porter, & Garst, 2005). Pain, fatigue, and dyspnea all were significantly correlated with sleep disturbance and, after controlling for all covariates, sleep disturbance was significantly associated with impaired cognitive function and poorer functional status in 115 patients receiving chemotherapy for lung cancer (Chen, Yu, & Yang, 2008). QOL in lung cancer survivors (n = 76) was not significantly different from non-cancer controls (n = 78), but lung cancer survivors with poorer sleep quality had significantly worse QOL than those with good sleep quality (Gooneratne et al., 2007). These studies suggest that newly diagnosed patients and those receiving treatment for lung cancer have increased symptoms and significantly worse QOL compared to controls. In addition, lung cancer survivors with poor quality sleep have significantly worse QOL than those with good sleep quality.
Much of the previous research on sleep in patients with lung cancer has focused on self-reported sleep measures. Few studies have used both subjective and objective measures to evaluate sleep. The purpose of this study was to characterize and compare relationships among sleep, mood, and QOL using both subjective and objective measures of sleep in patients receiving treatment for lung cancer. The authors hypothesized that some discrepancies would exist between objective and subjective measures (Schlesinger, Hering-Hanit, & Dagan, 2001; Vitiello, Larsen, & Moe, 2004), but associations also would exist between objective and subjective measures and that mood disorders and poorer QOL would be highly associated with self-reported sleep disturbance.
This study was guided by the Two Process Model of Sleep Regulation, one of the leading theories of sleep regulation (Borbely, 1982; Borbely & Achermann, 2005). This model suggests that sleep occurs when the homeostatic drive to sleep (Process S) approaches an upper threshold, and awakening from sleep occurs when Process S reaches a lower threshold. The need to sleep increases as the amount of prior wakefulness increases. In other words, an increase in sleep pressure occurs as wakefulness is prolonged and for a recovery process during sleep. The second process, Process C, signifies the circadian control of sleep propensity or chance for sleep. The circadian system communicates time of day (light and dark cues from the retina) and thereby regulates the change from wakefulness to sleep and the change from sleep to wakefulness. The interaction between these two processes forms the basis for a consistent period of sleep at night and a well-ordered period of wakefulness during the day.
Methods
Design and Sample
A cross-sectional, descriptive, correlational design was used to characterize and compare sleep using subjective and objective measures, mood, and QOL in patients receiving treatment for lung cancer. Subjective sleep was measured with the Pittsburgh Sleep Quality Index (PSQI) and sleep diary, whereas objective sleep was measured with actigraphy.
Sample size calculations were based on the dichotomous outcomes of poor sleep quality and poor sleep duration. Based on previous research, 55% of the sample was expected to have poor sleep quality or duration. This study was powered to be able to find moderate differences corresponding to an odds ratio of 3. Using logistic regression and assuming an alpha of 0.05, 80% power, and other risk factors accounting for about 30% of the variability in the data, 30 participants were required.
Procedures
Participants were recruited sequentially during regular clinic visits to the Philadelphia Veterans Administration Medical Center (PVAMC) and the Veterans Administration Western New York Healthcare System (VAWNYHS). The study was approved by the institutional review boards at each Veterans Administration (VA) hospital, the University of Pennsylvania, and the University at Buffalo. All participants provided written informed consent. Eligibility for the study included a diagnosis of small cell or non-small cell lung cancer, completion of one cycle of chemotherapy before recruitment, being at least 21 years of age, and a Karnofsky Performance Status (KPS) of greater than 70%. Patients with a KPS greater than 70% are unable to carry on normal activities or work but are able to care for themselves. Patients were excluded if they had known brain metastases or current treatment of another primary cancer except non-melanoma skin cancers and/or preexisting diagnosed sleep disorders. Medical oncologists and a nurse practitioner referred eligible patients to the principal investigator. Following informed consent, each patient was trained by the principal investigator on completion of the self-report surveys: PSQI, Hospital Anxiety and Depression Scale (HADS), and the Functional Assessment of Cancer Treatment–Lung (FACT-L) in the oncology clinics. If time did not permit survey completion, patients were allowed to complete surveys at home. Each patient also was trained to complete the seven-day sleep diary by recording the information on waking each morning and by wearing the motionlogger actigraph on the nondominant wrist continuously for seven days. The actigraph is a waterproof, wrist-worn device, similar in size and appearance to a wristwatch. A completed sleep diary and actigraph were returned to the principal investigator at the next clinic visit. Each patient was paid $30 for completing data collection materials.
Measures
Demographic, medical, and sleep history were collected using the sleep score questionnaire, a general demographic and medical history questionnaire developed by the Penn Center for Sleep Disorders. Information on age, gender, race, marital status, education, occupation, lung cancer diagnosis and treatment, comorbidities, and medication use were abstracted from participants’ medical records.
The PSQI, a 19-item self-report questionnaire requiring 5–10 minutes for completion, was used to assess subjective sleep quality (Buysse et al., 1991). Global scores as well as scores for seven subscales (subjective sleep quality, sleep latency, sleep efficiency, sleep duration, sleep disturbances, use of sleep medications, and daytime dysfunction) were calculated. The variables of interest were sleep efficiency, sleep onset latency, and total sleep time. The PSQI has been used in clinical and research settings to distinguish good sleepers (global score of 5 or less) and poor sleepers (score greater than 5), and is responsive to changes in insomnia (Dolberg, Hirschmann, & Grunhaus, 1998). Good measures of internal homogeneity (Cronbach alpha = 0.77–0.8), consistency (r = 0.86–0.9), and validity (low versus high fatigue, p < 0.001) have been obtained (Beck, Schwartz, Towsley, Dudley, & Barsevick, 2004; Gentili, Weiner, Kuchibhatla, & Edinger, 1995). The Cronbach alpha with this sample was 0.64.
The motionlogger actigraph, a wrist-worn device that monitors movement using a miniaturized accelerometer (sensitive to 0.003 g and higher), was used to measure objective sleep. Wrist actigraphy has been studied in comparison to the “gold standard” of polysomnography, and the correlation with EEG assessment of total sleep time was r = 0.91 (p < 0.001) for sum activity and r = 0.81 (p < 0.005) for maximum activity in community-dwelling patients (Ancoli-Israel et al., 2003). The variables of interest were sleep efficiency, sleep onset latency, total sleep time, and wake after sleep onset (WASO).
A sleep diary was completed for seven days in conjunction with actigraphy recordings to assess subjective sleep and to help in coding raw actigraph data. Patients were instructed to record the following information on paper logs on waking: time to fall asleep, duration of sleep, time in bed, time out of bed, wake time after sleep, and if they removed and/or replaced the actigraph. Daily data collection averaged about 3–5 minutes. The variables of interest were sleep efficiency, sleep onset latency, total sleep time, and WASO.
Mood was assessed with HADS, a 14-item, four-point intensity scale ranging from 0 (never) to 3 (most of the time) with total scores of 0–21 for anxiety and/or depression (Zigmond & Snaith, 1983). A score higher than a 7 on either HADS subscale is considered to be clinically significant. Research comparing HADS with structured clinical diagnostic interviews has demonstrated good predictive value and utility for HADS in patients with advanced cancer (Hopwood & Stephens, 2000; Snaith, 2003). Although adequate reliability scores had been documented previously (Faller & Schmidt, 2004), the Cronbach alpha with this sample was 0.71. Five to 10 minutes are required to complete HADS.
FACT-L, a 36-item self-report instrument consisting of 27-items from the FACT–General (FACT-G) and the nine-item lung cancer subscale (LCS) was used to measure QOL (Butt et al., 2005). The LCS monitors the severity of seven lung cancer symptoms (shortness of breath, cough, tightness in chest, difficulty breathing, appetite loss, weight loss, and lack of clear thinking), which are rated by the patient on a 0–4 Likert-type scale with a maximum (asymptomatic) score of 28. A total FACT-L score is obtained by summing the FACT-G with the LCS (two of the nine items are not scored). The maximum score obtainable for the complete FACT-L questionnaire is 136. In the FACT-L, a higher score corresponds to a higher (better) QOL, and a 2–3 point change in the FACT-L LCS reflected a minimally important difference associated with performance status, weight loss, objective tumor response, and time to progression (Cella et al., 2002). FACT-L was chosen for its wide use in lung cancer clinical trials and provides validated clinically meaningful score changes (an increase of more than two points in LCS and more than six points in FACT-L prospectively define improved symptom and QOL, respectively, whereas a decrease of two points or more on the LCS and a decrease of six points or more on the FACT-L prospectively define wors-ened symptom and QOL, respectively). The FACT-L has been used in a variety of studies, including patients receiving chemotherapy (Cella et al., 2002). Internal consistency of the five FACT-L subscales, physical well-being (PWB), functional well-being (FWB), social well-being (SWB), emotional well-being (EWB), and lung cancer symptoms (LCS), has ranged from 0.56–0.89 (Butt et al., 2005). The FACT-L takes about 15–20 minutes to complete. The Cronbach alpha with this sample was 0.81.
Data Analysis
Data were analyzed using SPSS®, version 18.0. Descriptive statistics and frequency distributions were computed for sample characteristics, PSQI, mood, and QOL data. Pearson correlations were calculated to examine the strength of the relationship between variables. For this analysis, the PSQI global score cut point of 5 or less was used to categorize a good sleeper and 5 or greater to categorize a poor sleeper to determine the average values and compare subjective and objective sleep parameters, mood, and QOL in good versus poor sleepers. Effect size estimates and their associated 95% confidence intervals (Cohen’s d statistic) also were calculated to provide a measure of the magnitude of differences according to the criteria suggested by Cohen (small, d = 0.2; medium, d = 0.5; and large, d = 0.8). Paired t tests were used to compare self-report and objective measures of sleep. All tests were two-tailed and a value of p < 0.05 was considered statistically significant. Adjustments were not made for missing data. Cases with missing values were excluded from the analyses.
Action®, version 3.8, analysis software was used to score actigraphy data in zero-crossing mode (Berger et al., 2008) in one-minute epochs. The Action 3.8 sleep scoring algorithm was used to provide summary measures of activity during time in bed for all patients. This software analyzes and reports a variety of sleep parameters, including sleep efficiency, sleep-onset latency, total sleep time, waking after sleep-onset, and others. Sleep efficiency is time asleep divided by time in bed as scored by the actigraphy sleep algorithm. Sleep latency is defined as the time in minutes to the first period of persistent inactivity (e.g., five minutes of inactivity). Total sleep time and waking after sleep-onset (during time in bed) were determined with the actigraphy sleep algorithm. The sleep diaries were used to determine “lights off” and “lights on” for the Action 3.8 “auto set down interval.” Sleep diary data were compared to the software selected periods to determine which epochs were inappropriate for measurement and should be deleted. Actigraphy recordings and sleep diary data were averaged during the seven-day data collection periods.
Results
Sample Characteristics
The initial sample included 40 participants. Five participants were excluded because of early death (n = 2) and three were lost to follow-up after signing consent but not returning data collection instruments. The final sample consisted of 35 patients. No significant differences were noted in demographic or disease-related characteristics between the five participants who were excluded and the 35 participants who completed the study. Although all patients were instructed to wear the actigraph for seven days, five patients (15%) had only four nights of actigraphic data for analysis. Patients reported removing the actigraph for a variety of reasons (i.e., working in the garden or bathing) and neglected to replace the actigraph after the activity was completed.
Table 1 provides descriptive data for the 35 participants in the study, of whom 37% were recruited from the PVAMC and 63% were recruited from VAWNYHS. The majority of the sample was male, married, and Caucasian. Most were diagnosed with advanced-stage non-small cell lung cancer.
Table 1. Sample Characteristics (N = 35).
| Characteristic | x̄ | SD | Range |
|---|---|---|---|
| Age (years) | 63.5 | 9.7 | 48–94 |
|
| |||
| Characteristic | n | ||
| Gender | |||
| Male | 34 | ||
| Female | 1 | ||
| Race | |||
| Caucasian | 23 | ||
| African American | 12 | ||
| Marital status | |||
| Married | 16 | ||
| Single | 5 | ||
| Separated or divorced | 7 | ||
| Widowed | 6 | ||
| Missing | 1 | ||
| Cancer stage | |||
| I | 1 | ||
| II | 1 | ||
| IIIA | 10 | ||
| IIIB | 8 | ||
| IV | 10 | ||
| Missing | 5 | ||
| Lung cancer type | |||
| Non-small cell | 21 | ||
| Small cell | 12 | ||
| Missing | 2 | ||
| Radiation therapy | |||
| Yes | 16 | ||
| No | 14 | ||
| Missing | 5 | ||
| Chemotherapy | |||
| Yes | 35 | ||
| Chemotherapy regimen | |||
| Carboplatin and paclitaxel | 15 | ||
| Carboplatin and gemcitabine | 3 | ||
| Carboplatin and etoposide | 2 | ||
| Cisplatin and etoposide | 12 | ||
| Missing | 3 | ||
Sleep Quality
Tables 2–5 provide descriptive statistics for sleep, QOL, and mood variables. PSQI results revealed that 60% (n = 21) of participants reported sleep latency of 30 minutes or longer; sleep duration was less than 6.5 hours per night in 77% (n = 27) of participants; sleep efficiency lower than 85% was reported by 69% (n = 24); and global sleep quality was higher than the cut point of 5 defined as a poor sleeper in 80% (n = 28) of the sample.
Table 2. Comparison of Sleep Measures in Good and Poor Sleepers Determined by the Pittsburgh Sleep Quality Index Global Score (N = 35).
| Total |
Good Sleep (n = 7) |
Poor Sleep (n = 28) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | x̄ | SD | Range | x̄ | SD | x̄ | SD | p | Effect Size |
| Sleep efficiency |
72.5 | 19.3 | 20–100 | 95 | 6.5 | 67 | 17.1 | 0.01 | 1.79 |
|
| |||||||||
| Sleep hours |
5.7 | 1.5 | 1.5–10 | 7.5 | 1.6 | 5.2 | 1.7 | 0.04 | 1.37 |
|
| |||||||||
| Sleep latency |
34.2 | 29.5 | 5–120 | 12 | 8.1 | 40 | 30.4 | 0.01 | 1 |
|
| |||||||||
| Global score | 9.1 | 3.9 | 1–17 | 3.9 | 1.5 | 10.5 | 3 | 0.01 | 2.34 |
Table 5. Quality-of-Life and Mood Scores in Good and Poor Sleepers Determined by the Pittsburgh Sleep Quality Index Global Score (N = 35).
| Total |
Good Sleep (n = 7) |
Poor Sleep (n = 28) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | x̄ | SD | Range | x̄ | SD | x̄ | SD | p | Effect Size |
| Functional Assessment of Cancer Therapy (FACT)-Lung | |||||||||
| Physical well-being |
18.8 | 6.1 | 2–28 | 21.6 | 5.5 | 18.1 | 6.1 | 0.18 | 0.57 |
|
| |||||||||
| Social well-being |
21.6 | 5.2 | 7–28 | 22.1 | 4.8 | 21.4 | 5.3 | 0.74 | 0.13 |
|
| |||||||||
| Emotional well-being |
17.3 | 5.2 | 1–24 | 19.1 | 4 | 16.8 | 5.4 | 0.29 | 0.45 |
|
| |||||||||
| Functional well-being |
15.9 | 7 | 5–2 7 | 18.4 | 4.1 | 15.2 | 7.5 | 0.14 | 0.68 |
|
| |||||||||
| Lung cancer symptoms |
18.3 | 5 | 8–28 | 22.4 | 3.8 | 17.2 | 4.8 | 0.02 | 1.12 |
|
| |||||||||
| FACT–General | 73.5 | 18 | 33–105 | 81.3 | 14.3 | 71.6 | 18.6 | 0.21 | 0.54 |
|
| |||||||||
| FACT–Lung | 91.8 | 21.6 | 42–132 | 103.7 | 16 | 88.8 | 22.1 | 0.1 | 0.85 |
|
| |||||||||
| Hospital Anxiety and Depression Scale | |||||||||
|
| |||||||||
| Anxiety | 6.4 | 4.4 | 0–19 | 6.6 | 5.8 | 6.3 | 4 | 0.91 | 0.05 |
|
| |||||||||
| Depression | 5.1 | 3.9 | 0–18 | 3.6 | 2.1 | 5.5 | 4.2 | 0.26 | 0.49 |
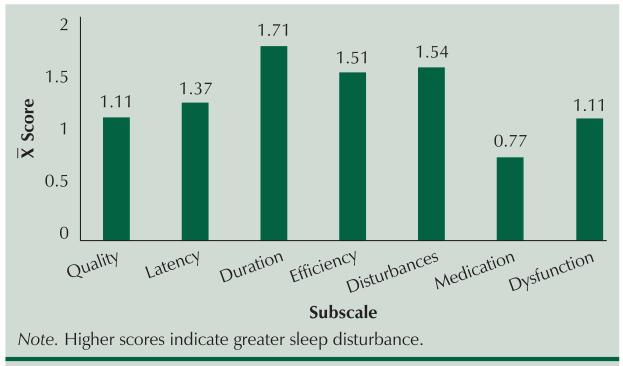
As shown in Figure 1, PSQI subscale scores revealed that sleep quality (76% rated good or fairly good) was better (received a lower score) than sleep latency, sleep duration, sleep efficiency, and sleep disturbance, indicating that despite these multiple sleep disturbances, the single-item measure of overall sleep quality was perceived as good. Only the sleep medications subscale was lower than sleep quality, indicating little use of sleep medications.
Figure 1.
Mean Scores for the Pittsburgh Sleep Quality Index Subscales
Sleep diary values for the study sample also were recorded. Seventy-seven percent (n = 27) of patients reported their sleep efficiency was less than 85%. Sleep latency of 30 minutes or more was prolonged in 43% (n = 15) of patients. Sleep duration was less than five hours in 35% (n = 12), whereas 17% (n = 6) were awake more than 30 minutes after sleep onset.
Actigraphy data revealed that 77% (n = 27) of participants experienced objective sleep latency longer than 30 minutes, and 61% (n = 21) were sleeping less than five hours per night, an amount associated with increased mortality in the general population (Kripke, Garfinkel, Wingard, Klauber, & Marler, 2002; Patel et al., 2004). Sleep efficiency of less than 85% was reported by 88% (n = 31), and 91% (n = 32) were awake more than 30 minutes after sleep onset.
Seventeen percent (n = 6) of participants had mean HADS anxiety scores between 8 and 10 (possible cases for anxiety), and 11% (n = 4) reported mean HADS anxiety scores of 11 or higher (cases for anxiety). For HADS depression, only 9% of participants reported mean scores of 8–10 (possible cases for depression) and another 9% (n = 3) reported mean scores of 11 or higher (cases for depression).
Quality of Life
Mean scores of the FACT-G, FACT-L, and subscale scores suggested overall poorer QOL, and results were similar to previous research in patients with lung cancer (Juliana, Jardim, Fernandes, Jamnik, & Santoro, 2010). The intraclass correlation coefficient values for the different scales of the FACT-L were excellent for PWB (0.85), FWB (0.89), SWB (0.81), and EWB (0.7), but not for LCS (0.65), which is consistent with previous research (Butt et al., 2005).
As expected, poor sleepers were significantly different from good sleepers on several of the PSQI’s measures, including sleep efficiency, sleep hours, sleep latency, and global sleep quality. The sleep diary data revealed a significant difference between good and poor sleepers for sleep efficiency and sleep latency. When comparing good and poor sleepers, actigraphy measures of sleep and measures of mood (HADS) were not significantly different. The FACT-L revealed a significant difference between good and poor sleepers on the lung cancer symptoms subscale. Additional analysis of the subscale revealed that three of the seven items were significantly worse in poor sleepers: breathing (p = 0.037), appetite (p = 0.043), and thinking (p = 0.017).
Large effects were found for sleep efficiency on the sleep diary, FACT-L, and the LCS subscale, whereas moderate effects were found for sleep hours and sleep latency on the sleep diary, the PWB and FWB subscales, and FACT-G.
Paired t tests were conducted to detect differences between subjective and objective measures. Significant differences were found with sleep efficiency (p = 0.002), sleep latency (p = 0.014), sleep hours (p < 0.001), and WASO (p < 0.001) between the two measures. Overall, patients’ self-reported sleep assessments were significantly better than the outcomes measured by the actigraph.
No significant relationships were identified between the sleep diary and actigraph sleep variables of interest, suggesting that objective and subjective measures are likely describing different aspects of sleep. Far more significant low-to-moderate correlations of subjective sleep measures were noted with mood and QOL than with objective sleep measures (see Table 6).
Table 6. Correlations Among Sleep, Mood, and Quality of Life.
| Variable | HADS-A | HADS-D | PWB | EWB | FWB | LCS | FACT-G | FACT-L |
|---|---|---|---|---|---|---|---|---|
| PSQI global | – | 0.381* | −0.446** | −0.386* | −0.49** | −0.425** | −0.56** | −0.586** |
|
| ||||||||
| PSQI latency | 0.344* | 0.429* | – | – | −0.44** | – | −0.458** | −0.408* |
|
| ||||||||
| SD latency | 0.375* | 0.424* | – | – | – | – | – | – |
|
| ||||||||
| AW2 sleep latency | – | – | −0.418* | −0.347* | – | – | −0.351* | −0.356* |
p < 0.05;
p < 0.01
AW2—motionlogger actigraph; EWB—emotional well-being subscale of the FACT-L; FACT–G—Functional Assessment of Cancer Therapy–General; FACT-L—Functional Assessment of Cancer Therapy–Lung; LCS—lung cancer symptoms subscale of the FACT-L; FWB—functional well-being subscale of the FACT-L; HADS-A—Hospital Anxiety and Depression Scale (anxiety); HADS-D—Hospital Anxiety and Depression Scale (depression); PSQI—Pittsburgh Sleep Quality Index; PWB—physical well-being subscale of the FACT-L; SD—sleep diary
Discussion
This study evaluated measures of sleep, mood, and QOL in patients receiving chemotherapy for lung cancer. The findings from this study demonstrated significant differences between subjective and objective measures of sleep; significant correlations among subjective sleep, mood, and QOL; and a significant difference in QOL scores (LCS subscale scores) between good and poor sleepers.
The mean total FACT-L scores reported in this study were consistent with previous research in patients with lung cancer (Browning, Ferketich, Otterson, Reynolds, & Wewers, 2009; John, 2001; Temel et al., 2010). The mean total FACT-L and LCS scores in the current study were similar to scores from newly diagnosed patients with non-small cell lung cancer who did not receive early palliative care and subsequently experienced significantly more depression and shorter survival than patients who did receive early palliative care (Temel et al., 2010). The LCS subscale scores in this study were significantly lower (worse) in poor sleepers and demonstrated a five-point difference. Previous research suggested a 2-3 point change in the LCS scores exposed a minimally important difference associated with performance status, objective tumor response, and time to progression (Cella et al., 2002). The larger effect sizes noted for lung cancer symptoms in terms of their relationship to good and poor sleep status, as compared to the effect sizes for anxiety and depression as defined by HADS, suggest that lung cancer symptoms (i.e., difficulty breathing) may have a more prominent association with sleep status than anxiety or depression.
Difficulty breathing is a complex and distressing symptom and is associated with reduced levels of physical functioning, role activities, cognitive and social functioning, and overall QOL (American Psychiatric Association, 2000; Langendijk et al., 2000; Sarna et al., 2004; Vena et al., 2006). The cause of dyspnea in lung cancer is multifactorial. Preexisting chronic obstructive pulmonary disease, pleural effusion, bronchial obstruction, lymphadenopathy from the disease process, and pulmonary fibrosis from chemotherapy or radiotherapy may result in decreased lung function and increased respiratory load (Dudgeon, 2002; Kvale, Selecky, & Prakash, 2007; Langendijk et al., 2000). For research related to an underlying physiologic mechanism, in the absence of airway obstruction or restrictive interstitial lung disease, dyspnea was related to ventilatory muscle weakness (Travers et al., 2008). Management of disease- and/or treatment-related symptoms may be the most effective interventions for sleep disturbances in patients receiving treatment for lung cancer (Degner & Sloan, 1995; Sarna et al., 2002, 2004; Vena et al., 2006).
In the current study, patients with lung cancer significantly overestimated sleep duration and sleep efficiency and underestimated sleep latency and WASO compared to concurrent objective actigraphic data recording. These findings are consistent with previous sleep research involving patients with lung cancer (Le Guen et al., 2007; Rumble et al., 2005; Silberfarb et al., 1993; Wang, Chang, & Lin, 2010). Silberfarb et al. (1993) reported mean sleep efficiency was 12% lower, mean total sleep time 1.5 hours less, and mean WASO 60 minutes longer in polysomnography recordings than sleep diaries. Le Guen et al. (2007) reported a slightly lower total sleep time in actigraphy versus PSQI. Wang et al. (2010) reported significantly more waking episodes in actigraphy versus sleep logs, but no significant differences in total sleep time, sleep latency, or sleep efficiency. Rumble et al. (2005) compared patients with lung cancer with and without insomnia and reported mean actigraphy total sleep time and sleep efficiency lower than sleep logs in the group without insomnia.
Discrepancies between subjective and objective measures may be related to sleep time misperception (Vanable, Aikens, Tadimeti, Caruana-Montaldo, & Mendelson, 2000). Previous research involving perceptions of sleep time stresses the potential role of physiologic (Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997), cognitive (Bonnet & Arand, 1997), and psychological factors (Vanable et al., 2000). However, patients with lung cancer may be a unique comorbid insomnia subgroup because of the magnitude of their tendency to under-report their sleep complaints (Gooneratne et al., 2007; Silberfarb et al., 1993). In addition, the lack of consistency between subjective and objective measures may be related to a gradual change in sleep quality over time. This gradual change may result in adaptation, and poor sleep is not recognized as disturbed. This may be related to a mediating factor such as a response shift—when a person’s health changes, a change may take place in their internal standards, values, or meanings of a construct that they are being asked to evaluate (Sprangers & Schwartz, 1999). Most patients with lung cancer are older adults and this age group may adjust their perception of acceptable sleep (Buysse et al., 1991).
The pattern of sleep disturbances in lung cancer suggests insomnia (Davidson et al., 2002; Ginsburg et al., 1995; Silberfarb et al., 1993). Objective measures revealed significantly longer sleep latency, shorter sleep duration, and poorer sleep efficiency than subjective measures. PWB and FWB subscales of the FACT-L were negatively correlated with global sleep quality, indicating daytime dysfunction was related to poorer sleep quality. In addition, PSQI subscale scores revealed that sleep latency, sleep duration, sleep efficiency, and sleep disturbance all were higher (worse) than sleep quality and the use of sleep medications. This suggests issues with sleep initiation and maintenance. In contrast, sleep-disordered breathing often results in shortened sleep latency as a result of increasing sleep pressure.
Based on self-report anxiety, 28% (n = 10) of this sample may benefit from referral for additional psychological evaluation. Although this level of anxiety is consistent with previous research (15%–40%) using the HADS anxiety scale in patients with lung cancer (Greer et al., 2008; Savard et al., 2009), anxiety scores were higher than depression scores. When poor sleepers were compared to good sleepers, as defined by the global PSQI score, no significant differences were noted in anxiety scores. Anxiety was significantly correlated to the PSQI sleep latency subscale, indicating anxiety may play a role in sleep initiation in patients with lung cancer.
Measures of depressed mood revealed that 18% of patients with lung cancer would meet the criteria for referral for additional evaluation. This level of depression is consistent with previous research (16%–38%) using the HADS scale in similar population samples (Lowe et al., 2004; Temel et al., 2010). When poor sleepers were compared to good sleepers, depression scores were lower in good sleepers but not significantly. Depression was significantly correlated to PSQI global and PSQI sleep latency and trending toward actigraphic sleep latency. This suggests that sleep initiation in patients with lung cancer may be more sensitive to depression than anxiety.
Limitations
This study has several limitations. The small cohort was collected through convenience sampling from Veterans Affairs clinics, thus limiting access to female patients. The cross-sectional nature of this study also prevents assessment of causality.
In addition, the data collection period with the actigraph and sleep diary was for seven days. Although longer observation periods, such as two weeks, have been used, a seven-day period provided a reasonable and complete picture of an individual’s sleep. Longer recording periods would increase participant burden and could result in an increased chance of data loss from patients forgetting to complete daily sleep diaries. The patients in the current study were particularly vulnerable to these additional study burdens because of the advanced cancer and chemotherapy treatments they were receiving.
Although long sleep latencies occurred with actigraphy, these findings should be interpreted with caution because the accuracy of actigraphy assessment of sleep latency can vary from patient to patient. Sleep latency assessment by actigraphy is generally considered to be one of the least accurate actigraphic sleep parameters.
Recommendations for Future Research
Additional investigation with a larger and more homogeneous sample including only patients with non-small cell lung cancer, for example, and conducting sleep-wake measures across the disease trajectory, including before treatment, would enhance the under-standing of sleep in this population. Although actigraphy can provide useful information about objective sleep and wakefulness, the gold standard measure for sleep parameters is polysomnography. Future sleep research in patients with lung cancer would benefit from the inclusion of polysomnography to determine the impact of disease and treatment on sleep stages, which is not provided by actigraphy. This would allow for assessment of other sleep disorders, including periodic limb movements and sleep apnea in this population. However, polysomnography is expensive and involves overnight study in a sleep laboratory or the use of expensive portable sleep devices for in-home monitoring.
Implications for Nursing Practice
This study provided several insights for clinical practice. The majority of patients receiving treatment for lung cancer reported poor sleep quality, which was related to daytime dysfunction and poorer QOL. Although sleep is not routinely assessed in oncology clinical practice, a growing body of evidence supports this important practice initiative (Berger & Mitchell, 2008; Dean et al., 2010; Page, Berger, & Johnson, 2006). Although using a single question to assess sleep is inadequate, it does provide the opportunity for a more targeted assessment similar to those used for pain and fatigue assessments. An excellent resource to guide nursing practice is the Oncology Nursing Society’s Putting Evidence Into Practice guidelines for sleep-wake disturbances (Page et al., 2006).
Conclusions
The findings from this study demonstrate that a large percentage of patients with lung cancer experience sleep disturbances during treatment. These sleep disturbances may be more strongly related to lung cancer symptoms than anxiety or depression. Significant discrepancies can occur between objective and subjective measures of sleep as well. Although patients may report an overall acceptable sleep quality when assessed by a single question, those same patients may still have markedly increased sleep latencies or reduced total sleep time. The clinical consequences of these objective impairments in sleep, despite minimal subjective complaints, remains unclear. Future research using prospective methods will help to elucidate their clinical significance.
Table 3. Comparison of Sleep Measures in Good and Poor Sleepers Determined by Sleep Diary (N = 35).
| Total |
Good Sleep (n = 7) |
Poor Sleep (n = 28) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | x̄ | SD | Range | x̄ | SD | x̄ | SD | p | Effect Size |
| Sleep efficiency |
74.3 | 13.6 | 50–98 | 84 | 11.6 | 71 | 12.9 | 0.03 | 1.01 |
|
| |||||||||
| Sleep hours |
5.8 | 1.5 | 3.7–9.7 | 6.6 | 1.2 | 5.6 | 1.5 | 0.13 | 0.68 |
|
| |||||||||
| Sleep latency |
48 | 50 | 0–230 | 23 | 17 | 54 | 54 | 0.03 | 0.64 |
|
| |||||||||
| Wake after sleep onset |
18 | 30 | 1–125 | 9 | 15 | 21 | 33 | 0.39 | 0.4 |
Table 4. Comparison of Sleep Measures in Good and Poor Sleepers Determined by Actigraphy (N = 35).
| Total |
Good Sleep (n = 7) |
Poor Sleep (n = 28) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | x̄ | SD | Range | x̄ | SD | x̄ | SD | p | Effect Size |
| Sleep efficiency |
61.9 | 17.6 | 22–95 | 63 | 18.8 | 61 | 17.6 | 0.79 | 0.11 |
|
| |||||||||
| Sleep hours |
4.4 | 2.1 | 0.5–8.1 | 4.3 | 1.7 | 4.4 | 2.2 | 0.95 | 0.02 |
|
| |||||||||
| Sleep latency |
96.3 | 92.3 | 12–466 | 82 | 70.3 | 100 | 98.6 | 0.57 | 0.2 |
|
| |||||||||
| Wake after sleep onset |
170 | 83 | 12–313 | 156 | 85 | 174 | 84 | 0.61 | 0.22 |
Acknowledgments
This research was funded, in part, by the University of Pennsylvania School of Nursing Postdoctoral Fellowship (No. 5T32HL007953), the University at Buffalo School of Nursing Garman Fund, and the Veterans Affairs Competitive Pilot Project Fund (VISN 4). The content of this article does not represent the views of the Department of Veterans Affairs or the U.S. government. Dean can be reached at gdean@buffalo.edu, with copy to editor at ONFEditor@ons.org.
References
- American Cancer Society Cancer facts and figures, 2013. 2013 Retrieved from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: 2000. text revision. [Google Scholar]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatrica Scandinavica. 2007;433(Supplement):104–115. doi: 10.1111/j.1600-0447.2007.00968.x. doi:10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. Journal of Pain Symptom Management. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. doi:10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Berger AM, Mitchell SA. Modifying cancer-related fatigue by optimizing sleep quality. Journal of the National Comprehensive Cancer Network. 2008;6:3–13. doi: 10.6004/jnccn.2008.0002. [DOI] [PubMed] [Google Scholar]
- Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. Journal of Pain and Symptom Management. 2008;36:191–199. doi: 10.1016/j.jpainsymman.2007.10.008. doi:10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Medicine Reviews. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. doi:10.1016/S1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Elsevier Saunders; Philadelphia, PA: 2005. pp. 405–417. [Google Scholar]
- Browning KK, Ferketich AK, Otterson GA, Reynolds NR, Wewers ME. A psychometric analysis of quality of life tools in lung cancer patients who smoke. Lung Cancer. 2009;66:134–139. doi: 10.1016/j.lungcan.2008.12.018. doi:10.1016/j.lungcan.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan D, Milroy R, Baker L, Thompson AM, Levack PA. Perceptions of anxiety in lung cancer patients and their support network. Supportive Care in Cancer. 2009;18:29–36. doi: 10.1007/s00520-009-0626-2. doi:10.1007/s00520-009-0626-2. [DOI] [PubMed] [Google Scholar]
- Butt Z, Webster K, Eisenstein AR, Beaumont J, Eton D, Masters GA, Cella D. Quality of life in lung cancer: The validity and cross-cultural applicability of the Functional Assessment of Cancer Therapy-Lung scale. Hematology Oncology Clinics of North America. 2005;19:389–420. doi: 10.1016/j.hoc.2005.02.009. doi:10.1016/j.hoc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- Cella D, Eton DT, Fairclough DL, Bonomi P, Heyes AE, Silberman C, Johnson DH. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) study 5592. Journal of Clinical Epidemiology. 2002;55:285–295. doi: 10.1016/s0895-4356(01)00477-2. doi:10.1016/S0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- Chen ML, Yu CT, Yang CH. Sleep disturbances and quality of life in lung cancer patients undergoing chemotherapy. Lung Cancer. 2008;62:391–400. doi: 10.1016/j.lungcan.2008.03.016. doi:10.1016/j.lungcan.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Social Science and Medicine. 2002;54:1309–1321. doi: 10.1016/s0277-9536(01)00043-0. doi:10.1016/S0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- Dean GE, Finnell DS, Scribner M, Wang YJ, Steinbrenner LM, Gooneratne NS. Sleep in lung cancer: The role of anxiety, alcohol and tobacco. Journal of Addictions Nursing. 2010;21:130–138. doi:10.3109/10884601003777620. [Google Scholar]
- Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. Journal of Pain and Symptom Management. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. doi:10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- Dolberg OT, Hirschmann S, Grunhaus L. Melatonin for the treatment of sleep disturbances in major depressive disorder. American Journal of Psychiatry. 1998;155:1119–1121. doi: 10.1176/ajp.155.8.1119. [DOI] [PubMed] [Google Scholar]
- Dudgeon DJ. Managing dyspnea and cough. Hematology Oncology Clinics of North America. 2002;16:557–577. doi: 10.1016/s0889-8588(02)00019-9. doi:10.1016/S0889-8588(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. PsychoOncology. 2004;13:359–363. doi: 10.1002/pon.783. doi:10.1002/pon.783. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Gentili A, Weiner DK, Kuchibhatla M, Edinger JD. Test-retest reliability of the Pittsburgh Sleep Quality Index in nursing home residents. Journal of the American Geriatric Society. 1995;43:1317–1318. doi: 10.1111/j.1532-5415.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg ML, Quirt C, Ginsburg AD, MacKillop WJ. Psychiatric illness and psychosocial concerns of patients with newly diagnosed lung cancer. Canadian Medical Association Journal. 1995;152:701–708. [PMC free article] [PubMed] [Google Scholar]
- Gooneratne NS, Dean GE, Rogers AE, Nkwuo JE, Coyne JC, Kaiser LR. Sleep and quality of life in long-term lung cancer survivors. Lung Cancer. 2007;58:403–410. doi: 10.1016/j.lungcan.2007.07.011. doi:10.1016/j.lungcan.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JA, Pirl WF, Park ER, Lynch TJ, Temel JS. Behavioral and psychological predictors of chemotherapy adherence in patients with advanced non-small cell lung cancer. Journal of Psychosomatic Research. 2008;65:549–552. doi: 10.1016/j.jpsychores.2008.03.005. doi:10.1016/j.jpsy chores.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: Implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. British Journal of Cancer. 1995;71:633–636. doi: 10.1038/bjc.1995.124. doi:10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood P, Stephens RJ. Depression in patients with lung cancer: Prevalence and risk factors derived from quality-of-life data. Journal of Clinical Oncology. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- John LD. Quality of life in patients receiving radiation therapy for non-small cell lung cancer. Oncology Nursing Forum. 2001;28:807–813. [PubMed] [Google Scholar]
- Juliana F, Jardim JR, Fernandes AL, Jamnik S, Santoro IL. Reliability of the Brazilian version of the Functional Assessment of Cancer Therapy-Lung (FACT-L) and the FACT-Lung Symptom Index (FLSI) Clinics (Sao Paulo) 2010;65:1247–1251. doi: 10.1590/S1807-59322010001200005. doi:10.1590/S1807-59322010001200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives in General Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. doi:10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Kvale PA, Selecky PA, Prakash UB. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd ed.) Chest. 2007;132(3, Suppl.):368S–403S. doi: 10.1378/chest.07-1391. doi:10.1378/chest.07-1391. [DOI] [PubMed] [Google Scholar]
- Langendijk JA, Aaronson NK, ten Velde GP, de Jong JM, Muller MJ, Wouters EF. Pretreatment quality of life of inoperable non-small cell lung cancer patients referred for primary radiotherapy. Acta Oncologica. 2000;39:949–958. doi: 10.1080/02841860050215936. doi:10.1080/02 841860050215936. [DOI] [PubMed] [Google Scholar]
- Le Guen Y, Gagnadoux F, Hureaux J, Jeanfaivre T, Meslier N, Racineux JL, Urban T. Sleep disturbances and impaired daytime functioning in outpatients with newly diagnosed lung cancer. Lung Cancer. 2007;58:139–143. doi: 10.1016/j.lungcan.2007.05.021. doi:10.1016/j.lungcan.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lowe B, Grafe K, Ufer C, Kroenke K, Grunig E, Herzog W, Borst MM. Anxiety and depression in patients with pulmonary hypertension. Psychosomatic Medicine. 2004;66:831–836. doi: 10.1097/01.psy.0000145593.37594.39. doi:10.1097/01.psy.0000145593.37594.39. [DOI] [PubMed] [Google Scholar]
- Page MS, Berger AM, Johnson LB. Putting Evidence Into Practice: Evidence-based interventions for sleep-wake disturbances. CJON. 2006:753–767. doi: 10.1188/06.CJON.753-767. [DOI] [PubMed] [Google Scholar]
- Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, Morrow GR. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer. Journal of Clinical Oncology. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. doi:10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: The behavioural model and a neurocognitive perspective. Journal of Sleep Research. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. doi:10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Rumble ME, Keefe FJ, Edinger JD, Porter LS, Garst JL. A pilot study investigating the utility of the cognitive-behavioral model of insomnia in early-stage lung cancer patients. Journal of Pain and Symptom Management. 2005;30:160–169. doi: 10.1016/j.jpainsymman.2005.02.013. doi:10.1016/j.j painsymman.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Sarna L, Evangelista L, Tashkin D, Padilla G, Holmes C, Brecht ML, Grannis F. Impact of respiratory symptoms and pulmonary function on quality of life of long-term survivors of non-small cell lung cancer. Chest. 2004;125:439–445. doi: 10.1378/chest.125.2.439. doi:10.1378/chest.125.2.439. [DOI] [PubMed] [Google Scholar]
- Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of life of long-term survivors of non-small cell lung cancer. Journal of Clinical Oncology. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. doi:10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a two-month period. Journal of Clinical Oncology. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. doi:10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- Schlesinger I, Hering-Hanit R, Dagan Y. Sleep disturbances after whiplash injury: Objective and subjective findings. Headache. 2001;41:586–589. doi: 10.1046/j.1526-4610.2001.041006586.x. doi:10.1046/j.1526-4610.2001.041006586.x. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. doi:10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Silberfarb PM, Hauri PJ, Oxman TE, Schnurr P. Assessment of sleep in patients with lung cancer and breast cancer. Journal of Clinical Oncology. 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- Snaith RP. The Hospital Anxiety and Depression Scale. Health Quality Life Outcomes. 2003 doi: 10.1186/1477-7525-1-29. Retrieved from http://www.hqlo.com/content/1/1/29. [DOI] [PMC free article] [PubMed]
- Sprangers MA, Schwartz CE. Integrating response shift into health-related quality-of-life research: A theoretical model. Social Science and Medicine. 1999;48:1507–1515. doi: 10.1016/s0277-9536(99)00045-3. doi:10.1016/S0277-9536 (99)00045-3. [DOI] [PubMed] [Google Scholar]
- Stein MB, Mellman TA. Anxiety disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 4th ed. Elsevier Saunders; Philadelphia, PA: 2005. pp. 1297–1310. [Google Scholar]
- Steinberg T, Roseman M, Kasymjanova G, Dobson S, Lajeunesse L, Dajczman E, Small D. Prevalence of emotional distress in newly diagnosed lung cancer patients. Supportive Care in Cancer. 2009;17:1493–1497. doi: 10.1007/s00520-009-0614-6. doi:10.1007/s00520-009-0614-6. [DOI] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Lynch TJ. Early palliative care for patients with metastatic non-small cell lung cancer. New England Journal Medicine. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. doi:10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- Travers J, Dudgeon DJ, Amjadi K, McBride I, Dillon K, Laveneziana P, O’Donnell DE. Mechanisms of exertional dyspnea in patients with cancer. Journal of Applied Physiology. 2008;104:57–66. doi: 10.1152/japplphysiol.00653.2007. doi:10.1152/japplphysiol.00653.2007. [DOI] [PubMed] [Google Scholar]
- Turner NJ, Muers MF, Haward RA, Mulley GP. Psychological distress and concerns of elderly patients treated with palliative radiotherapy for lung cancer. Psycho-Oncology. 2007;16:707–713. doi: 10.1002/pon.1109. doi:10.1002/pon.1109. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: Variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:71–79. [PubMed] [Google Scholar]
- Vena C, Parker K, Allen R, Bliwise D, Jain S, Kimble L. Sleep-wake disturbances and quality of life in patients with advanced lung cancer. Oncology Nursing Forum. 2006;33:761–769. doi: 10.1188/06.ONF.761-769. doi:10.1188/06.ONF.761-769. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Larsen LH, Moe KE. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, noncomplaining older men and women. Journal of Psychosomatic Research. 2004;56:503–510. doi: 10.1016/S0022-3999(04)00023-6. doi:10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Wang SY, Chang HJ, Lin CC. Sleep disturbances among patients with non-small cell lung cancer in Taiwan: Congruence between sleep log and actigraphy. Cancer Nursing. 2010;33:E11–E17. doi: 10.1097/NCC.0b013e3181b3278e. doi:10.1097/NCC.0b013e3181b3278e. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. doi:10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]