Abstract
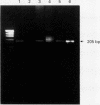
DNA extraction and PCR amplification from paraffin wax embedded bone tumour specimens present several difficulties, firstly, because of the abundant matrix they contain and, secondly, because decalcification often causes degradation of DNA. In this report, comparative studies were carried out to determine the most efficient method for DNA extraction and PCR amplification from such specimens. The results indicated that nested PCR produced appropriate strong reaction products with minimal background contamination. A method for DNA extraction from paraffin wax embedded bone tissue and a nested PCR-SSCP technique have been developed for use in such diagnostic specimens.
Keywords: DNA extraction
Keywords: PCR amplification
Keywords: SSCP
Keywords: paraffin wax embedded bone tumour specimens
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassam B. J., Caetano-Anollés G., Gresshoff P. M. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem. 1991 Jul;196(1):80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- Bosari S., Viale G. The clinical significance of p53 aberrations in human tumours. Virchows Arch. 1995;427(3):229–241. doi: 10.1007/BF00203389. [DOI] [PubMed] [Google Scholar]
- Cha R. S., Zarbl H., Keohavong P., Thilly W. G. Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl. 1992 Aug;2(1):14–20. doi: 10.1101/gr.2.1.14. [DOI] [PubMed] [Google Scholar]
- Koshiba M., Ogawa K., Hamazaki S., Sugiyama T., Ogawa O., Kitajima T. The effect of formalin fixation on DNA and the extraction of high-molecular-weight DNA from fixed and embedded tissues. Pathol Res Pract. 1993 Feb;189(1):66–72. doi: 10.1016/S0344-0338(11)80118-4. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Porter-Jordan K., Rosenberg E. I., Keiser J. F., Gross J. D., Ross A. M., Nasim S., Garrett C. T. Nested polymerase chain reaction assay for the detection of cytomegalovirus overcomes false positives caused by contamination with fragmented DNA. J Med Virol. 1990 Feb;30(2):85–91. doi: 10.1002/jmv.1890300202. [DOI] [PubMed] [Google Scholar]
- Sparrow L. E., Soong R., Dawkins H. J., Iacopetta B. J., Heenan P. J. p53 gene mutation and expression in naevi and melanomas. Melanoma Res. 1995 Apr;5(2):93–100. doi: 10.1097/00008390-199504000-00004. [DOI] [PubMed] [Google Scholar]