Abstract
Background: Inborn errors of metabolism (IEM) are mostly transmitted as autosomal recessive disorders and are therefore more frequent in countries with high consanguinity rates such as in the Arab world. Objective: To study the socio-demographic characteristics and the clinical presentation of IEM in Libyan children and to shed light on our experience in dealing with these disorders. Methods: This is a descriptive case series hospital-based study of 107 children attending the Metabolic Unit at El-Khadra Teaching Hospital (MUKH) in Tripoli, Libya. The study took place between January 2001 and December 2012. Information was collected from caregivers and from all available hospital records on the following variables: age, sex, birth order, place of residence, age at onset, presenting complaints and family history of the same illness. Results: During the 12-year study period, there were 55,422 live births at El-Khadra Teaching Hospital and 107 children were diagnosed with 46 different metabolic disorders. A significantly high consanguinity rate was observed (86.9%) among parents of the affected children. Family history of previous affected children was noted in 63.5% of cases. Male to female ratio was 1.18:1. The most frequent IEM cases were amino acids disorders (25%), carbohydrate disorders (14.9%), lysosomal storage diseases (14%), organic aciduria and energy metabolic defects (9.3% each). The main clinical presentations were jaundice, hepatomegaly and seizures. Most children presented between one and six months of age (43.4%); whereas the median age at diagnosis was eight months. Thirty-eight children (35.5%) were born at El-Khadra Hospital with IEM giving a birth prevalence of 1:1458 live births, (1:6158 for aminoaciduria and 1:6927 for carbohydrate disorders). Conclusion: IEM disorders are common in Libya. Efforts to enhance awareness among pediatricians and primary healthcare providers should be supported and encouraged as many diseases are still undiagnosed. It is very important to consider IEM among all children when they present any worrying or suspicious symptoms or signs which do not respond to conventional treatment. Although our findings are preliminary, and probably the first to be conducted in Libya, they suggest ideas for decision makers to plan services including newborn screening programs and country-wide research of IEM diseases.
Keywords: inborn errors of metabolism, Libya, consanguinity
Introduction
Libya is situated in North Africa, stretching along the Mediterranean shoreline for nearly 2,000 kilometers. Its surface area is 1,775,500 square kilometers making it the fourth largest country in Africa. Egypt is on its eastern border, Sudan, Chad and Niger are to the south and Tunisia and Algeria lie to the west. Tripoli (the capital) is the country's major city. The total population of Libya is 6.18 million, with 39% of the population below the age of 15. More than half of the population is urban; most live in the coastal area in the main cities, namely Tripoli (2.4 million) and Benghazi (0.75 million).
The estimated birth rate is ∼25.6 births/1000 populations (∼158,000 births per annum) with a growth rate of 1.83%. Life expectancy is approximately 72.5 years. Approximately 98.3% of all births are in health establishments. The infant mortality rate is 17.6/1000 live births; under-five mortality is 20.1/1000 populations, (1) the perinatal mortality rate is 25.8/1000 total births. (2)
Causes of mortality in neonatal intensive care units are prematurity (43.9%), hypoxic ischemic encephalopathy (16.7%), congenital malformations (15.9%), neonatal infections (14.6%) and other reasons (4.6%). (3) Due to limited diagnostic facilities and lack of experienced pediatricians trained in metabolic diseases, many unexplained deaths have been reported and are likely to have been misdiagnosed. High rates of consanguineous marriages in Libya (48.4% as inter-marriages between relatives including 30% between first cousins) (1,4,5) play an important role in increasing expression of autosomal recessive disorders. In Libya there is limited data explaining the incidence and clinical presentation of IEM. This study reports our local experience in the field of IEM over the last 12 years.
Material and Methods
A study of children attending the Metabolic Unit at El-Khadra Teaching Hospital (MUKH) in Tripoli, took place between 1st January 2001 and 31st December 2012. Patients recruited for this study were either born in the hospital or referred from primary and secondary health centers in Tripoli and its neighboring areas. All affected children were admitted to and treated at the El-Khadra Hospital's Department of Pediatrics in Tripoli, Libya. El-Khadra Hospital is one of the main teaching hospitals in Western Libya. The Department of Pediatrics provides healthcare services to all pediatrics age groups between 0 to16 years.
Prior to the commencement of the study, official approval was obtained from the hospital ethical committee granting permission for the study. Following this, data was collected from the medical records of patients already diagnosed with IEM over the study period. Children with clinical symptoms were reviewed and classified according to the type of their metabolic disorder: carbohydrate, amino acids (AA), organic acids (OA), fatty acids, energy defects, purine pyrimidine, vitamins and metal transport, lipoprotein, bile acid and the organelle-related disorders. Other variables that were collected and analyzed included: age at presentation, age at diagnosis, sex, consanguinity, family history of previous IEM, clinical presentation, and current treatment and the outcome.
Initial and baseline biochemical investigations which were sent to local laboratories included complete blood count, blood ammonia (B.NH3), arterial blood gas (ABG), blood sugar, ketones, urea and electrolyte, liver function tests, lipid profile, lactate, ferritin, alpha fetoprotein, urine and stool analysis. Between 2001–2005 blood and urine samples were sent for scheduled evaluation of IEM to Bioscientia Laboratory in Germany. Since 2006, confirmatory samples were scheduled for evaluation of IEM in collaboration with expert laboratories such as Bioscientia, Gutenberg Universitat Mainz (Germany), Saint Joseph University (Lebanon) and Cerba Laboratory (France).
From 2009, it became easier to send samples as dried blood spots (filter paper cards). Collection of the samples were consistent with the recommendations of the Clinical Laboratory Standards Institute Blood Collection on Filter Paper for Newborn Screening Programs. Blood specimens were allowed to air dry thoroughly on a horizontal level for a minimum of three hours at ambient temperature and were kept away from direct sunlight. Within 24 hours of collection, the dried blood collection card was prepared for shipment to the laboratory and samples were packaged in a protective envelope and sent by courier. Results were reported electronically via email.
SPSS Statistics version 17.0 was used to analyze the data.
Results
Over the 12-year study period, the total admission to the Department of Pediatrics was 19,938 children (11.7% of total hospital admission), of which 107 patients were diagnosed with 46 different types of IEM diseases. The total number of live births at El-Khadra Hospital was 55,422. Consanguinity rate was 86.9% among parents of affected children. Thirty-three patients died. Twelve families had two affected children. Family history of previously affected children was noted in 63.5% of cases. Family history of infantile death with similar illnesses was 52.3% (56 families). One effected child reached adulthood at the end of the study period. The sex distribution was 58 males and 49 females. The frequency of disorders, the main clinical features, age at onset, age at diagnosis and the outcome of the 107 diagnosed children are summarized in Tables 1–8. The most frequent disorders were amino acid (25%), carbohydrate (14.9%), lysosomal storage disease (14%), organic acids and energy defects (9.3% each).
Table 1.
Types of metabolic disorders and numbers of patients.
| Type of disorder | Numbers (Total 107) |
|
| |
| Amino acids | 27 |
|
| |
| Organic acids | 10 |
|
| |
| Urea cycle defect | 3 |
|
| |
| Carbohydrate | 16 |
|
| |
| Fatty acid oxidation | 4 |
|
| |
| Lysosomal storage | 15 |
|
| |
| Energy defect | 10 |
|
| |
| Purine Pyrimidine | 2 |
|
| |
| Bile acid | 6 |
|
| |
| Lipoprotein | 1 |
|
| |
| Trace elements, Iron and Vitamins | 7 |
|
| |
| Peroxisomal | 5 |
|
| |
| Congenital disorder of glycosylation | 1 |
|
| |
Table 2.
Clinical presentation and outcomes of patients with disorders of Amino acids.
| Type of disorder numbers (%) | Main features | Outcome alive/died | Age at onset median (range) | Age at diagnosis median (range) |
|
| ||||
| AA 27 (25) | ||||
|
| ||||
| -MSUD 5(18.5) | Lethargy, seizures | 3 alive with developmental delay | 4d (3–7) | 2m (1–8) |
|
| ||||
| -HCY 1(3.7) | Epilepsy, myopia, long, thin | Alive with schooling difficulty | 6m | 64m |
|
| ||||
| -Hartnup 1(3.7) | Ataxia, dermatitis | Alive and well | 12m | 24m |
|
| ||||
| -HTI 17(62.9) | Hepatomegaly, rickets | 13 alive | 4.5m (1w-8m) | 8m (1w-40m) |
|
| ||||
| -NKHG 3(3.7) | Seizures, coma | All died | 10d (7–14) | 1m |
|
| ||||
Note: AA: Amino acid, MSUD: Maple syrup urine disease, HCY: Homocystinuria, HTI: Hereditary Tyrosinemia type I, NKHG: Non-ketotic hyperglycinemia.
Table 3.
Clinical presentation and outcomes of patients with Organic acid disorders and Urea cycle defects.
| Type of disorder No. (%) | Main features | Outcome alive/died | Age at onset median (range) | Age at diagnosis median (range) |
|
| ||||
| OA 10(9.3%) | ||||
|
| ||||
| -PA 4(4o) | Vomiting, dehydration, lethargy acidosis | Alive 2 with mild global delay | 14d (10–40) | 1.5m (1–4) |
|
| ||||
| -MMA 4(40) | Vomiting, reluctant to feed, acidosis, hypotonia | Alive, well | 1.5m (1–4) | 2.5m (2–5) |
|
| ||||
| -IVA 1(10) | Feeding difficulties, acidosis | Died in 1 month | 2m | 3m |
|
| ||||
| -GA 1(10) | Seizures, dystonic movements | Well no more crisis | By newborn screen | 1m |
|
| ||||
| UCD 3(2.8%) | ||||
|
| ||||
| -OTC 2(66.6) | Seizures | Developmental delay | 12m (10–14) | 15m (12–18) |
|
| ||||
| Citrullinemia type 1 1(33.3) | Episodes of vomiting, failure to thrive | Alive | 8m | 10m |
|
| ||||
Note: OA: Organic acid, PA: Propionic aciduria, MMA: Methylmalonic aciduria, IVA: Isovaleric aciduria, GA: Gluteric aciduria, UCD: Urea cycle defect and OTC: Ornithine transcarbamylase deficiency.
Table 4.
Clinical presentation of patients with Carbohydrate disorders and Fatty acid oxidation disorders.
| Type of disorder No. (%) | Main features | Outcomes alive/died | Age at onset median (rang) | Age at diagnosis median (range) |
|
| ||||
| Carbohydrate 16 (14.9) | ||||
|
| ||||
| -Galactosemia 4 (25) | Jaundice, hepatomegaly, failure to thrive | 2 alive | 4w (3–8) | 12w (8–12) |
|
| ||||
| -GSD Ia 7 (43,7) | Hypoglycemia, convulsion hepatomegaly | 5 alive | 8w (3–32) | 4m (2–48) |
|
| ||||
| -GSD XI 4 (25) | vit.D resistant rickets | alive, 3 bone deformity | 4.5m (3–12) | 10.5m (6–12) |
|
| ||||
| -GSD III 1(6.2) | Hepatomegaly, hypoglycemia | Alive healthy | 7m | 12m |
|
| ||||
| FOD 4 (3.7) | ||||
|
| ||||
| -CPT I 1 (25) | Coma, hypoglycemia, hepatomegaly | Alive, well | 9m | 24m |
|
| ||||
| -VLCAD 2 (50) | Hepatomegaly, cardiomyopathy | Died | 5.5m (5–6) | 8.5m (8–9) |
|
| ||||
| -GA II 1 (25) | Hypotonia, hypoglycemia, dysmorphic | Died | 2w | 4m |
|
| ||||
Note: GSD: Glycogen storage disease, FOD: Fatty acid oxidation deficiencies, CPT: Carnitine palmitoyl transferase deficiency, VLCAD: Very long chain acyl-CoA dehydogenase deficiency, GA II: Gluteric aciduria.
Table 5.
Clinical presentation and outcomes of patients with Lysosomal diseases.
| Type of disorder No. (%) | Main features | Outcomes alive/died | Age at onset median (rang) | Age at diagnosis median (range) |
|
| ||||
| LSD 15 (14) | ||||
|
| ||||
| -GM I 1 (6.6) | Hypotonia | Blind, MR | 1m | 9m |
|
| ||||
| -Tay Sachs 2 (13.3) | Hypotonia, eyes cherry red spots | MR | 3m | 10.5m (9–12) |
|
| ||||
| -NPC 1(6.6) | Jaundice, ataxia, hepatosplenomagly | Alive | 1m | 2.5y |
|
| ||||
| -Gaucher 4(26.6) | Hepatosplenmegly, pancytopenia | 1 alive | 2.5m (1–3) | 8m (7–9) |
|
| ||||
| -Farber 1(6.6) | Joint pain, skin nodules | Died | 9m | 12m |
|
| ||||
| -MPS I 3 (20) | Dysmorphic, respiratory infection | All died | 10m (9–11) | 2y (2–3) |
|
| ||||
| -MPS III 1 (6.6) | Slightly course features and development delay | Alive | 8m | 7y |
|
| ||||
| -MPS VI 1 (6.6) | Hypotonia and sever chest dysfunction | Died | 6m | 1y |
|
| ||||
| -Cystinosis 1 (6.6) | Polyuria, dehydration, rickets, failure to thrive | Alive | 6m | 9m |
|
| ||||
Note: LSD: Lysosomal storage disease, GM I: Gangliosidosis type I, NPC: Niemann Pick C disease, MPS: Mucopolysacchardoses, MR: mental retarded.
Table 6.
Clinical presentation and outcomes of patients with disorders of Energy defects.
| Type of disorder No. (%) | Main features | Outcomes alive/died | Age at onset median (rang) | Age at diagnosis median (rang) |
|
| ||||
| Energy defect 10 (9.3) | ||||
|
| ||||
| -PDH 2 (20) | Hypotonia, seizures, high lactate | Died | 2.5m (2–3) | 4.5m (4–5) |
|
| ||||
| -PC 1 (10) | Hypotonia, high lactate, hypoglycemia. Hypotonia, seizures | Died | 3m | 4m |
|
| ||||
| -Fumaric aciduria 1(10) | Microcephaly, seizures | MR | 3m | 9m |
|
| ||||
| -Leigh syndrome 3(30) | Nystagmus, MRI: tbl2 basal ganglia brain stem. hyperintensity | Died | 4m (3–6) | 9m (8–9) |
|
| ||||
| -Mitochondrial DNA depletion 1(10) | Failure to Thrive, hypotonia, hypoglycmia | Global development delay | 6m | 2y |
|
| ||||
| -Creatine 2(20) biosynthesis GAMT deficiency | Autistic behaviors | Alive | 1.5y (1–2) | 5.5y (5–6) |
|
| ||||
Note: PDH: Pyruvate dehydrogenase deficiency, PC: Pyruvate carboxylase deficiency, GAMT: Guanidinoacetate methyltransferase deficiency.
Table 7.
Clinical presentation and outcomes of patients with Purine Pyrimidine disorders, Bile acid disorder and Lipoprotein disorder.
| Type of disorder No. (%) | Main features | Outcomes live/died | Age at onset median (rang) | Age at diagnosis median (range) |
|
| ||||
| Purine Pyrimidine disorders 2(1.8) | ||||
|
| ||||
| -Lesch Nyhan 1(50) syndrome | Self mutilation, hypotonia, dystonia | Alive MR | 6m | 1y |
|
| ||||
| - Orotic aciduria 1(50) | Megaloplastic anaemia | Died | 1m | 2m |
|
| ||||
| Bile acid disorder 6(5.6) | ||||
|
| ||||
| -PFIC | Hepatomegaly, jaundice, pruritus rickets | Alive | 2.5w (2–4) | 6w (4–8) |
|
| ||||
| Lipoprotein 1(0.9) | ||||
|
| ||||
| Mixed hyperlipidmia | Sepsis, lipiemic blood sample | Alive, well | 2w | 3w |
|
| ||||
Note: PFIC: Progressive familial intrahepatic cholestasis.
Table 8.
Clinical presentation and outcomes of patients with variable metabolic disorders.
| Type of disorder No. (%) | Main features | Outcomes live/died | Age at onset median (range) | Age at diagnosis median (range) |
|
| ||||
| Trace elements, Vitamins and Iron 7 (6.5) | ||||
|
| ||||
| -Copper Wilson 3(42.8) | Hepatomegaly, ataxia, dysarthria | Alive, one liver transplanted | 4y (3–6) | 7y (6–9) |
|
| ||||
| Hypomagnesamia 1(14.2) | Hypocalcemia, convulsion | Alive and well | 6m | 7m |
|
| ||||
| -Biotinidase 2(28.5) | Skin rash, acidosis, convulsion | Alive | 6.5m (6–7) | 8.5m (8–9) |
|
| ||||
| -Iron hemochromatosis 1(14.2) | Prolonged jaundice | Alive | 2w | 4w |
|
| ||||
| Peroxisomal 5(4.6) | ||||
|
| ||||
| -Zellweger 1(20) | Hypotonia, jaundice convulsion | Died | 1w | 8m |
|
| ||||
| -Rufsum 1(20) | Ichthyosis.ataxia | Missed | 7y | 8y |
|
| ||||
| -ALD 1(20) | Ataxia, hypoglycemia | Died | 8y | 8y |
|
| ||||
| -Hyperoxaluria I 2(40) | Recurrent UTI nephrocalcinosis | Alive, one transplanted | 3.5m (3–4) | 6.5m (6–7) |
|
| ||||
| CDG 1(0.9) | Hepatomagaly, FTT coagulopathy, deaf | Alive | 4m | 2.5y |
|
| ||||
Note: ALD: Adrenoleukodystrophy, CDG: Congenital disorder of glycosylation, UTI: urinary tract infection, FTT failure to thrive.
The main clinical presentation at onset was jaundice and hepatomegaly at 32.6%. Of all diagnosed patients, seizures were the second most alarming sign at 23.9%. Age at presentation: 12 diseases were seen in the first month of life (26%), 20 diseases were seen between one and six months of age (43.4%), seven diseases were seen between seven and twelve months of age (15.2%) and the remainder of the children (15.2% – 7 diseases) were presented to us at an age of one year or greater. The median age at diagnosis was eight months (range was 1–96 months), 13% (14 diseases) were diagnosed at an age of six months or younger.
The study sample was believed to be representative of the Libyan population because, despite the large size of Libya, it is mostly desert with few medical services and thus most of the health services and specialized clinics in which these children and families are seen are concentrated in Tripoli.
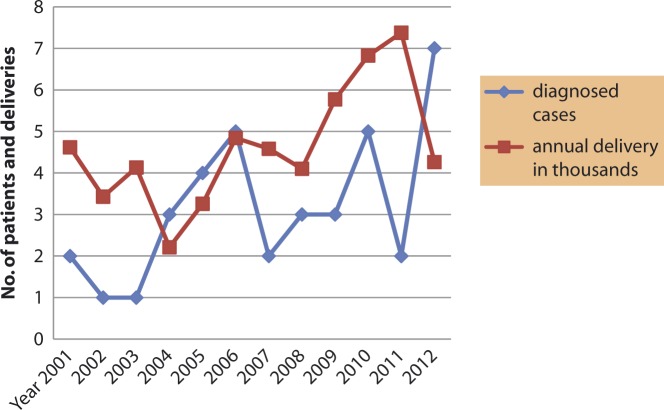
El-Khadra hospital is a referral teaching hospital in Tripoli serving the Libyan population from different areas and because the study itself is large with time trend results, one should generalize the findings with reservation. The total diagnosed patients were 107 of which 38 patients (35.5%) were born at El-Khadra Hospital (birth prevalence 1:1458 live births) (Figure 1). The highest incidence rate was 16/100,000 for AA disorders (birth prevalence 1:6158 live births) and was 14/100,000 live births for carbohydrate disorders (birth prevalence 1:6927 live births).
Figure 1.
The time trend of diagnosed patients with IEM who were born at the El-Khadra Hospital during the 12 years with annual hospital deliveries.
Discussion
In many countries the majority of IEM disorders with small molecule disorders are diagnosed by newborn screening, most often pre-symptomatically. Newborn screening (NBS) is an effective and important preventive measure; it is an integration of efforts from financial, political and cultural factions. (6–8) In general, the Libyan healthcare system has been suffering for decades because the Libyan government has not dedicated enough resources to dealing with this problem. In the year 2010, a national committee for NBS was established with no defined budget or financial support, furthermore, the country's poor facilities and infrastructure do not allow people in rural areas to seek proper healthcare. The newborn screening program has not been introduced nationally. Only where siblings have a history of an IEM disorder, will the newborn be offered testing.
The introduction of tandem mass spectrometry (MS/MS) in the mid-1990s started a new era by offering the benefit of detecting large numbers of metabolic diseases. There are a few Arab countries in the Middle East and North Africa (MENA) region that have started a NBS program, while others have either a limited hospital-based selective NBS program or have just completed pilot studies. (7–15) Determining the prevalence and incidence among Libyan children with different types of metabolic disorders will help in setting the first Libyan reference values and provides useful information for starting a newborn screening program to include treatable disorders such as AA, OA, carbohydrate and MPS.
High consanguineous marriages (48.4%) in the Libyan population increase the risk of metabolic disorders as most are inherited as autosomal recessives. (1) The consanguinity was significantly higher in our study (86.9%) and 62% of marriages were between first cousins which is twice the rate seen in the normal Libyan population. In 63.5% of the sample, there was a positive family history of either affected alive or previously dead siblings with a similar illness. The Libyan population (similar to populations in other Arab countries) is characterized by a high level of inbreeding, particularly among the Tabou and Touareg people living in the isolated oases of Southern Libya, the Barbar people in the Nafossa mountains of Western Libya and the Green Mountain (the Jebel Akhdar) area of Eastern Libya. In our cohort we did not observe that these disorders affected specific families, clans or tribes.
The perinatal mortality rate at El-Khadra hospital was 25.8/1000 total births during the mid- 1990s (2) which was high in comparison to Western European and North American countries. (16) Causes of neonatal death were recorded as respiratory distress (79%), sepsis (32%) and stillbirths (38.35%); most deaths were in the first week of life (61.65%). A study in Tripoli during mid-2000 (3) has shown that the associated causes of neonatal death were respiratory distress (40.4%) and infection (18.5%); the majority of deaths (63.1%) occurred during the early neonatal period whereas (30.8%) were during the late neonatal period and (6.2%) during the post-neonatal period. In both of these studies, (2,3) no metabolic disorders were mentioned as possible causes of death in this critical age which might indicate poor facilities for identifying these diseases.
Approximately 70% of children in our study presented with IEM between 0 and six months of life. The earliest age at presentation was for children with maple syrup urine disease (MSUD) for which the median age was four days. The earliest age at diagnosis was in non-ketotic hyperglycinemia (NKH) and haemochromatosis for which the median age was one month. Children presenting with MSUD had the worst prognosis even with an early start to treatment; 87.5% of children were developmentally delayed. Similar results were seen in a Japanese study. (17) Two of the three NKH patients died before diagnosis. Jaundice and hepatomegaly was the main clinical picture of carbohydrate disorders or fatty acid oxidation deficiency. CDG (congenital disorder of glycosylation) presented with a wide range of signs and symptoms. Seizures were the main presentation of the (AA) disorders or urea cycle defect. Hyperoxaluria type I was presented in two brothers both of whom were referred to Geneva, Switzerland in 1998 and 2004 respectively for diagnosis and metabolic workup due to the lack of diagnostic facilities for IEM at the hospital. The older brother was kidney transplanted two years ago.
Treatment success for HTI was 86.6% with Nitisinone and 66.6% of progressive familial intrahepatic cholestasis (PFIC) patients responded well with bile acid substitution and diet. Although 70% of patients with different types of OA disorders survived, we are still facing crises that require emergency treatment, especially in children up to the age of two years, mostly due to poor dietary treatment. Success in treatment was seen in carbohydrate disorders, the best results observed were for treatment of GSD Ia. In spite of aggressive supplementation and dietary treatment in GSDXI, three children survived to have bone deformities and required surgical correction. Seven children with different IEM diseases (15.2%) were older than one year when presented to us and had alarming signs of unexplained neurological complaints or developmental delay, these diseases were Wilson disease, creatine biosynthesis (GAMT) deficiency, Rufsum, Adrenoleukodystrophy, Hartnup, Ornithine transcarbamylase deficiency and Niemann-Pick type C disease (newly diagnosed patient at the age of four years currently on Miglustat (Zavesca) therapy).
Diet management is essential for IEM treatment where general dietitians should work in collaboration with the medical team to plan a restricted diet for the patient that provides adequate nutrition. The main disorders treated with combination of diet and medication were tyrosinemia type I, MSUD, UCD, OA, GSD, galactosemia, FOD, hyperlipidemia and Wilson disease. Diets normally consist mainly of natural food and special milk formulas (in the last five years, special food products have been introduced). Nasogastric tubes are used if food is not tolerated orally.
A selective screening of aminoacidopathies and organic acidurias over 23 years in Tunisia has reported findings similar to our results. It reported a higher incidence of AA disorders (excluding PKU) of 57.3%; MSUD and HTI being the most frequent as individual diseases, followed by OA disorders (42.7%), with the most diagnosed diseases similar to our results being MMA and PA. 10 Tunisia lies on Libya's north-west border. Intermarriage between the people of Libya and Tunisia is common, which might explain the similarity of the distribution of these diseases in both countries.
Conclusion
In Libya, the diagnosis of IEM disorders has increased in recent years. El-Khadra Hospital is a referral center for metabolic disorders where no neonatal screening is available. Over the past 12 years we have diagnosed 107 patients with 46 different types of IEM disorders, with 86.9% born to consanguineous parents. Family history of similar illnesses was seen in 63.5%. The most frequent disorders were AA, carbohydrate, LSD, OA and energy defects. The main clinical presentations were jaundice, hepatomegaly and seizures. Thirty eight children (35.5%) were born with IEM at El-Khadra Hospital in the study period (birth prevalence 1:1458 live birth).
Our study provides useful information for health policy and planning services for future metabolic newborn screening programs, which should include screening for the most frequent treatable disorders. The results reflected increased medical awareness, clinical interest and improving diagnostic facilities. All this was studied in an area which still suffers from a lack of therapeutic and diagnostic resources, including confirmatory DNA testing, metabolic laboratories, specialized metabolic dietitians and in-country collaboration.
Acknowledgements
I thank Dr. Omar Ibrahim Abusnena from the department of Family Community Medicine, Faculty of Medicine Tripoli University for his expert advice on the statistical analysis.
Conflict of interest
None.
References
- 1. Ben Omran T. Genetic disorders in Libya Ahmad TS, ed. Genetic disorders among Arab populations 2nd ed. Berlin Heidelberg: Springer-Verlag; 2010. 444 445 [Google Scholar]
- 2.Alobaidy H, Kreasta M, Dekna M, Juma MR. Perinatal Mortality and Morbidity at Elkhadra Hospital Tripoli Libya. JMJ. 2004;3(1):48–51. [Google Scholar]
- 3.Abushhaiwia AME, Ziyani MMN, Dekna M. Mortality in the special care baby unit of the main children's hospital in Tripoli Libyan Arab Jamahiriya. East Mediterr Health J. 2010;16(11):1137–1142. [PubMed] [Google Scholar]
- 4. Social Indicators. United Nation Statistical Division. (Internet). Update June 2011. Available from: http://unstats.un.org/unsd/demographic/products/socind/health.htm.
- 5.Broadhead RL, Sehgal KC. Consanguinity and congenital abnormalities in East Libya. Garyounis Med J. 1981;4:3–5. [Google Scholar]
- 6.Wilcken B. Expanded newborn screening: reducing harm, asserting benefit. J Inherit Metab Dis. 2010;33:205–210. doi: 10.1007/s10545-010-9106-6. [DOI] [PubMed] [Google Scholar]
- 7.Saadallah AA, Rashed MS. Newborn screening: Experiences in the Middle East and North Africa. J Inherit Metab Dis. 2007;30:482–489. doi: 10.1007/s10545-007-0660-5. [DOI] [PubMed] [Google Scholar]
- 8.Krotoski D, Namasta S, MHS, Raouf RK, El Nekhely I, Hindi-Alexander M, Engelson G, Hanson JW, Howell RR. Conference report: Second conference of the Middle East and North Africa newborn screening initiative: Partnerships for sustainable newborn screening infrastructure and research opportunities. Genet Med. 2009;9:1–6. doi: 10.1097/GIM.0b013e3181ab2277. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Hamid M, Tisocki K, Sharaf L, Ramadan D. Development, validation and application of tandem mass spectrometry for screening of inborn metabolic disorders in Kuwaiti infants. Med Princ Pract. 2007;16(3):215–221. doi: 10.1159/000100393. [DOI] [PubMed] [Google Scholar]
- 10.Hadg-Taieb S, Naasrallah F, Hammami MB, Elasmi M, Sanhaji H, Moncef F, Kaabachi N. Aminoacidopathies and organic aciduria in Tunisia: a retrospective survey over 23 years. Tunis Med. 2012;90:259–262. [PubMed] [Google Scholar]
- 11.Al-Gazali L, Hamamy H, Al-Arrayad S. Genetic disorders in the Arab world. BMJ. 2006;333(21):831–834. doi: 10.1136/bmj.38982.704931.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olayiwola N. /Staff Reporter. Neonatal screening ‘helps prevent impaired growth’ [Newborn screening in India web site]. Available at: http:// www.NeoGenLabs.com, posted by Neha on November 2, 2012.
- 13.Moammar H, Cheriyan G, Mathew R, Al-Sanaa N. Incidence and patterns of inborn errors of metabolism in the Eastern province of Saudi Arabia, 1983–2008. Ann Saudi Med. 2010;30(4):271–277. doi: 10.4103/0256-4947.65254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi SN, Hashim J, Venugopalan P. Pattern of inborn errors of metabolism in an Omani population of the Arabian Peninsula. Ann Trop Peadiat. 2002;22:93–96. doi: 10.1179/027249302125000238. [DOI] [PubMed] [Google Scholar]
- 15.Hassan F, Mougy FE, Mandour I, Abdel Hady S, Selim L, Abdel Atty S, Morgan M, Salem F, Oraby A, Mehaney D, Abdel Monem M, El Nekhely I, Moharam N. Preliminary results of Egypt experience for use of Tandem Mass Spectrometry for expanded metabolic screening. J Appl Sci Res. 2009;5:1425–1435. [Google Scholar]
- 16.Barton L, Hodgman JE. The contribution of withholding or withdrawing care to newborn mortality. Pediatrics. 2005;116(6):1487–1491. doi: 10.1542/peds.2005-0392. [DOI] [PubMed] [Google Scholar]
- 17.Aoki K. Long term follow-up of patients with inborn errors of metabolism detected by the newborn screening program in Japan. Southeast Asian J Trop Med public health. 2003;34(suppl 3):19–23. [PubMed] [Google Scholar]