Abstract
Background:
Agarwood is a priceless non-timber forest product from Aquilaria species belonging to the Thymelaeaceae family. As a result of a defence mechanism to fend off pathogens, Aquilaria species develop agarwood or resin which can be used for incense, perfumery, and traditional medicines. Evidences from ethnopharmacological practices showed that Aquilaria spp. have been traditionally used in the Ayurvedic practice and Chinese medicine to treat various diseases particularly the inflammatory-associated diseases. There have been no reports on traditional use of agarwood towards cancer treatment. However, this is most probably due to the fact that cancer nomenclature is used in modern medicine to describe the diseases associated with unregulated cell growth in which inflammation and body pain are involved.
Objective:
The aim of this current study was therefore to investigate the potential anticancer properties of agarwood essential oil obtained from distillation of agarwood (resin) towards MCF-7 breast cancer cells.
Materials and Methods:
The essential oil was subjected to screening assays namely cell viability, cell attachment and sulforhodamine B (SRB)-based cytotoxicity assay to determine the IC50 value.
Results:
The agarwood essential oil caused reduction of the cell number in both the cell viability and attachment assay suggesting a cumulative effect of the cell killing, inhibition of the cell attachment and or causing cells to detach. The agarwood essential oil showed IC50 value of 900 μg/ml towards the cancer cells.
Conclusion:
The agarwood essential oil exhibited anticancer activity which supports the traditional use against the inflammatory-associated diseases. This warrants further investigation towards the development of alternative remedy towards cancer.
Keywords: Aquilaria, agarwood, breast cancer, MCF-7, SRB, resin
INTRODUCTION
The sought-after fragrant resinous wood of Aquilaria species is known by many names. They are called as gaharu in Indonesia, Malaysia and Papua New Guinea, jin-koh in Japan, Ch΄en Hsiang or Ch΄en Xiang in China, Chim-Hyuang in Korea, Kritsana noi in Thailand, Tram Huong in Vietnam; Bols d’agle, Bols d’aloes, calambac or calambour in French and oud in the Middle East.[1,2,3,4] Agarwood is predominantly used to fulfil the demand in religious, aromatic and medicinal preparation. High grade agarwood powder is prescribed in Chinese medicine and used in the production of pharmaceutical tinctures.[5] An ethno-medicinal study conducted in Bangladesh revealed that Aquilaria spp. was used to treat rheumatism.[6] The agarwood resin was also reported to be traditionally used to treat snakebite, rheumatism, vomiting, paralysis, gout, diarrhea and others.[7]
Agarwood has been traditionally used, particularly in the East Asian region, as sedative, analgesic and digestive medicine.[8]
Although, agarwood has been reported to have traditional medicinal purposes towards various kinds of diseases, information on its anticancer properties is very scarce. Nevertheless, the potential anticancer activity of agarwood resin and its essential oil should not be underestimated as they contain various constituents that may have anticancer properties. The constituents in agarwood include epoxychromones and syringaresinol,[8] sesquiterpenoids and their oxygenated derivatives,[9] 3-phenyl-2-butanone, alpha-guaiene, alpha-agarofuran, beta-agarofuran,[10] kusunol[11,12] as well as agarospirol and jinkoh-eremol.[10,11,12]
A limited study on anticancer effects of agarwood was conducted by Gunasekara et al.[13] where it was reported to have significant activity against Eagle’s carcinoma of the nasopharynx. In this current study, agarwood essential oil was screened for its anticancer activity towards the breast cancer cells.
MATERIALS AND METHODS
Materials
Agarwood essential oil (AEO) was obtained from Malaysian market, weighted and dissolved in 10% (v/v) culture grade dimethyl sulfoxide (DMSO; Fisher Scientific).
Cell line and culture medium
MCF-7 breast adenocarcinoma cells (ATCC® HTB-22™) were used in this study. The cells were maintained in Dulbecco’s modification of Eagle’s medium, DMEM with high glucose and L-glutamine (Gibco®) and 10% v/v fetal bovine serum (FBS) at 37°C/5% CO2.
Cell viability assay
In this study, the assay was used to study the cumulative cytotoxic effects of AEO at various concentrations on the cell viability and ability to cause loss of the cell adhesion from plastic surface. Confluent MCF-7 cells were harvested and seeded into the new T-25 cm2 tissue culture flasks at the concentration of 1 × 105 cells/ml in 3 ml culture media. The flasks were then incubated for 24 h at 37°C in a humidified incubator with 5% CO2. After 24 h, the spent media was discarded and new media containing AEO at various concentrations was added while 10% (v/v) DMSO was added into the control flask. The cells were subjected to final incubation for 24 h. Finally, the cells were washed with phosphate buffered saline (PBS), trypsinized using the accutase and then subjected to the cell counting procedure using trypan blue dye exclusion method.
Cell attachment assay
In this assay, AEO at adjusted concentrations were added to the culture media in the flask at the time of the cell inoculation to study the ability of the essential oil to inhibit cell attachment and or cause cell detachment from substrate. 10% (v/v) DMSO was added into the control flask. After 24 h incubation at 37°C in a humidified incubator with 5% CO2 , cells were washed with phosphate buffered saline (PBS), trypsinized using accutase and then subjected to the cell counting procedure using the trypan blue dye exclusion method.
Sulforhodamine B colorimetric assay (modification of Vichai and Kirtikara)
The assay is based on the method of Vichai and Kirtikara,[14] and relies on the ability of SRB, a bright-pink aminoxanthene dye with two sulfonic groups to bind to protein components of the cells that have been fixed to the tissue culture plates by trichloroacetic acid (TCA). AEO in 10% (v/v) DMSO was prepared in twofold serial dilutions with an initial concentration of 1000 μg/ml. Confluent MCF-7 cells were trypsinized, counted and seeded into 96-well tissue culture plate at 1 × 105 cells/ml in 190 μl culture media in each well. Plates were then incubated at 37°C/5% CO2 for 24 h. Subsequently, 10 μl of each dilution of AEO was added into each well and incubated for 72 h. Next, without removing the cell culture supernatant, 100 μl of cold TCA was added into each well and plates were incubated at 4°C for an hour followed by the washing step using slow-running tap water. Plates were then allowed to dry at room temperature. For the staining step, 100 μl of 0.057% (w/v) SRB solution was added into each well and left at room temperature for 30 minutes. Immediately afterwards, plates were rinsed four times with 1% (v/v) acetic acid to remove unbound dye. Then, 100 μl of 10 mM Tris base solution was added into each well containing the protein-bound dye for solubilisation and plates were then placed on a gyratory shaker for 10 minutes. Optical density (OD) reading was measured for all plates at 510 nm wavelength. IC50 value (concentration of compound that yields 50% less cells compared to the control) was derived from curve-fitting methods. OD data was used to plot the dose-response between the compound concentration and growth inhibition percentage. The percentage of the control cell growth is determined as follows:
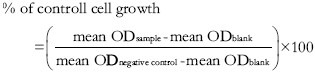
RESULTS AND DISCUSSION
Effects of agarwood essential oil on cell viability and attachment
Figure 1 shows the percentage of viable cell number of MCF-7 cells following two different experimental procedures respectively where AEO was added onto (i) already adherent cells (cell viability assay) and (ii) cells at the time of inoculation (cell attachment assay). In both assays, AEO caused similar trend of reduction in cell number after 24 h of exposure. More than 50% cell kill was observed when cells were treated with 0.2 mg/ml AEO suggesting that AEO may have cytotoxic activity (see cell viability). Nevertheless, the reduction of cell number seen in the cell viability study could also be a cumulative effect of AEO as anti-attachment agent (preventing cells to attach to substrate and or causing cells to detach from substrate). Taken together, the observed cumulative effect is particularly interesting for the development of anticancer treatment or prevention at pre-metastatic level. Previous studies also showed that the plant extracts possess anti-attachment effects against the cancer cells. For instance, extracts from Maclura amboinensis B1. roots showed anti-attachment effects towards B16F10 melanoma cells[15] while Leucobryum bowringii Mitt. showed anti-attachment effects towards MCF-7 human breast cancer cells in vitro[16] [Figure 1].
Figure 1.
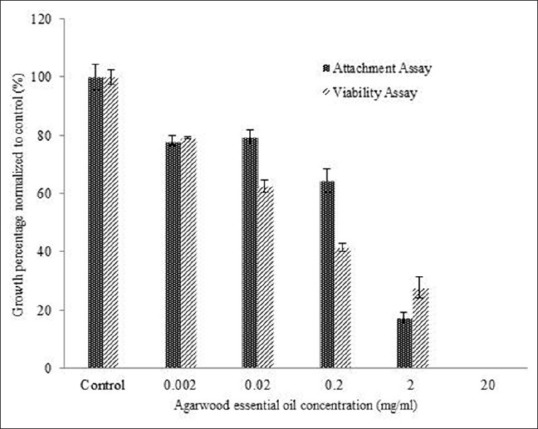
Viable MCF-7 cells after 24 hours of exposure to various concentration of agarwood essential oil following procedures in cell viability and attachment assays respectively. Results are expressed as mean ± SD; n = 3
Anti-cancer effects of agarwood essential oil
Figure 2 shows the dose-response plot of agarwood essential oil against MCF-7 cells. From the fitted curve, it is observed that at 44 μg/ml, AEO was able to obtain 50% cell inhibition (IC50) suggesting that agarwood essential oil has anticancer activity. Other essential oils from plants have also been shown to have anticancer activity. Two different essential oil fractions of Pulicaria jaubertie were reported to have anticancer activities against MCF-7 and HEPG2 (human liver cancer) cells.[17] In another study, myrrh essential oil had been shown to have anticancer properties against MCF-7 cells.[18] The authors suggested that the anticancer effects could be due the presence of sesquiterpenoid compounds. Sesquiterpenoid such as guaiene compounds are commonly reported to be present in AEO.[10] In line with this, the anticancer effects of AEO may have also been attributed to the presence of sesquiterpenoid compounds [Figure 2].
Figure 2.
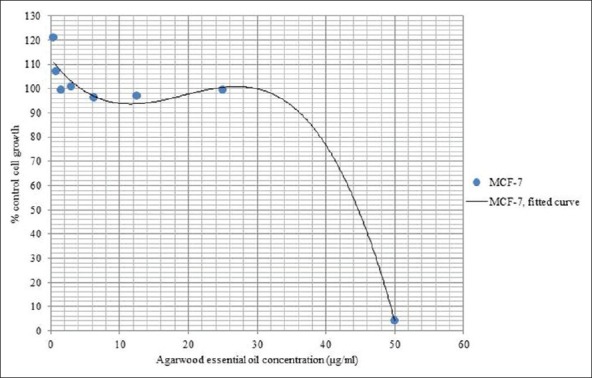
Dose-response analysis of agarwood essential oil (AEO) against MCF-7 cells using SRB assay in 96-well format. AEO concentration at 44 μg/ml was able to inhibit 50% of control cell growth
CONCLUSIONS
In conclusion, based on the simple screening procedures (cell viability assay and cell attachment assay) as well as cytotoxicity assay (SRB assay), AEO was observed to possess anticancer activity towards MCF-7 breast cancer cells. The findings offer evidence to further support the traditional uses of AEO to treat inflammatory related diseases. As such, this warrants further research to investigate the mechanism of actions of the AEO for better understanding and positioning of the essential oil as potential remedy for cancer.
Footnotes
Source of Support: International Islamic University Malaysia (IIUM) Special Grant and Kayu Gaharu (M) Sdn. Bhd
Conflict of Interest: None declared
REFERENCES
- 1.Ng LT, Chang YS, Kadir AA. A review of Agar (Gaharu) producing Aquilaria spp. J Trop For Prod. 1997;2:272–85. [Google Scholar]
- 2.Yamada I. Aloeswood forest and the maritime world. Southeast Asian Stud. 1995;33:181–96. [Google Scholar]
- 3.Chakrabarty K, Kumar A, Menon V. India: WWF-TRAFFIC(World Wide Fund for Nature-The Wildlife Trade Monitoring Network); 1994. Trade in Agarwood. [Google Scholar]
- 4.Sidiyasa K. Jenis-jenis Gaharu di Indonesia (Types of Gaharu in Indonesia) Jurnal Penelitian dan Pengembangan Kehutanan (Journal of Forestry Research and Development) 1986;2:7–16. [Google Scholar]
- 5.Yaacob S. Malaysia: Agarwood: Trade and CITES Implementation in Malaysia. Unpublished report prepared for TRAFFIC Southeast Asia; 1999. [Google Scholar]
- 6.Rana MP, Sohel MS, Akhter S, Islam MJ. Ethno-medicinal Plants Use by the Manipuri Tribal Community in Bangladesh. J For Res. 2010;21:85–92. [Google Scholar]
- 7.Borris RP, Blasko G, Cordell GA. Ethnopharmacologic and phytochemical studies of the thymelaeaceae. J Ethnopharmacol. 1988;24:41–91. doi: 10.1016/0378-8741(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 8.Yagura T, Shibayama N, Ito M, Kiuchi F, Honda H. Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett. 2005;46:4395–8. [Google Scholar]
- 9.Nor Azah MA, Chang YS, Mailina J, Abu Said A, Abd Majid J, Saidatul Husni S, et al. Comparison of chemical profile of selected gaharu oils from Peninsular Malaysia. Malays J Anal Sci. 2008;12:338–40. [Google Scholar]
- 10.Chang YS, Nor-Azah MA, Abu-Said A, Lok EH, Reader S, Spiers A. Gaharu. Kuala Lumpur, Malaysia: Forest Research Institute Malaysia; 2002. FRIM Technical Information Forest Research Institute Malaysia. No. 69. Pub. [Google Scholar]
- 11.Nakanishi T, Yamagata E, Yoneda K, Nagashima T, Kawasaki I, Yoshida T, et al. Three fragrant sesquiterpenes of agarwood. Phytochem. 1984;23:2066–7. [Google Scholar]
- 12.Ishihara M, Tsuneya T, Uneyama K. Guaiane sesquiterpenes from agarwood. Phytochem. 1991;30:3343–7. [Google Scholar]
- 13.Gunasekara SP, Kinghorn AD, Cordell GA, Farnsworth NR. Plant Anticancer Agents. XIX. Constituents of Aquilaria malaccensis. J Nat Prod. 1981;44:569–72. doi: 10.1021/np50017a010. [DOI] [PubMed] [Google Scholar]
- 14.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–6. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 15.Siripong P, Rassame K, Piyaviriyakul S, Yahuafai J, Kanokmedhakul K. Anti-metastatic effects on B16F10 melanoma cells of extracts and two prenylated xanthones isolated from Maclura amboinensis B1. roots. Asian Pac J Cancer Prev. 2012;13:3519–28. doi: 10.7314/apjcp.2012.13.7.3519. [DOI] [PubMed] [Google Scholar]
- 16.Manoj GS, Santosh Kumar TR, Varghese S, Murugan K. Effects of methanolic and water extract of Leucobryum bowringii Mitt. on growth, migration and invasion of MCF7 human breast cancer cells in vitro. Indian J Exp Biol. 2012;50:602–11. [PubMed] [Google Scholar]
- 17.Fawzy GA, Al Ati HY, El Gamal AA. Chemical composition and biological evaluation of essential oils of Pucilaria jaubertii. Pharmacogn Mag. 2013;9:28–32. doi: 10.4103/0973-1296.108133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Zhou C, Ge Z, Liu Y, Liu Y, Feng W, et al. Composition and potential anticancer activities of essential oils obtained from myrrh and frankincense. Oncol Lett. 2013;6:1140–6. doi: 10.3892/ol.2013.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]


