Abstract
Background:
Herbal medicines have been long used for antioxidant properties. The purpose of this study was to investigate the effect of hydroalcholic extract Matricaria chamomilla. L (M. chamomilla) against Paraquat (PQ) induced pulmonary injury in association with its antioxidant activity.
Materials and Methods:
Effective doses of PQ (5 mg/kg/day) and M. chamomilla (50 mg/kg/day) were administered alone or in combination for 7 days. At the end of the experiment, lung tissue of the animals was separated. The activity of enzymatic scavengers such as glutathione peroxidase (GPx) and superoxide dismutase (SOD), lipid peroxidation (LPO) and total antioxidant power (TAP) were measured.
Results:
In these samples, the LPO, SOD, and GPx were higher in the PQ group as compared with controls. M. chamomilla extract ameliorated LPO, SOD, GPx and increased TAP in plasma and lung tissue of PQ induced changes. Co administration of PQ with M. chamomilla improved LPO and SOD, and GPx.
Conclusion:
M. chamomilla as natural antioxidant may be considered beneficial for the protection oxidative lung injury in PQ poisoning.
Keywords: Lung, Matricaria chamomilla. L, Oxidative stress, paraquat, Rat
INTRODUCTION
Paraquat (PQ; 1, 1’- dimethyl-4, 4’- bipyridinium) is an herbicide that preferentially accumulates in the lung and exerts its cytotoxic effects via the generation of reactive oxygen species (ROS).[1,2] Currently, the exact mechanism of PQ poisoning is unclear, but most investigators conclude that the oxidation-reduction reaction induced by PQ produces large amounts of oxygen-free radicals, which leads to a lipid peroxidation (LPO) that produces oxidative damage in the lungs.[3,4]
Many studies have focused on increasing the antioxidant status in the lung to protect against PQ injury using various antioxidants, including antioxidant enzymes (e.g. superoxide dismutase (SOD), vitamins (e.g. ascorbic acid, α-tocopherol), and low-molecular-weight thiol-containing antioxidants (e.g. glutathione (GSH), N-acetylcysteine (NAC) and other materials such as quercitin[5,6] but the outcomes from such treatments are limited or without success.
Herbal medicines have long been used for antioxidant properties. Matricaria chamomilla. L (M. chamomilla) is a well-known medicinal plant as carminative, analgesic, and anticonvulsant in traditional medicine.[7,8] The use of M. chamomilla of the Asteraceae (Compositae) family as a medicinal plant dates back to ancient many countries.[9] It is believed to possess anti-inflammatory vulnerary, deodorant, bacteriostatic, antimicrobial, anticatarrhal, carminative, sedative, antiseptic and spasmolytic properties.[10,11] Animal model studies indicate potent antioxidant, anti-inflammatory action, some antimutagenic and cholesterol-lowering activities, as well as antispasmotic and anxiolytic effects.[8,9,12]
Based on the role of ROS, antioxidants may be an important tool against PQ-induced toxicity due to the lack of effective treatments or a specific antidote.[13] The effects of PQ on antioxidant system and the role of antioxidants in PQ toxicity have been evaluated.[6,14] Therefore, we investigated whether PQ-induced lung toxicity is involved in oxidative injuries and whether M. chamomilla protects rats from PQ-induced lung toxicity. The results of this study will aid in further understanding of PQ-induced toxicity in the rat lung and may provide a new therapeutic strategy.
MATERIALS AND METHODS
Chemicals
Ethyl enediamine tetra acet i c acid (EDTA), dithiobis 2 nitrobenzoic acid (DTNB), tri s base and 2, 4, 6 tripyridyl S triazine (TPTZ), comassie blue, bovine serum album in (BSA), 4,5 (dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), glutathione peroxidase (GPx) and SOD (Ransel kit, Randox Laboratories Ltd, Crumlin, UK), bioxytech GSH kit (Oxis Research, USA) were used in this study. All other chemicals were obtained from Sigma.
Animal treatment
Male Wistar rats (180-250 g) were obtained from the animal colony of the Pastor Institute, Iran. Animals were maintained under standard conditions of temperature (22 ± 1˚C), humidity (45-55%) and light (12/12-h light/dark cycle). The rats in the control group (n = 5) were treated with the saline solution. The rats in the PQ-treated group (n = 5) were orally given solution of PQ (5 mg/kg/day) by gastric gavage. The rats in hydroalcholic extract M. chamomilla were orally given by gastric gavage (50 mg/kg/day) (n = 5). The rats in the PQ (5 mg/kg) + M. chamomilla flower (50 mg/kg) group (n = 5) were orally given aqueous solution by gastric gavage was administered for seven consecutive days. The experiments were conducted according to the ethical rules approved by Institutional Review Board (IRB).
Sample collection
To distinguish the rats’ left and right lungs, 300 g of each lower right lung was placed in a frozen pipette and stored at a −70°C liquid nitrogen freezer to detect measurement of biomarkers of oxidative stress in lung rats.
PLANT MATERIALS AND EXTRACTION PROCEDURE
Plant material
The powder of M. chamomilla (Asteraceae) were collected from the region of Hamadan, Iran, and air-dried at 40°C. The plant was deposited at the herbarium of the Faculty of Pharmacy in Hamadan University.
Preparation of the aqueous extract
Dried and finely powdered aerial parts (1000 g) were extracted with ethanol 50% (3 × 5 L) at room temperature for 4 weeks. After removal of the solvent in vacuum at 50˚C, the residue (300 g, 30%, w/w) was stored at 4°C in sealed vials until usage.[15]
Measurement of biomarkers of oxidative stress
Measurement of total antioxidant power
It was measured by FRAP assay (Ferric Reducing Antioxidant Power), which depends upon the reducing of ferric tripyridyltriazine [Fe (III)- TPTZ] complex to the ferrous tripyridyltriazine [Fe (II)-TPTZ] at low pH; was used to measure the in vitro antioxidant power of three extract of M. chamomilla and in vivo: plasma and lung tissue samples. The Fe (II)-TPTZ complex gives a blue color with absorbance maximum at 593 nm.[16]
Measurement of Cu/Zn-SOD activity
The activity of Cu/Zn-SOD was measured using a commercial kit (Ransod kit, Randox Laboratories Ltd, Crumlin, UK). Measurement of the enzyme was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase and reacted with 2-(4-iodophenyl)-3-(4-nitrofenol) 5-phenyltetrazolium chloride (INT) to form a red formazan dye. The formazan was read at 505 nm. One unit of Cu/Zn-SOD was defined as the amount of enzyme necessary to produce 50% inhibition in the INT reduction rate.
Measurement of GPx activity
The amount of GPx was determined using a commercially available kit (Ransel kit, Randox Laboratories Ltd, Crumlin, UK) by measuring the rate of oxidation of NADPH at 340 nm. A unit of enzyme was expressed as the amount of enzyme needed to oxidize 1 nmol of NADPH oxidase/minute.
Measurement of the lipid peroxidation
The LPO product in blood and tissues was determined by TBA reagent, expressed as the extent of malondialdehyde (MDA) productions during an acid heating reaction. Briefly, the samples were diluted by 1.5 ml TCA (20% w/v) added to 250 μl of this sample and centrifuged in 3000 g for 10 min. Then, the precipitation was dissolved in sulfuric acid and 1.5 ml of the mixture was added to 1.5 ml of TBA (0.2% w/v). The mixture was then incubated for 1 h in a boiling water bath. Following incubation, 2 m l of n-butanol was added, the solution centrifuged, cooled and the absorption of the supernatant was recorded in 532 nm. The calibration curve of tetraethoxypropane standard solutions was used to determine the concentrations of TBA + MDA adducts in samples.[17]
Total Protein
The protein content was quantified by the method of Bradford. Concentrated Coomassie blue (G250) was diluted in 250 μl distilled water, and then 750 μl of this diluted dye was added to 50 μl of sample. The mixture was incubated at room temperature for 10 min and an absorbance measurement was taken at 595 nm by a spectrophotometer. A standard curve was constructed using BSA ranging between 0.25 and 1 mg/ml.[18]
Statistical analysis
Mean and standard error values were determined for all the parameters and the results were expressed as Mean ± SEM. All data were analyzed with SPSS Version 11.5 employing one-way ANOVA followed by the Tukey post hoc test. Differences between groups were considered significant when P < 0.05.
RESULTS
Lipid peroxidation
PQ caused a significant increase in LPO when compared to control (P < 0.05). M. chamomilla caused a significant decrease in LPO when compared to the PQ group (P < 0.05). Co-administration of M. chamomilla with PQ significantly reduced PQ-induced LPO (P < 0.05); Figure 1.
Figure 1.
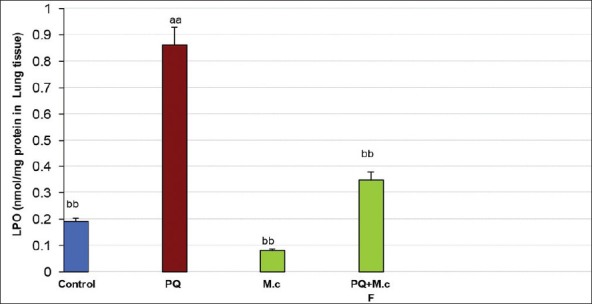
Lipid peroxidation (LPO) in lung tissue of rats. aaSignificantly different from control group at P < 0.05. bbSignificantly different from the PQ group at P < 0.05. PQ, paraquat; M.c, M. chamomilla; PQ + M.c F, paraquat + flower of M. chamomilla)
Superoxide dismutase
PQ caused a significant increase in SOD activity when compared to control (P < 0.05). M. chamomilla caused a significant decrease in SOD activity when compared to the PQ group (P < 0.05). Co-administration of M. chamomilla with PQ significantly reduced PQ-induced SOD activity (P < 0.05); Figure 2.
Figure 2.
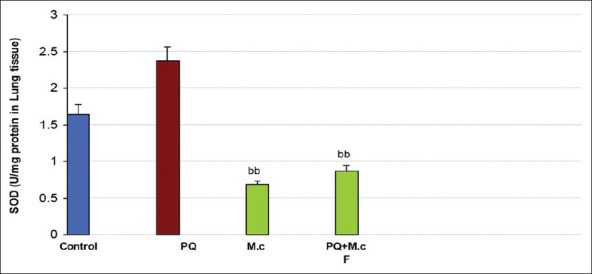
Superoxide dismutase (SOD) activity in lung tissue of rats. aaSignificantly different from control group at P < 0.05. bbSignificantly different from the PQ group at P< 0.05. PQ, paraquat; M.c,M. chamomilla; PQ + M.c F, paraquat + flower of M. chamomilla)
Glutathione peroxidase
No significant difference was observed in GPx activity between groups; Figure 3.
Figure 3.
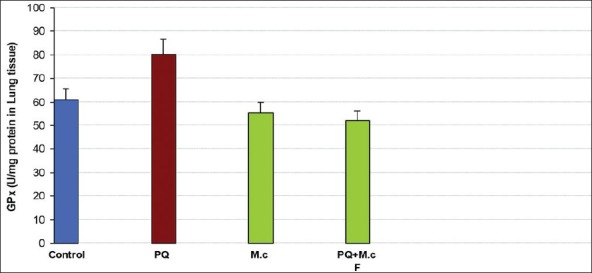
Glutathione peroxidase (GPx) activity in lung tissue of rats. aaSignificantly different from control group at P < 0.05. bbSignificantly different from the PQ group at P < 0.05. PQ, paraquat; M.c,M. chamomilla; PQ + M.c F, paraquat + flower of M. chamomilla)
Total antioxidant power
Treatment with PQ decreased TAP as compared to control (P < 0.05). M. chamomilla increased TAP as compared to the PQ group (P < 0.05). Co-administration of PQ with M. chamomilla significantly increased PQ-induced TAP level (P < 0.05); Figure 4.
Figure 4.
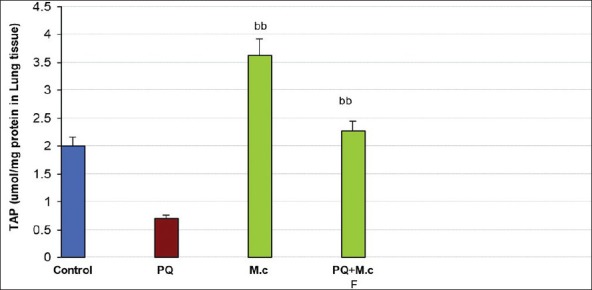
Total antioxidant capacity (TAC) in lung tissue of rats. aaSignificantly different from control group at P < 0.05. bbSignificantly different from the PQ group at P <0.05. PQ, paraquat; M.c,M. chamomilla; PQ + M.c F, paraquat + flower of M. chamomilla)
DISCUSSION
Our study was carried out to evaluate the possible protective effect of hydroalcholic extract of M. chamomilla against PQ-induced oxidative stress and lung toxicity. The findings suggest that the extract possesses a protective action against lung dysfunction inflicted by the potent toxin PQ. It is well documented that free radicals are implicated in a large number of metabolic alterations and/or disturbances and diseases; these toxic effects of PQ in vivo are well known to be mediated through radical reaction.[5,19]
The main target organs involved in PQ toxicity are the lung. The lungs are preferentially targeted because of PQ’s rapid uptake and accumulation in this organ through polyamine transporters.[20,21] Severe PQ poisoning produces adult respiratory distress syndrome, pulmonary hypertension, edema and progressive lung fibrosis. Free radicals are known to play a crucial role in PQ-induced lung toxicity.[22,23] It has been reported that the toxicity is related to redox cycling of an iron PQ complex, which in turn catalyzes the formation of ROS with the ultimate progression of LPO.[24,25] In this study, M. chamomilla reduced the lung injury induced by PQ intoxication, and the LPO concentration, as a marker of LPO, was lower in the PQ + M. chamomilla-treated group than in the PQ-treated group. The PQ-induced biochemical changes as indicated by significant decrease of SOD and GPx activities increase of LPO levels in lung tissue were alleviated by M. chamomilla. In these results, we could infer that M. chamomilla reduced lung injury by attenuation of free radicals-induced LPO.
Lipid peroxidation of the membranous system initiates and propagates endogenous toxicants that can readily react with adjacent molecules like membrane proteins.[26] In the present study, PQ induced lung injuries, and interestingly, M. chamomilla prevented the lung damages.
Antioxidant enzymes works in a coordinated fashion to prevent the oxidative stress.[27] The metabolic role of liver in detoxification of xenobiotics results in the generation of ROS, where SOD, CAT, GSH-Px, GST and GR play an important role in prevention of oxidative stress in various tissues samples.[27,28] In this study, PQ induced oxidative stress as was evidenced by a significant increase in SOD activity. M. chamomilla reduced the oxidative stress possibly by scavenging of free radicals produced by PQ with subsequent restoration in enzymatic activities and oxidative stress indicators, LPO and TAP, concentration in lung homogenates. From previous studies similar results were reported while studying the protective effects of C-phycocyanin[29] and quercetin[5] against PQ-induced lung injury in rat most probably via its antioxidant activity.
These findings suggested that M. chamomile might exert its protective effects on PQ-induced damage via oxidative stress in rat lung. Future studies are warranted to further investigation the underlying mechanisms involved in this complicated process.
ACKNOWLEDGEMENT
This study was supported by a grant from Vice Chancellor of Research of Hamadan University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Abdollahi M, Chan TS, Subrahmanyam V, O’Brien PJ. Effects of phosphodiesterase 3, 4, 5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and suceptibility to a mitochondrial toxin. Mol Cell Biochem. 2003;252:5–11. doi: 10.1023/a:1025568714217. [DOI] [PubMed] [Google Scholar]
- 2.Smith P, Heath D. The pathology of the lung in paraquat poisoning. J Clin Pathol Suppl (R Coll Pathol) 1975;9:81–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974;252:314–5. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- 4.Seidenfeld J, Wycoff D, Zavala D, Richerson H. Paraquat lung injury in rabbits. Br J Ind Med. 1978;35:245–57. doi: 10.1136/oem.35.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HK, Kim SJ, Kwon DY, Park JH, Kim YC. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010;78:181–6. doi: 10.1016/j.lfs.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 7.Costescu C, Hadaruga N, Rivis A, Hadaruga D, Lupea A, Parvu D. Antioxidant activity evaluation of some Matricaria chamomilla L. extracts. J Agroal Proc Technol. 2008;14:417–32. [Google Scholar]
- 8.Romeilah RM. Anticancer and antioxidant activities of Matricaria chamomilla L. and Marjorana hortensis essential oils. Res J Med Med Sci. 2009;4:332–9. [Google Scholar]
- 9.Singh O, Khanam Z, Misra N, Srivastava MK. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011;5:82–95. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cemek M, Yilmaz E, Büyükokuroglu ME. Protective effect of Matricaria chamomilla on ethanol-induced acute gastric mucosal injury in rats. Pharm Biol. 2010;48:757–63. doi: 10.3109/13880200903296147. [DOI] [PubMed] [Google Scholar]
- 11.Abdoul-Latif FM, Nabil M, Edou P, Ali AA, Djama SO, Obame LC, et al. Antimicrobial and antioxidant activities of essential oil and methanol extract of Matricaria chamomilla L. from Djibouti. J Med Plants Res. 2011;5:1512–7. [Google Scholar]
- 12.Karbalay-Doust S, Noorafshan A. Antiulcerogenic effects of matricaria chamomilla extract in experimental gastric ulcer in mice. Iran J Med Sci. 2009;34:198–203. [Google Scholar]
- 13.Daisley H, Hutchinson G. Paraquat poisoning. Lancet. 1998;352:1393–4. doi: 10.1016/S0140-6736(05)60796-9. [DOI] [PubMed] [Google Scholar]
- 14.Ray S, Sengupta A, Ray A. Effects of paraquat on anti-oxidant system in rats. Indian J Exp Biol. 2007;45:432–8. [PubMed] [Google Scholar]
- 15.Macchioni F, Perrucci S, Cecchi F, Cioni P, Morelli I, Pampiglione S. Acaricidal activity of aqueous extracts of camomile flowers, Matricaria chamomilla, against the mite Psoroptes cuniculi. Med Vet Entomol. 2004;18:205–7. doi: 10.1111/j.0269-283X.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 16.Benzie IF, Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Yang W-l, Sun AY. Paraquat-induced free radical reaction in mouse brain microsomes. Neurochem Res. 1998;23:47–53. doi: 10.1023/a:1022497319548. [DOI] [PubMed] [Google Scholar]
- 20.Forman H, Aldrich TK, Posner MA, Fisher AB. Differential paraquat uptake and redox kinetics of rat granular pneumocytes and alveolar macrophages. J Pharmacol Exp Ther. 1982;221:428–33. [PubMed] [Google Scholar]
- 21.Chen CM, Lua AC. Lung toxicity of paraquat in the rat. J Toxicol Environ Health A. 2000;60:477–87. doi: 10.1080/00984100050079548. [DOI] [PubMed] [Google Scholar]
- 22.Tampo Y, Tsukamoto M, Yonaha M. Superoxide production from paraquat evoked by exogenous NADPH in pulmonary endothelial cells. Free Radic Biol Med. 1999;27:588–95. doi: 10.1016/s0891-5849(99)00110-0. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed A. Protective effect of montelukast on paraquat-induced lung toxicity in rats. Biosci Trends. 2009;3:63–72. [PubMed] [Google Scholar]
- 24.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–6. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Bus J, Aust S, Gibson J. Lipid peroxidation: A possible mechanism for paraquat toxicity. Res Commun Chem Pathol Pharmacol. 1975;11:31–8. [PubMed] [Google Scholar]
- 26.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: A review. Med Sci Monit. 2004;10:RA141–7. [PubMed] [Google Scholar]
- 27.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 28.Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 2009;674:137–47. doi: 10.1016/j.mrgentox.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Zhang J, Yan Y, Chi M, Chen W, Sun P, et al. The protective effect of C-phycocyanin on paraquat-induced acute lung injury in rats. Environ Toxicol Pharmacol. 2011;32:168–74. doi: 10.1016/j.etap.2011.04.008. [DOI] [PubMed] [Google Scholar]


