Abstract
Background:
Ulcerations have been effecting humans and causing major damage in the gastro intestinal tract. A need for development of a flawless anti-ulcer medication was always in the agenda. Thus, the need to conduct a study was provoked.
Objectives:
The major objective of the present study is to screen the antiulcer activity of the ethanolic extract of Talinum portulacifolium (Forsk) plant.
Materials and Methods:
The antiulcer activity of the ethanolic extract of T.Z portulacifolium was performed on albino rats. Gastric ulcers were induced by ethanol, pylorus ligated aspirin and histamine induced ulcer models. Omeprazole was used as a standard drug for comparison.
Results:
Treatment with the T. portulacifolium plant extract significantly protected the ulceration induced by the three models. The gastric volume, pH of gastric juice, total acidity, free acidity and ulcer index were the parameters estimated and did show significant results. 800 mg/kg extracts of T. portulacifolium did show high significant results as that of standard drug. Percentage protection of 800 mg/kg was 79.9% and the standard drug-treated group did show 84%. So was with total acidity (38.1 + 1.45) and free acidity (16.5 + 0.76)
Conclusion:
The ethanolic extract of T. portulacifolium plant was found to possess a significant antiulcer activity.
Keywords: Ethanol, histamine, omeprazole, pylorus-ligated aspirin, Talinum portulacifolium
INTRODUCTION
Ulcers are sores or open wounds that occur on the skin or along the lining of the digestive tract due to loss of tissue. They are also defined histologically as a breach in the mucosa of the alimentary tract that extends through the muscularis mucosa into the sub-mucosal layer or even deeper.[1] Ulcers most often occur at the junction between the body and antrum of the stomach on the anterior or non-acid-secreting side, and 1 cm proximal or distal to the pylorus. Ulcers within 1 cm proximal to the pylorus are called pre-pyloric ulcers. Ulcers occurring more proximally in the stomach is called gastric ulcer.[2] Ulcers are caused by various factors like microorganisms (Heliobacter pylori), lifestyle, drugs (NSAID’s), age, gender and ethnic background.
There are many kinds of medications for treating peptic ulcers like H2-receptor antagonists, proton pump inhibitors, anti-cholinergics, prostaglandin analogs etc., But unfortunately, these medications are found to generate many adverse effects on the body due to the synthetic or chemical origin. So herbal formulations are chosen to inhibit these adverse effects and deliver a safe medication to the individuals. Talinum portulacifolium is a herbaceous plant found in the family Talinaceae (formerly in the family Portulacaceae) whose common names include fame flower, water leaf etc., Several species bear edible leaves and Talinum fruticosum is widely grown in tropical regions as a leaf vegetable. Talinum paniculatum is grown as an ornamental plant. The family is cosmopolitan and it has 19 genera and more centered in South Africa and America.[3] It is distributed from Ethiopia to the Eastern Cape South Africa, China and also in India and Nepal. It is widely distributed in Talakona forest in Chittoor District of Andhra Pradesh. It is distributed from Rajasthan to India south words to the peninsular region.[4]
For a long time, the tribal people of the Rayalaseema region in Andhra Pradesh, India, have used the leaves of the plant T. portulacifolium [Figure 1] (Forssk: Portulacaceae) to keep away from diabetes, ulcer, and fevers. The leaf powder of this plant mixed with boiled milk is used to treat diabetes.[5] Since ages, the plant was used as an aphrodisiac. The leaves of T. portulacifolium was used against stomach ache as an ancient medicine.[6] In places like Uganda, this antecedent of Mother Nature was employed against demonic effects of malaria and mouth ulcers. There were even evidences of isolation of flavanoids from this plant.[7] As flavanoids are the chemical constituents which play a prime role in anti-ulcer activity, thus this plant was chosen for this particular study.
Figure 1.

Image of Talinum portulacifolium
MATERIAL AND METHODS
Collection of plant materials
Plant material of T. portulacifolium was collected from the moderate-sized deciduous plants found widely in the Talakona forest of Andhra Pradesh, India. The plant material was identified taxonomically and authenticated by Dr. S. Madhava Chetty, Asst. Professor Dept. of Botany, Sri Venkateshwara University, Tirupati, Andhra Pradesh. The voucher was preserved in the laboratory.
Preparation of plant extract
The plant was isolated, chopped into small pieces and dried under shade at room temperature for 7 days. The dried plant was powdered. This powder was used for the ethanolic extract. A 95% w/v ethanolic extract was prepared by the soxhlet extraction method. The dried powdered plant of T. portulacifolium (100 g) was extracted with 95%v/v ethanol for 24 hrs, using a soxhlet extractor. The extract was concentrated at 40°C to obtain dark greenish residue. The yield obtained from the above process was found to be 15% w/w. The extract was preserved in a refrigerator.
Experimental animals
Albino rats (200 ± 50 g) were procured from Mahaveer Enterprises, Hyderabad, India, and used for the experiment. Rats were maintained in an air conditioned room (25 ± 2°C) with a normal night and day cycle. Rats were feed with standard pellet diet and demineralized drinking water libitum. The rats were allowed to acclimatize to the laboratory environment for a week before the start of the experiment. All experimental procedures were conducted in conformity with Animal Ethics committee (Reg. No. number 769/2010/CPCSEA) for the care and use of animals and were strictly followed throughout the study.
Procedure and experimental design
Inducing ulcer by ethanol[8] (Robert, 1979)
The ulcer was induced by administering ethanol. All the animals were fasted for 36 hours before administration of ethanol. The Albino rats of either sex were divided into five groups, each consisting of six rats (n = 6). Group I represented the control group, which received ethanol, omeprazole in the dose of 20 mg/kg was administered orally for Group II which was referred as the standard drug. Group III (200 mg/kg), Group IV (400 mg/kg) and Group V (800 mg/kg) received the ethanolic extract of T. portulacifolium. The gastric ulcers were induced in rats by administrating absolute ethanol (90%) 0.2 ml/100g orally, after 45 min of ethanolic extract and omeprazole treatment. They were kept in specially constructed cages to prevent coprophagia during and after the experiment. The animals were anaesthetized 1 hr later with anesthetic ether and stomachs were incised along the greater curvature and ulcerations were scored. Gastric juice was collected into a graduated centrifugation tube and was centrifuged at 1000 rpm for 10 min and gastric volume was noted. The pH of the gastric juice was recorded by PH meter. The centrifuged supernatant contents were then subjected to analysis for free and total acidity. The stomach was opened along the greater curvature and washed with running water to see for ulcers in glandular portion of the stomach. The total severity of the ulcers was determined by recording the severity of each ulcer, and sample was send to further histopathological study.
The number of ulcers per stomach was noted and severity of the ulcers of the ulcers scored microscopically with the help of hand lens (10X) and scoring was done as following:
0 = normal stomach
0.5 = red coloration
1.0 = spot ulcers
1.5 = hemorrhagic streaks
2.0 = ulcer >3 but <5
3.0 = ulcer >5
Mean ulcer score for each animal is expressed as ulcer index. The percentage protection was calculated using the formula:

Screening of Anti-ulcer Activity in pylorus ligation aspirin induced ulcer model:
The ethanolic extract of T. portulacifolium (T.P), aspirin and standard antiulcer drug, Omeprazole were prepared in DMSO suspension as vehicle and administered orally once daily at a volume of 10 ml/kg body weight. In the present study, albino rats weighing about 200-250 gms were selected and divided into five groups containing six animals in each group. Group-I animals were treated with normal saline orally for 7 days. Group-II animals were treated with omeprazole 20 mg/kg for 7 days. Similarly, Group-III , Group-IV and Group-V animals were treated with 200 mg and 400 mg and 800 mg of plant extract for 7 days, respectively. From days 5 to 7, animals of all the groups received aspirin orally as an aqueous suspension at a dose of 300 mg/kg, 2 h after the administration of respective drug treatment. Animals in all the groups were fasted for 18 hrs after the respective assigned treatment and were anaesthetized with anesthetic ether. Pyloric ligation was performed and pyloric ligation was done by ligating the pyloric end of stomach. After 4 hrs of pyloric ligation, the animals of all the groups were sacrificed and gastric contents were collected. The free acidity and total acidity were determined. In addition, the ulcer index was determined by opening the stomach on greater curvature and the scores were given 0 to 3 depending upon the severity of ulcers. Mean ulcer score was calculated as mentioned above.
Screening of anti-ulcer activity by histamine induced ulcerations
Albino rats of either sex weighing between 200 and 250 g were divided into five groups of six animals in each group.
In this method, male Albino rats of around 200-250 mg were used and grouped into five groups, each group consisting of six animals. Animals were fasted for 24 hrs before treatment and water was at libidum. Pretreatment of vehicle (distilled water 1 ml/animal) orally (p.o) was given to Group I which served as the control group. The plant extract was given at the doses of Group III (200 mg/kg), Group IV (400 mg/kg) and Group V (800 mg/kg) p.o to their respective groups of animals and Group II was induced with omeprazole (20 mg/kg po) which served as the standard drug-treated group. After 1 hr, histamine (7.5 mg/kg) was given to all groups of animals. After 6 hrs, stomachs of all the animals were isolated, opened along greater curvature, observed under a Stereo microscope and photographs were taken.
RESULTS
Parameters estimated
Measurement of pH
The pH of gastric juice was measured by using a pH strips.
Determination of free acidity and total acidity
One milliter of gastric juice was pipetted into a 100 ml conical flask, 2 to 3 drops of Topfer’s reagent was added and was titrated with 0.01N NaOH until all traces of red color disappeared and the color of the solution turned into yellowish orange. The volume of the alkali added was noted. This volume corresponds to free acidity. Then 2 to 3 drops of phenolphthalein solution was added and titration was continued until a definite red tinge reappeared. The total volume of alkali added was noted. This volume corresponded to total acidity. Acidity was calculated by the following formula:
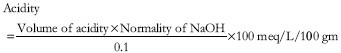
In this method, the following parameters were studied.
pH of gastric juice
Volume of gastric secretion
Free acidity
Total acidity
Ulcer index
Percentage protection.
Histopathology evaluation
The stomachs were immersed in a 10% formalin solution for histopathological examination. These tissues were processed and embedded in paraffin wax. The central part of damaged or ulcerated tissue (if present) was cut on half along the long diameter. If the stomach was protected from the damage, then the section was taken from basal part using a rotary microtome. Sections of about 5 μm thickness were cut and stained with hematoxylin and eosin. These were examined under the microscope for histopathological changes such as congestion, hemorrhage, necrosis, inflammation, infiltration, erosion, and ulcer and then the photographs were taken.
Table 1 is stating the efficiency of the Talinum portulacifolium against the ulcerations produced by the ethanol in the stomach. The extract of the plant 800 mg/kg did show high significance and was found to be efficient. The standard drug did show significant anti-ulcer activity. This parameter in assessed for the evident effect of the T.P against the ulcerations produced by the ethanol induced ulcers. This tabular column [Table 2] shows the extent of protection of the tissue by the plant extract.
Table 1.
Screening of anti-ulcer activity T.P on ethanol-induced ulcers in rats
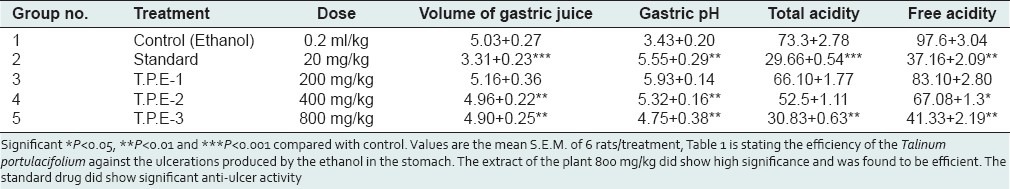
Table 2.
Effects of ethanolic extract of T.P plant on ulcer index and percentage of protection against ulcers following ethanol induction in rats
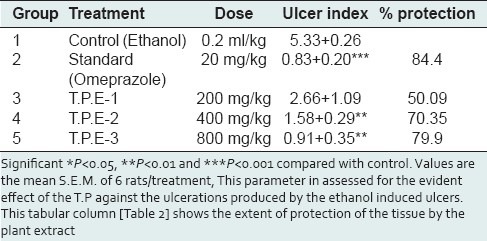
Figure 2 elucidates the stomach samples of the animal where in which the ulcerations are found to be less in plant extract-treated groups when compared to the control animal treated groups. In control treated group, the ulcerations were evident but whereas the disruption in the stomach tissue was far reduced in plant extract treated stomachs and standard treated stomach tissues. In this case, the drug treated (800 mg/kg) plant extract was found to be more significant in minimizing the ulcers than the standard drug (Omeprazole)-treated group.
Figure 2.
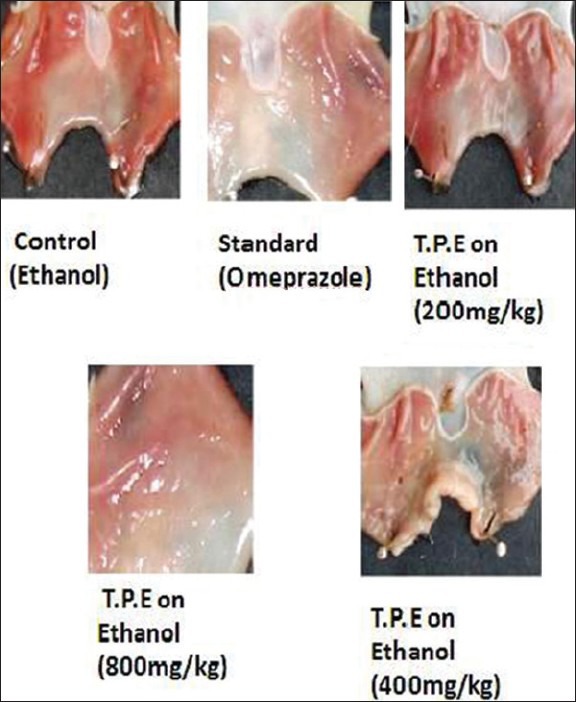
Effect of ethanolic extract of T.P on ethanol-induced ulcer in rat
Histopahtological studies of Pyloris Ligated Aspirin-Induced Ulcer in Rats
Group I: In this study, the mucosa in the control group (300 mg/kg) did shown redness, congestion, hemorrhagic sticks, necrosis and dilation of blood vessels.
Group II: In Omeprazole (20 mg/kg)-treated group, mucosa showed the mild redness, but the inflammation was absent.
Group III: At 200 mg/kg, the ethanolic extract of T.P did show hyperplasia of the mucosa, mild inflammation and congested blood vessels.
Group IV: At 400 mg/kg, the ethanolic extract T. portulacifolium-treated group did elucidate mild inflammation.
Group V: At 800 mg/kg, the ethanolic extract T. portulacifolium plant-treated group did evidently elucidate slight redness in the mucosa but there was no inflammation or congestion recorded.
Table 3 illustrates the potential of the Talinum portulacifolium plant extract against the pyrloris ligation induced ulcerations. The significance of the 800mg/kg plant extract was comparable with that of the anti-ulcerative potential of standard drug.
Table 3.
Screening of anti-ulcer activity T.P on pyloris ligation-induced ulcers in rats
Figure 3 discloses the histopathological evaluation images of the pyloris ligated aspirin induced ulcers, 3 doses of T. portulacifolium plant extract and standard drug-treated group. The 800 mg/kg treated group elucidates histopathology in comparison to standard drug-treated group. The damage was far reduced by the 800 mg/kg treated group.
Figure 3.
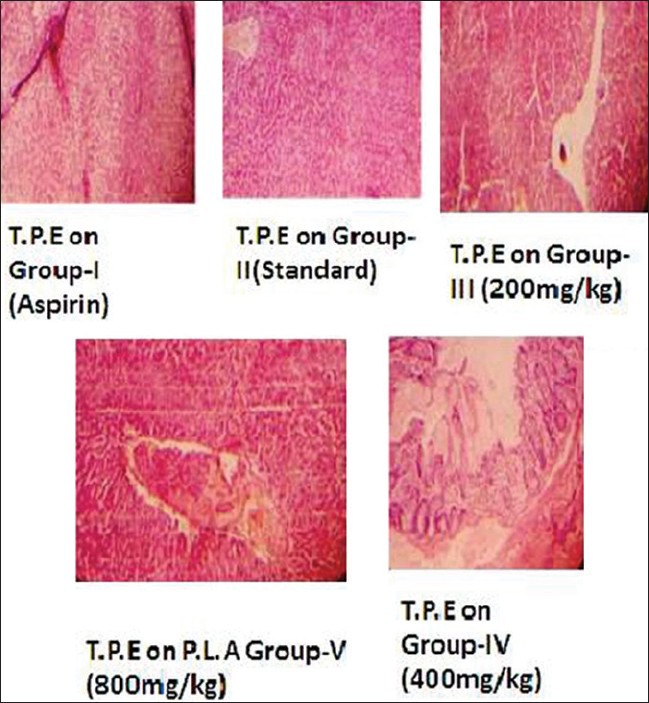
Histopathological evaluation of pylorus ligated aspirin-induced ulcers
Histopathological studies of Histamine Induced Ulcerations in Rats
Group I: In this study, the mucosa in control group-treated animals (7.5 mg/kg) did exhibit redness, congestion, hemorrhagic sticks and dilation of blood vessels.
Group II: In the Omeprazole (20 mg/kg)-treated group, the mucosa of the stomach has shown no evident inflammation.
Group III: This group was treated with 200 mg/kg of the ethanolic extract of T.P, did exhibit mild inflammation in the mucosa and congestion of blood vessels.
Group IV: At 400 mg/kg, in the ethanolic extract T.P plant-treated group has shown mild inflammation.
Group V: 800 mg/kg of ethanolic extract T.P plant-treated group has evidently shown de-generation but no inflammation of tissue.
Table 4 delineates the anti ulcer potential of Talinum portulacifolium against histamine induced ulcerations in animals. As before present, the 800mg/kg of plant extract did manifest significant anti-ulcerogenic potential in comparison to the standard drug.
Table 4.
Screening of anti-ulcer activity of T.P against histamine-induced ulcer activity in rats
The Table 5 emphasizes the effect of plant extract on ulcer index and percentage of protection. In either of the conditions, the plant extract did exhibit more significant effect than that of the standard drug. There was a significant difference of 0.17 differences in the ulcer index.
Table 5.
Effect of ethanolic extract of T.P plant on histamine-induced ulcer index and percentage protection ulceration in rats
Figure 4 elucidates the histopathological evaluation of histamine induced ulcers. The above images show the microscopic images of the stomach tissues of control-treated, plant-treated and standard drug-treated groups. The evident damage caused due to disruptions in the gastric tissue in the histamine-treated group can be compared to that of the 800 mg/kg plant extract-treated group and the standard drug-treated group.
Figure 4.
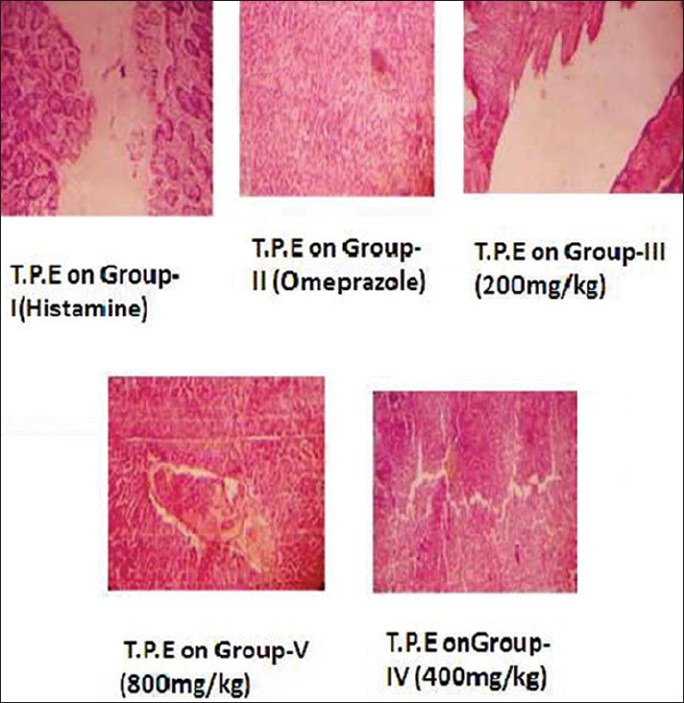
Histopathology evaluation of histamine-induced ulcers
DISCUSSION
Peptic ulcers are caused when the natural imbalances between the aggressive factors of acid, pepsin, defensive mechanisms of mucus, bicarbonate, mucosal turnover and blood supply (mucosal barrier) are disturbed.[9] It was reported that acid and pepsin are relatively less important as causative agents and that a defect in the defensive mechanism of gastric mucosa is the first step toward ulcer formation.[10] Pyloric ligation-induced ulcers are due to imbalance between offensive and defensive mucosal factors and is the ideal model to infer the mechanism by which a drug works as an anti-ulcerogenic agent. Gastric ulcer in this model occurs because of an increase in acid pepsin accumulation due to pyloric obstruction and subsequent mucosal digestion and breakdown of the gastric mucosal barrier.[11] A copious amount of mucus is secreted during superficial damage and provides favorable microenvironment for repair process. Hence, estimation of gastric acidity, pepsin and mucin content is a valuable part of the study to clarify the mechanism of action of the drug under trial. Studies have shown alterations in the antioxidant status following ulceration, indicating that free radicals seem to be associated with the pylorus ligation-induced ulceration in rats.[12] T. portulacifolium with multiple mechanisms of protective action, including antioxidant properties, may be one way forward in minimizing tissue injury in human disease. Ethanolic extract of T. portulacifolium inhibited ulcerogenic activity of pylorus ligation significantly when compared to control group rats and the effects compared with that of the standard drug ranitidine. EETp (400 mg/kg and 800 mg/kg b.w.) reduced volume of gastric juice and total acidity significantly (P < 0.01) and elevated the mean pH significantly (P < 0.001). EETp at a dose of 800 mg/kg b.w. inhibited ulcer index significantly (P < 0.001) by 74.2%, MEGS at a dose of 400 mg/kg and 200 mg/kg b.w. inhibited ulcer index significantly (P < 0.05) by 60.2% and 55.4%, respectively. EETp has also showed significant results against histamine-induced and ethanol-induced ulcerations. Omeprazole provided a fairly protective effect on the gastric mucosa while 800 mg/kg of the extract offered a highly protective effect with the result that the rat gastric mucosa remained without ulcerative lesions after exposure to the damaging effects of histamine. The production of gastric HCl is mediated through histamine which in-turn is produced by enterochromaffin-like cell or mast cell.[13] Treatment with 400 and 800 mg/kg of the extract significantly inhibited the formation of stomach ulcers caused by histamine administration in rats; it therefore suggests that the crude extract of T. portulacifolium leaves can suppress gastric damage induced by one of the aggressive factors. The activity of the extract could have been mediated through blockage of histamine H2-receptors or by inhibition of hydrogen, potassium, adenosine triphosphatase (H+/K+-ATpase) or proton pump activity. The effects leading to ulcerogenesis are mediated at the parietal cells by the transmitter substances Histamines, gastrin and acetylcholine which through a common path involving cyclic Adenosine monophosphate (cAmp) and calcium ions, interact with the gastric proton pump (H+/K+-ATpase) that is ultimately responsible for secreting acid into the lumen of the stomach.[11] Histamine appears to be necessary for the action of gastrin and acetylcholine and it is the critical regulator of gastric acid secretion.[14] The possible mechanisms of EETp antiulcer benefit may be due to its oxygen radicals scavenging by inhibition of lipid peroxidation, preventing the loss of gastric mucus and NPSH, and blockade of ethanol-induced apoptosis.[15]
CONCLUSION
In congruence with this study, we can say that EETp could evidently inhibit the ulcerations induced by Pyloris-ligated, histamine-induced and ethanol-induced ulcerations. The data clearly states that the ethanolic extract of this traditional Chinese herb could successfully inhibit the ulcerations at 800 mg/kg body weight. Thus, this study has proved that T. portulacifolium is found to have significant anti-ulcer potential and this study will act as a key in endorsing researchers for going in depth and understanding the mechanism of ulcerations or peptic ulcers.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kumar V, Abbas KA, Fausto N. 7th ed, Updated Edition. New Delhi, India: Elseiver; 2011. Text book of Pathologic Basis of Disease; pp. 816–20. [Google Scholar]
- 2.Smith LH, Their SO. International text book of medicine. 2nd ed. Philadelphia: W B Saunders company; 1985. Pathophysiology, the principles of disease; pp. 139–51. [Google Scholar]
- 3.Heywood VH. U.K: Oxford University Press; 1978. Flowering plants of the World; p. 336. [Google Scholar]
- 4.Rao NT, Kumarappana CT, Lakshmib MS, Mandal SC. Antidiabetic activity of leaves of Talinum portulacifolium (forssk) in alloxan – induced diabetic rats. Pharmacologyonline. 2009;2:407–17. [Google Scholar]
- 5.Seetharami Reddi TV, Ramarao Naidu BVA, Prasanthi S. Herbal remedies for diabetes. In: Alikhan I, Khanum A, editors. Ethnomedicine and human Welfare. 3rd ed. Hyderabad: Ukaaz Publications; 2009. p. 44. [Google Scholar]
- 6.Yoganarasimhan SN. Vol. 1. Bangalore: Interline Publishing Pvt Ltd; 2000. Medicinal plants of India. Karnataka; p. 85. [Google Scholar]
- 7.Sunil J, Sanjith Nath M, Raja S, Vinatha B. Phytochemical studies on Talinum Portulacifolium (forsk) Sci J Pharm. 2010;1:1–4. [Google Scholar]
- 8.Nartey ET, Ofosuhene M, Kudzi W, Agbale CM. Antioxidant and gastric cytoprotective prostaglandins properties of Cassia sieberiana roots bark extract as an anti-ulcerogenic agent. BMC Complement Altern Med. 2012;12:65. doi: 10.1186/1472-6882-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Khajuria A, Taneja SC, Khajuria RK, Singh J, Johri RK, et al. The gastric ulcer protective effect of boswellic acids, a leukotriene Inhibitor from Boswellia serrata, in rats. Phytomedicine. 2011;15:408–15. doi: 10.1016/j.phymed.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Gregory M, Vithalrao KP, Franklin G, Kalaichelavan V. Antiulcer (ulcer-preventive) activity of Ficus arnottiana Miq (Moraceae) leaf methanolic extract. Am J Pharm Toxicol. 2010;4:89–93. [Google Scholar]
- 11.Goel RK, Sairam K. Antiulcer drugs from indigenous sources with emphasis on Musa sapientum, Tamrabhasna, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol. 2010;34:100–10. [Google Scholar]
- 12.Alvarez-Suarez JM, Dekanski D, Ristic S, Radonjic NV, Petronijevic ND, Giampieri F, et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One. 2011;6:e25878. doi: 10.1371/journal.pone.0025878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munson PL, Mueller RA, Breese GR. United States: Chapman Hall; 2009. Principles of pharmacology: Basic concepts and clinical applications; pp. 1063–81. [Google Scholar]
- 14.Laurence DR, Bennett PN, Brown MJ. 8th ed. London: Churchill Livingstone; 1997. Clinical Pharmacology; pp. 567–78. [Google Scholar]
- 15.Wang Q, Zhao WZ, Ma CG. Protective effects of Gingko biloba extract on gastric mucosa. Acta Pharmacol Sin. 2000;21:1153–6. [PubMed] [Google Scholar]


