Abstract
Background:
The effects and benefits of Acacia auriculiformis on health are not well established. This study was planned to evaluate the effect of ethanolic extract of Acacia auriculiformis leaves on learning and memory in rats.
Materials and Methods:
Learning and memory were evaluated using passive avoidance paradigm and rewarded alternation test (T-maze) after the oral administration of two doses (200mg/kg and 400mg/kg) of ethanolic extract of Acacia auriculiformis with rivastigmine as positive control. Forty eight rats were divided into 4 groups in each study model. Estimation of brain cholinesterase activity was done to substantiate the results of the above mentioned tests. Data was analyzed using one way Analysis of Variance (ANOVA) followed by Tukey's post-hoc test using GraphPad InStat software, version 3.06.
Results:
The extract produced a dose-dependent improvement in the memory score namely the step through latency in passive avoidance model (P < 0.001) and the percentage of correct responses in rewarded alternation test (P < 0.05). Dose-dependent inhibition of brain cholinesterase activity (P < 0.001) was also noted.
Conclusion:
The acetylcholinesterase inhibiting property of Acacia auriculiformis contributes to its memory enhancing potential. Further large scale studies are required to elucidate its benefits on cognitive function. This may offer a promising new option for the treatment of dementia and other cognitive deficits.
Keywords: Acacia auriculiformis, acetylcholinesterase, memory, passive avoidance, rewarded alternation
INTRODUCTION
Dementia is a condition characterized by impairment of memory and general intellectual functions along with cognitive dysfunction and loss of skills in the performance of the activities of daily living. The occurrence of neuropsychiatric symptoms is common and while this leads to a lowering of the quality of life of patients, it also poses a challenge to the family, caretakers, health services and the general public. Alzheimer's disease (AD) is the leading cause of dementia worldwide.[1,2] Though 100 years have passed since its first description, AD continues to be one of the most onerous and disabling diseases, defying the tireless efforts to produce a cure. It is estimated that about 81.1 million people will be affected by this condition globally by the year 2040.[3]
The cognitive deterioration seen in patients with AD is found to be associated with a progressive loss of cholinergic neurons and a consequent decline in levels of acetylcholine (ACh) in the brain, particularly in the nucleus basalis of Meynert.[4,5] ACh replacement therapy has been shown to be beneficial but is likely to provide symptomatic relief only.[6,7] Among the various factors contributing to the development of Alzheimer's disease, oxidative stress is considered to play a key role by provoking the cell signalling pathways. It is one of the earliest changes occurring in the neurons. Also the incidence of oxidative stress is found to increase with advancing age.[8,9]
Acacia auriculiformis (Family: Fabaceae; Common name - Black Wattle) is a common Indian plant which is known by a variety of names in various countries such as ‘Akasia’ (Indonesia), ‘Australian babul’, ‘Australian wattle’, ‘Acacia’, ‘Kasia’ (India), ‘Darwin black wattle’ and ‘Tan wattle’ (Australia). It has shown significant anti-helminthic, anti-filarial and microbicidal activity.[10,11] An extract produced from the root is known to be useful in the treatment of various aches, pains and sore eyes. The Aborigines of Australia have been known to use the bark extract to treat rheumatism.[12] Various extracts of this plant have shown antioxidant benefit.[13,14] Acacia nilotica, another species belonging to the same family has shown acetylcholinesterase (AChE) inhibitory activities along with anti-inflammatory and antioxidant properties which could be of potential benefit for treatment of AD.[15,16] There is no scientific study data reported about the effect of Acacia auriculiformis on cognition. Considering the beneficial potential of Acacia auriculiformis, this study was planned to evaluate its effect on learning and memory in rats using two experimental models-passive avoidance (PA) and rewarded alternation test (RA).
MATERIALS AND METHODS
Animals
Forty eight healthy male inbred albino rats of Wistar strain, weighing 200-300g were used. The rats were maintained under a reverse photo cycle of 12 h day and 12 h night in temperature and humidity controlled environment with free access to food and water. All experiments were conducted between 9:00 am and 12:00 pm in a noise free environment. The study was approved by Institutional Animal Ethics Committee (IAEC/KMC/51/2012 dated May 18th, 2012).
Drugs and chemicals
Following drugs and chemicals were used in the study: rivastigmine (Sun pharmaceuticals, Ind. Ltd), DTNB [(5, 5’-dithiobis-2-nitrobenzoic acid), Sigma chemicals, St Louis, MO, USA], acetylthiocholine [(ATC), Sigma chemicals, St Louis, MO, USA]. Buffers and other reagents used were of analytical grade.
Method of preparation of ethanolic extract
Leaves of Acacia auriculiformis were obtained locally (from Udupi, a district in Karnataka) and its authenticity was confirmed by Dr. K. Gopalkrishna Bhat, Professor of Botany, Poorna Prajna College, Udupi. Fresh leaves were collected in the morning in the month of June and care was taken to collect leaves of uniform growth (15 to 20 days old). These leaves were shade dried and powdered. The powder was loaded into Soxhlet extractor in batches of 200g each and was subjected to extraction for 30 to 40 h with 95% ethanol. After extraction, the solvent was distilled off and the extract was concentrated under reduced pressure on a water bath at a temperature below 50°C to syrup consistency. Then it was dried in a dessicator. The final yield of extract obtained was 80g.
Acute toxicity study
The acute toxicity studies were performed in accordance with the Organization for Economic Co-operation and Development (OECD) Test Guideline 425 (Up-and-Down Procedure).[17] Adult female rats were used and a limit test was done using a limit dose of 2000mg/kg. Animals were observed individually, for mortality and general behaviour, at least once during the first 30 min after dosing, periodically during the first 24 h (with special attention given during the first 4 h), and daily thereafter for a total of 14 days. No death was observed till the end of the study. The test samples were safe up to the dose of 2000mg/kg, and from the results, 400mg/kg dose was chosen as the maximum dose for further experimentation, along with a dose of 200mg/kg.
Experimental protocol
Learning and memory was assessed with two behavioural paradigms viz. passive avoidance and rewarded alternation test. Four study groups with six animals in each were tested at the end of a treatment period of 15 days. Groups were as follows:
Group I (control): Equivolume of normal saline p.o.
Group II (200mg): Ethanolic extract of Acacia auriculiformis 200mg/kg p.o.
Group III (400mg): Ethanolic extract of Acacia auriculiformis 400mg/kg p.o.
Group IV (rivastigmine): Suspension of rivastigmine 5mg/kg p.o.
Two compartment passive avoidance test (modified from Bures et al.)
The apparatus consists of a square box with a grid floor (50 × 50 cm) and wooden walls of 35cm height. This box is illuminated with a 100 W bulb placed 150cm above the centre of the compartment. In the centre of one of the walls, there is an opening (6 × 6 cm) which can be opened or closed using a transparent plexy glass sliding door that leads to a small dark compartment (15 × 15 cm). This compartment is provided with an electrifiable grid floor which can be connected to a shock source and a removable ceiling.
The experiment was performed in three stages. On the first day, the animal was placed at the centre of the illuminated box facing away from the entrance to the small compartment. The door between the two compartments was kept open. The rat was allowed to explore the apparatus for 3 min and then returned to home cage. Twenty four hours later, the rat was placed in the illuminated chamber and time to enter the dark compartment was measured as step through latency (STL). Sliding door between the two compartments was closed as the rat entered dark chamber and unavoidable footshock (1.5mA, 50 Hz, 2s) was delivered (acquisition trial). The ceiling was opened and the rat was returned to home cage. Retention was tested after 24 h and STL was recorded. Cut off time allotted was 3 min. Increase in the STL was considered as an index of improvement of memory.[18]
Rewarded alternation test (T-maze)
During this test, rats were subjected to 6 trials on each day for 4 days. Each trial had 2 runs, a forced run and a choice run. In the forced run, the rat was forced to one of the arms by blocking the other arm and was allowed to consume the pellet in that goal area. The rat was placed back into the start box, immediately after it had consumed the pellet in the goal area for a choice run. In this choice run, the goal area of the forced arm was kept empty and pellets were placed in the goal area of the opposite arm. Both the arms were free for the rat to choose. Each forced run and the choice run were separated by an interval of one minute. The inter-trial interval was also one minute. The sequence of the forced arm was predetermined and was the same for all the rats for a given day. During the choice run, if the rat entered the arm opposite to the forced arm, then that response was considered as “correct response”. If it entered the same arm to which it was forced during the forced run, it was considered as a “wrong response”. Using the following formula, the percentage of correct responses was calculated for each rat as follows: percentage of correct responses = total number of correct responses ×100/total number of trials. An increase in the percentage of correct response was considered as an index of improved learning and memory.[19,20]
Collection of brain samples
The animals were sacrificed by cervical decapitation under excessive ether anaesthesia. Immediately after decapitation, whole brain was carefully removed from the skull, weighed and then kept in cold normal saline.
In vitro cholinesterase assay
The activity of brain AChE was measured according to Ellman's method with some modification. Rat brain was homogenized under 37°C in 0.1M KH2PO4 buffer (30 mg/ml rat brain wt/ml of buffer) at pH = 8 and was kept frozen in an ice chest. The homogenate was then centrifuged at 3000 rpm for 10 min and the resultant cloudy supernatant was used for estimation of brain AChE activity. A volume of 3 ml of phosphate buffer (pH = 8) was added to the test tubes labelled as sample blank and test to which 200μL of supernatant was added and the mixture was vortexed. Susequently DTNB was added to both the test tubes and incubated for 5 min at room temperature and absorbance was read at 412nm using spectrophotometer and was set at zero absorbance when the enzyme activity had stopped increasing. Finally 20μL of ATC was added to the test sample, and 20μL of phosphate buffer (pH = 8) to sample blank. Mixture was vortexed and absorbance was read at 412nm for 10 min at 37°C at 1 min interval. The enzyme activity was calculated based on the changes in absorbance/min.[21,22]
Statistical analysis
Data was analyzed using one way analysis of variance (ANOVA) followed by Tukey's post-hoc test using GraphPad InStat (GPIS) software, version 3.06. The level of significance was set at P < 0.05.
RESULTS
Effect on step through latency using passive avoidance paradigm
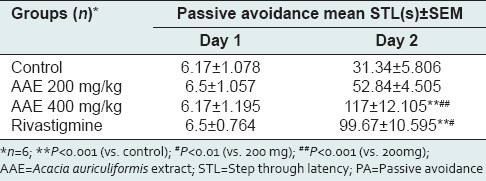
The results of acquisition and retention performance in PA model are depicted in Table 1. Mean STL on acquisition trial among the groups as compared to control was statistically insignificant. In retention performance, highly significant improvement in memory was noted in all the groups as compared to the acquisition trials (P < 0.001) whereas only 400 mg/kg dose of Acacia auriculiformis extract (AAE) and rivastigmine group showed significant improvement when compared to control (P < 0.001). Although memory improvement in 400 mg/kg AAE group was qualitatively comparable to rivastigmine group, it was not supported by statistical significance. When compared to 200 mg/kg AAE group, the 400 mg/kg AAE group showed highly significant improvement in memory (P < 0.001) and rivastigmine group also showed significant result (P < 0.01).
Table 1.
Effect of Acacia auriculiformis extract on learning and memory (PA model)
Rewarded alternation test
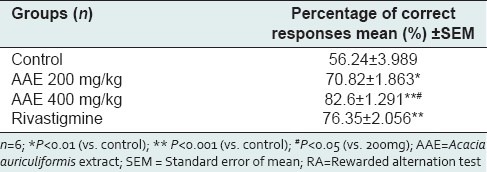
Table 2 shows the results of RA model. AAE showed dose-dependent increase in percentage of correct responses as compared to control [200 mg/kg AAE vs. control (P < 0.01); 400 mg/kg AAE vs. control (P < 0.001)]. Rivastigmine group also showed highly significant increase in percentage of correct responses as compared to control (P < 0.001).
Table 2.
Effect of Acacia auriculiformis extract on learning and memory (RA model)
Effect on Acetylcholinesterase enzyme activity (Unit - Moles of substrate hydrolyzed ×10-6/min/g of brain tissue)
All the study groups inhibited brain AChE enzyme activity significantly as compared to control (P < 0.001) in both PA and RA models. Figure 1 depicts the effect of drugs on the brain AChE activity in the PA model where a highly significant difference was seen between the extract treated groups (P < 0.01) and also between 400 mg/kg dose of AAE and rivastigmine group (P < 0.001). Similarly in Figure 2, we can see the effect of drugs on brain AChE activity in the RA model. Again a dose-dependent inhibition of AChE was seen in the extract treated groups (P < 0.001). Rivastigmine group showed a highly significant difference as compared to 400 mg/kg AAE group (P < 0.001).
Figure 1.
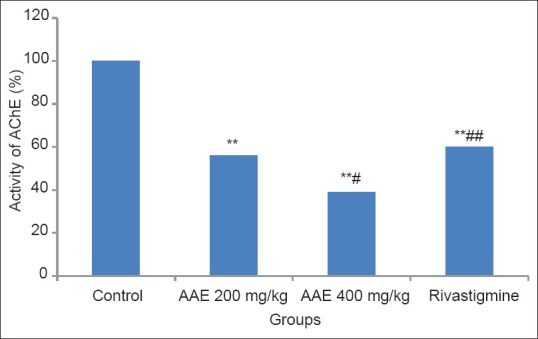
Effect of Acacia auriculiformis extract on acetylcholinesterase activity (PA model). ** P< 0.001 (vs. control); # P< 0.01 (vs. 200mg); ## P< 0.001 (vs. 400mg); AAE = Acacia auriculiformis extract;AChE = acetylcholinesterase
Figure 2.
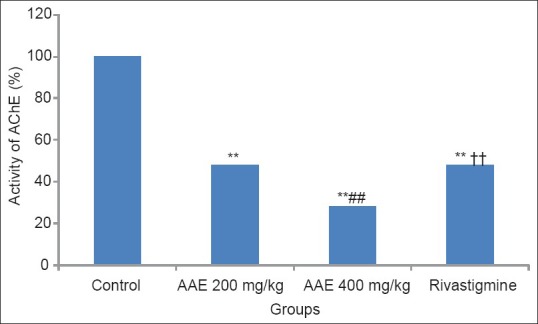
Effect of Acacia auriculiformis extract on acetylcholinesterase activity (RA model). **P< 0.001 (vs. control); ## P< 0.001 (vs. 200mg); ††P< 0.001 (vs. 400mg); AAE = Acacia auriculiformis extract;AChE = acetylcholinesterase
DISCUSSION
The management of AD and other age-related neurodegenerative disorders is posing a great challenge to health care systems worldwide because of the complexity in the underlying pathological mechanisms and availability of a handful of drugs which enable the slowing of progression but do not modify the course of the disease. With increasing life expectancy, it is the need of the hour to discover an efficient cure for this disease. Furthermore, the cholinergic hypothesis of AD proposes that low synaptic levels of ACh resulting from loss of cholinergic neurons lead to cognitive decline.[23] This hypothesis resulted in the exploration of various strategies to increase the brain levels of ACh. The usage of AChE inhibitors like donepezil, rivastigmine and galantamine has emerged as a successful treatment option to this end. However, they can produce nasty adverse effects due to increased ACh levels like vomiting, diarrhoea, sweating, bradycardia and headache.
Study conducted by Mukherjee et al. has demonstrated the usefulness of phytotherapy in treatment of AD.[24] Moreover, the chemicals derived from plants provide protection against a wide range of etiological factors. The study conducted by Crowch et al. demonstrated acetylcholinesterase inhibitory property of Acacia nilotica in in vitro models.[15] No study has been conducted so far to demonstrate such an effect of Acacia auriculiformis that is of benefit in the treatment of AD.
In the current study, the extract treated groups showed a dose-dependent improvement in memory as compared to control in both PA and RA models. Extract treated groups also demonstrated inhibition of AChE which was dose-dependent and superior to that produced by rivastigmine. Acacia auriculiformis exhibited a consistent enzyme inhibition activity when measured on two separate occasions. This can be taken as an indirect evidence of its ability to cross blood brain barrier effectively. These biochemical observations further strengthen our earlier findings of dose-dependent improvement in memory.
To the best of our knowledge, this is the first study of its kind where Acacia auriculiformis has been evaluated for learning and memory enhancing potential in rats. It is evident from the above findings that the beneficial effects of Acacia auriculiformis on memory can be attributed to facilitation of cholinergic transmission due to inhibition of AChE in rat brain. Hence it can be added as an adjuvant to existing therapies for the treatment of dementia. However, studies employing other memory models, with large sample size and characterization of active principles will be required to prove the benefits conclusively.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES
- 1.Burns A, Iliffe S. Dementia. British Medical Journal. 2009;338:405–9. [Google Scholar]
- 2.Bruvik FK, Ulstein ID, Ranhoff AH, Engedal K. The quality of life of people with dementia and their family carers. Dement Geriatr Cogn Disord. 2012;34:7–14. doi: 10.1159/000341584. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: Loss of neurons in the basal forebrain. Science. 1982;215:1237–9. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 5.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 6.Ellis JM. Cholinesterase inhibitors in the treatment of dementia. J Am Osteopath Assoc. 2005;105:145–58. [PubMed] [Google Scholar]
- 7.Holden M, Kelly C. Use of cholinesterase inhibitors in dementia. Advances in Psychiatric Treatment. 2002;8:89–96. [Google Scholar]
- 8.Srinivasan V. Melatonin oxidative stress and neurodegenerative diseases. Indian J Exp Biol. 2002;40:668–79. [PubMed] [Google Scholar]
- 9.Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, et al. Oxidative Stress Signaling in Alzheimer's Disease. Curr Alzheimer Res. 2008;5:525–32. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandal P, SinhaBbbu SP, Mandal NC. Antimicrobial activity of saponins from Acacia auriculiformis. Fitoterapia. 2005;76:462–5. doi: 10.1016/j.fitote.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh M, Sinhababu SP, Sukul NC. Antifilarial effect of two triterpenoid saponins isolated from Acacia auriculiformis. Indian J Exp Biol. 1993;31:604–6. [PubMed] [Google Scholar]
- 12.Girijashankar V. Micropropagation of multipurpose medicinal tree Acacia auriculiformis. J Med Plants Res. 2011;5:462–6. [Google Scholar]
- 13.Singh R, Singh S, Kumar S, Arora S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis. A Cunn Food Chem Toxicol. 2007;45:1216–23. doi: 10.1016/j.fct.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Singh S, Kumar S, Arora S. Free radical-scavenging activity of acetone extract/fractions of Acacia auriculiformis. A Cunn Food Chemistry. 2007;103:1403–10. [Google Scholar]
- 15.Crowch CM, Okello EJ. Kinetics of acetylcholinesterase inhibitory activities by aqueous extracts of Acacia nilotica (L.) and Rhamnus prinoides (L’Hér.) Afr J Pharm Pharmacol. 2009;3:469–75. [Google Scholar]
- 16.Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Investigation of Ethyl Acetate Extract/Fractions of Acacia nilotica wild. Ex Del as Potent Antioxidant. Rec Nat Prod. 2009;3:131–8. [Google Scholar]
- 17.Acute Oral Toxicity – Up-and-Down-Procedure (UDP). OECD guidelines for the testing of chemicals. OECD. 2008. [Last accessed on 2013 Oct 27]. Available from: http://browse.oecdbookshop.org/oecd/pdfs/free/9742501e.pdf .
- 18.Buresova O, Bures J. Learning and memory. In: Bures J, Buresova O, Huston JP, editors. Techniques and basic experiments for the study of brain and behaviour. Amsterdam: Elsevier; 1983. pp. 148–52. [Google Scholar]
- 19.Rao MK, Rao MS, Rao GS. Treatment with Centalla asiatica (Linn) fresh leaf extract enhances learning ability and memory retention power in rats. Neurosciences. 2007;12:236–41. [PubMed] [Google Scholar]
- 20.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker M. Methods in enzymatic Analysis. In: Bergmeyer J, GraBel M, editors. Enzymes- 2: Estreases, glycosidases, lyases, ligases. Florida: Verlang Chemie; 1984. pp. 57–62. [Google Scholar]
- 23.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]


