Abstract
Early growth response 1 (EGR1) is a multifunctional transcription factor; Positive and negative functions of EGR1 in various tumors rely on the integrated functions of various genes it regulates. In this study, we observed the role of EGR1 in non-small-cell lung carcinoma (NSCLC) and identified genes that influence cell fate and tumor development. Various assays showed that EGR1 arrested cell mobility, inhibited migration, and induced apoptosis. Microarray analysis revealed that 100 genes, including CDKN1C, CDC27 and PRKDC, changed their mRNA expressions with the increase of EGR1 and contributed to intervention of tumor progression. Bioinformatics analysis and promoter analysis indicated that an EGR1 binding site was situated in the promoter of KRT18 (also named CK18) and KRT18 could assist in inhibition of NSCLC development. The expression level of EGR1 and KRT18 in NSCLC clinical cases was investigated by immunohistochemistry, in which the protein expression of KRT18 was found to be significantly associated with EGR1 and lymph node metastasis. The results collectively confirm that EGR1 functions as a tumor suppressor in NSCLC. This study is the first to report KRT18 expression is directly regulated by EGR1, and contributes to decrease malignancy of NSCLC.
Early growth response 1 (EGR1) is a nuclear transcriptional factor that belongs to a family of early response genes1. It contains a highly conserved DNA-binding domain that binds to the GC-rich consensus sequence GCG (G/T) GGGCG2,3,4. Its promoter contains several important elements, such serum response elements (SREs), cAMP response element(CRE), nuclear factor binding domains, and EGR1 binding site(EBS), to provide a negative feedback loop for controlling EGR1 expression5,6,7. Multiple factors stimulate the transcription of the EGR1 gene, such as growth factors, cytokines, UV, and hypoxia7,8. EGR1 gene expression is mediated through subgroups of MAPKs, including ERK, JNK, and p38 pathways9,10.
EGR1 is crucial in the development of various carcinomas with opposing biofunctions. Some reports regard the gene to be a positive impact factor in prostate cancer11. Recent studies have described EGR1 as a tumor repressor that directly or indirectly upregulates multiple tumor suppressors, including PTEN, TP53, fibronectin, BCL-2, and TGFβ1, to inhibit cell growth, proliferation, and metastasis, as well as induce apoptosis12.
We surmised that the positive and negative functions of EGR1 in tumor development relied on the integrated result of its gene regulation functions. A previous study summarized the functions of all known and putative target genes of EGR1 in proliferation/transforming, survival/differentiation, apoptosis, tumor progression/angiogenesis, and grown inhibition in different cells or tissues8. Many studies showed the evident correlation between EGR1 and PTEN expression. PTEN activation was an important tumor-suppressing process that required p14ARF-mediated EGR1 sumoylation, which contributed to the Akt-EGR1-ARF-PTEN axis and stimulated growth or apoptosis13. PTEN was shown to be mutated or deleted in many types of prostate tumors, disabling its tumor-suppressing function in carcinoma development regardless of EGR1 expression14
Ferraro et al.15 reported that EGR1 was underexpressed in non-small cell lung carcinoma (NSCLC) than in normal lung tissue and low EGR1 expression was predictive of poor survival regardless of tumor stage in a stratified log-rank test. In vitro assays performed in the present study confirmed that EGR1 was able to arrest lung cancer cell mobility and induce cell apoptosis. Microarray and bioinformatic analyses were applied to characterize the direct or indirect target genes of EGR1 and provided a general view of the functions of EGR1 in NSCLC. We further confirmed by the testing of clinical samples that the promoter of KRT18 contained EGR1 binding sites and KRT18 expression was directly regulated by EGR1, contributing to decrease malignancy of NSCLC.
Results
Upregulation of EGR1 decreases NSCLC proliferation in vitro
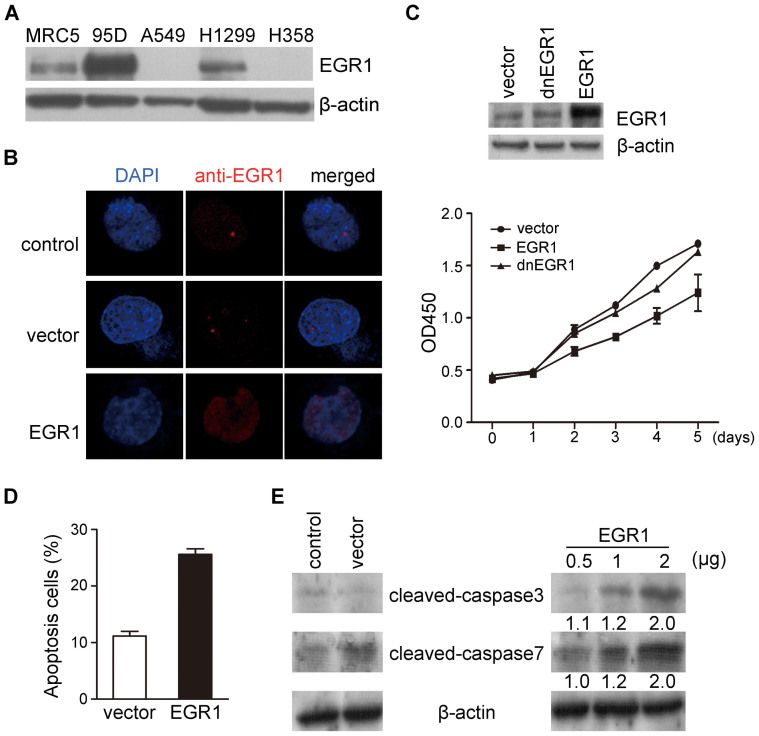
Basing on the report of Ferraro et al.15 that EGR1 was underexpressed in NSCLC compared with that in normal lung tissue, we detected the expression level of EGR1 in several lung cancer cell lines. EGR1 was highly expressed in giant cell lung carcinoma cell line 95D, but it was not significantly different among the NSCLC cell lines H1299 and H358 and human fetal lung fibroblast cell line MRC5. EGR1 expression was clearly undetectable by western blot assay in the human adenocarcinoma cell line A549 (Fig. 1A). The results indicated that EGR1 was not upregulation in most lung cancer cell lines.
Figure 1. Upregulation of EGR1 decreases NSCLC proliferation in vitro.
(A) Total cell lysates from MRC5 and other indicated NSCLC cell lines were immunoblotted with anti-EGR1. β-actin was used as loading control. (B) Representative of H1299 cells with or without overexpressed EGR1 immunofluorescence-stained with antibodies against EGR1 (red color). The nuclei were counterstained with DAPI (blue color). (C) The growth of H1299 cells infected with CD513B-1-EGR1 or -dnEGR1 was analyzed by CCK8 assay. Up panel: western blot for EGR1 expression levels in H1299 cells transfected with EGR1 or dnEGR1. (D) Histogram showing the percentages of apoptotic cells by flow cytometric staining with Annexin V-PE/7-AAD. (E) Western blot for apoptotic caspase-3 and -7 in H1299 cells with increasing quantities of EGR1 compared with control. Numbers presented the fold change compared with the vector. Relative values ± SD were obtained from three independent assays. The full-length blots of Fig. 1A, 1C and 1E were presented in Supplementary Fig. S1, S3 and S4.
We evaluated cell proliferation rate by infecting lentiviral vector CD513B-1-EGR1 or dnEGR1 (dominant negative EGR1, EGR1 inhibitor)16,17 in H1299 cells to determine whether EGR1 had a negative effect on cell growth. Compared with the mock control, the cells with EGR1 had significantly lower growth rate, whereas the cells with dnEGR1 had no significant difference in cell proliferation (Fig. 1C). Therefore, CDK family members CDK2 and CDK6 were detected to show the inhibition of EGR1 on tumor cell growth. CDK2 was almost the same between the mock control and EGR1-overexpressing H1299 cells, while CDK6 in EGR1-overexpressing cells was significantly lower than control (Supplementary Fig. S2A).
Further study using H1299 cells showed that EGR1 induced cell apoptosis at a rate of 25% relative to the mock control (Fig. 1D). We then detected the caspase signals in the EGR1 induced H1299 cells. An evident increase in the activity of cleaved-caspase-3 and -7 was observed in the EGR1-overexpressing H1299 cells as compared with the mock control in an EGR1 dose-dependent manner (Fig. 1E).
EGR1 upregulation decreases NSCLC migration in vitro
Wound-healing and migration assays were performed to evaluate the effect of EGR1 on cancer cell metastasis. In the wound-healing assay, EGR1 dramatically decreased cell mobility in both H1299 and A549 cells within 72 h as compared with the mock control. No significant difference was observed in the dnEGR1 group of H1299 and A549 cells at the same treatment period (Fig. 2A). In the migration assay, EGR1 decreased the movement of the lung cancer cells. The EGR1-positive group showed a decreased migration by 41% and 50% in the H1299 and A549 cells, respectively (P < 0.01), relative to control. By contrast, the dnEGR1 group showed no significant difference in cell migration rate in both lung cancer cell lines (Fig. 2B). These results suggested that EGR1 arrested cell movement.
Figure 2. EGR1 decreases cell mobility and migration.
(A) The effect of EGR1 on cell migration was determined by wound healing assay. The spreading speed of EGR1-transfected cells along the wound edge was slower than that of vector- or dnEGR1-transfected cells over a period of 72 h. (C) Representative images showing the transfected cells that migrated through the cell. The number of migrated tumor cells was quantified and is shown in the right panel. Columns, mean of triplicate experiments; *P < 0.05.
Genes differentially expressed in EGR1-overexpressing NSCLC cells
The specific functions of EGR1 as a tumor suppressor in NSCLC were explored by determining the differentially expressed genes in the EGR1-overexpressed group and mock control group of H1299 cells. Gene expression profiles were obtained by microarray analysis using Affymetrix GeneChip PrimeView™ human gene expression arrays of 48937 probes. A total of 100 genes were identified as significantly differentially expressed in EGR1-overexpressing cells (ratio ≥ 2.0 or ≤0.5) (Fig. 3A). 76 upregulated genes (76%) and 24 downregulated genes (24%) were detected in EGR1-overexpressing cells as compared with control (Supplementary Table S3). A visualization of the differential expression pattern of the 100 genes is shown in Fig. 3 through a hierarchical clustering heat map. IPA launches revealed the bio-function enrichment profiles of these genes (Fig. 3C). Terms closely related to cellular growth and proliferation, cellular movement, and gene expression were also found (Supplementary Tables S4 to S6). Microarray data were validated by qPCR through the comparison of the expression levels of 14 randomly selected genes. The qPCR differential gene expression pattern in the EGR1-overexpressed cells was consistent with the microarray results (Figs. 3B and 3D).
Figure 3. mRNA expression profile of cells with re-expressed EGR1.
(A and B) Hierarchical clustering of 100 genes that exhibited significantly altered expression in EGR1-transfected H1299 cells as compared with vector-transfected H1299 cells. The color bar indicates the fold change (log2). (C) IPA launch of bio-function enrichment profiles, plotted by relative statistical significance. P value, Fisher's exact test. (D) The expression of 15 upregulated genes (ARC, GDF15, CDKN1C, TGM2, HES1, EFNA1, NR4A1, TRIB1, IGFBP6, ITGB8, PDGFA, KRT18, TIMP1, and ID2) was analyzed by qPCR. Relative values ± SD were obtained from three independent assays.
EGR1 influences cell growth and proliferation
Many genes can influence cell growth by differential expression: (1) CDKN1C, CDC27, and PRKDC are regarded as cell cycle-related genes. CDKN1C expression increased up to 9.7-fold, whereas CDC27 and PRKDC decreased by 2.2- and 2.7-fold respectively. (2) CDKN1C, FGFR3, GDF15, HES1, LIF, DDIT3, and TOP1 are thought to arrest the growth of tumor cell lines. All these genes, except for TOP1, increased in expression in the EGR1-overexpressing cells. (3) FOS, GADD45B, RASD1, SERPINA1, SERTAD1, and VEGFA genes are attributed for colony formation of many different tumor cell lines, which were all upregulated in the experimental cell groups. (4) CD24, KCTD11, LIF, and TOP1, which contributes to growth inhibition or cytostasis, also demonstrated modified expression levels (Table 1 and Supplementary Table S3). The cell proliferation assay and cytometry analysis showed that EGR1 significantly inhibited cell growth (Fig. 1C–E and Supplementary Fig. S2A).
Table 1. Genes differentially expressed between EGR1-overexpressed H1299 cells and control cells.
| Gene Symbol | Gene Title | Fold-Change | Probe Set ID | Entrez Gene |
|---|---|---|---|---|
| Cellular Growth and Proliferation | ||||
| Upregulated genes | ||||
| MGP | Matrix Gla protein | 9.75 | 11716203_a_at | 4256 |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | 9.07 | 11763717_a_at | 1028 |
| GDF15 | Growth differentiation factor 15 | 7.83 | 11716663_a_at | 9518 |
| SERPINA1 | Serpin peptidase inhibitor, clade A (alpha-1 Antiproteinase, antitrypsin), member 1 | 4.42 | 11721455_s_at | 5265 |
| ITGB8 | Integrin, beta 8 | 4.32 | 11722503_at | 3696 |
| HEY1 | Hairy/enhancer-of-split related with YRPW motif 1 | 4.14 | 11718201_at | 23462 |
| IL11 | Interleukin 11 | 3.92 | 11727690_at | 3589 |
| FGFR3 | Fibroblast growth factor receptor 3 | 3.83 | 11755786_a_at | 2261 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | 3.74 | 11717994_a_at | 3164 |
| ID2 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | 3.68 | 11715994_x_at | 3398 |
| KRT8 | keratin 8 | 3.54 | 11756989_x_at | 3856 |
| KRT18 | keratin 18 | 3.39 | 11757905_x_at | 3875 |
| TGM2 | Transglutaminase 2 (C polypeptide, protein-glutamine-Gamma-glutamyltransferase) | 3.29 | 11718399_s_at | 7052 |
| IER3 | Immediate early response 3 | 2.99 | 11720062_s_at | 8870 |
| ITGA7 | Integrin, alpha 7 | 2.86 | 11718243_a_at | 3679 |
| CD24 | CD24 molecule | 2.78 | 11715918_s_at | 1E + 08 |
| TRIB1 | Tribbles homolog 1 (Drosophila) | 2.72 | 11716048_at | 10221 |
| RASD1 | RAS, dexamethasone-induced 1 | 2.68 | 11716839_a_at | 51655 |
| RAPGEF3 | Rap guanine nucleotide exchange factor (GEF) 3 | 2.68 | 11760609_at | 10411 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 2.62 | 11715360_x_at | 7076 |
| SERPINB2 | Serpin peptidase inhibitor, clade B (ovalbumin), member 2 | 2.53 | 11716631_s_at | 5055 |
| VEGFA | Vascular endothelial growth factor A | 2.51 | 11738414_a_at | 7422 |
| FBXO2 | F-box protein 2 | 2.51 | 11722236_at | 26232 |
| HES1 | Hairy and enhancer of split 1, (Drosophila) | 2.42 | 11716551_s_at | 3280 |
| HEYL | Hairy/enhancer-of-split related with YRPW motif-like | 2.28 | 11722074_s_at | 26508 |
| CAMK2N1 | Calcium/calmodulin-dependent protein kinase II inhibitor 1 | 2.25 | 11719242_s_at | 55450 |
| NPTX2 | Neuronal pentraxin II | 2.24 | 11720005_at | 4885 |
| EFNA1 | Ephrin-A1 | 2.21 | 11716341_s_at | 1942 |
| IGFBP6 | Insulin-like growth factor binding protein 6 | 2.21 | 11717909_at | 3489 |
| PDGFA | Platelet-derived growth factor alpha polypeptide | 2.19 | 11729062_a_at | 5154 |
| GADD45B | Growth arrest and DNA-damage-inducible, beta | 2.18 | 11757865_a_at | 4616 |
| DDIT3 | DNA-damage-inducible transcript 3 | 2.17 | 11758198_s_at | 1649 |
| SERTAD1 | SERTA domain containing 1 | 2.15 | 11718631_at | 29950 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | 2.15 | 11734659_a_at | 2353 |
| MMP9 | Matrix metallopeptidase 9 (gelatinase B, 92kDa Gelatinase, 92kDa type IV collagenase) | 2.13 | 11719657_a_at | 4318 |
| GNG7 | Guanine nucleotide binding protein (G protein), gamma 7 | 2.11 | 11723239_a_at | 2788 |
| LIF | Leukemia inhibitory factor (cholinergic differentiation factor) | 2.10 | 11758619_s_at | 3976 |
| KCTD11 | Potassium channel tetramerisation domain containing 11 | 2.09 | 11725178_at | 147040 |
| FST | Follistatin | 2.08 | 11732713_at | 10468 |
| LEF1 | Lymphoid enhancer-binding factor 1 | 2.03 | 11726333_s_at | 51176 |
| Downregulated genes | ||||
| PRKDC | Protein kinase, DNA-activated, catalytic polypeptide | −2.97 | 11719391_a_at | 5591 |
| PUS7L | Pseudouridylate synthase 7 homolog (S. cerevisiae)-like | −2.81 | 11727136_a_at | 83448 |
| TOP1 | Topoisomerase (DNA) I | −2.51 | 11717437_x_at | 7150 |
| CDC27 | Cell division cycle 27 homolog (S. cerevisiae) | −2.22 | 11743401_x_at | 996 |
| PPP1R1C | Protein phosphatase 1, regulatory (inhibitor) subunit 1C | −2.04 | 11763834_a_at | 151242 |
| Cellular movement | ||||
| Upregulated genes | ||||
| MGP | Matrix Gla protein | 9.75 | 11716203_a_at | 4256 |
| GDF15 | Growth differentiation factor 15 | 7.83 | 11716663_a_at | 9518 |
| NPTX1 | Neuronal pentraxin I | 7.50 | 11755325_s_at | 4884 |
| SERPINA1 | Serpin peptidase inhibitor, clade A (alpha-1 Antiproteinase, antitrypsin), member 1 | 4.42 | 11721455_s_at | 5265 |
| ITGB8 | Integrin, beta 8 | 4.32 | 11722503_at | 3696 |
| HEY1 | Hairy/enhancer-of-split related with YRPW motif 1 | 4.14 | 11718201_at | 23462 |
| IL11 | Interleukin 11 | 3.92 | 11727690_at | 3589 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | 3.74 | 11717994_a_at | 3164 |
| ID2 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | 3.68 | 11715994_x_at | 3398 |
| TGM2 | Transglutaminase 2 (C polypeptide, protein-glutamine-gamma-glutamyltransferase) | 3.29 | 11718399_s_at | 7052 |
| IER3 | Immediate early response 3 | 2.99 | 11720062_s_at | 8870 |
| CD24 | CD24 molecule | 2.78 | 11715918_s_at | 1E + 08 |
| TRIB1 | Tribbles homolog 1 (Drosophila) | 2.72 | 11716048_at | 10221 |
| RAPGEF3 | Rap guanine nucleotide exchange factor (GEF) 3 | 2.68 | 11760609_at | 10411 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 2.62 | 11715360_x_at | 7076 |
| SERPINB2 | Serpin peptidase inhibitor, clade B (ovalbumin), member 2 | 2.53 | 11716631_s_at | 5055 |
| VEGFA | Vascular endothelial growth factor A | 2.51 | 11738414_a_at | 7422 |
| NPTX2 | Neuronal pentraxin II | 2.24 | 11720005_at | 4885 |
| EFNA1 | Ephrin-A1 | 2.21 | 11716341_s_at | 1942 |
| IGFBP6 | Insulin-like growth factor binding protein 6 | 2.21 | 11717909_at | 3489 |
| PDGFA | Platelet-derived growth factor alpha polypeptide | 2.19 | 11729062_a_at | 5154 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | 2.15 | 11734659_a_at | 2353 |
| MMP9 | Matrix metallopeptidase 9 (gelatinase B, 92kDa Gelatinase, 92kDa type IV collagenase) | 2.13 | 11719657_a_at | 4318 |
| LIF | Leukemia inhibitory factor (cholinergic differentiation factor) | 2.10 | 11758619_s_at | 3976 |
| FST | Follistatin | 2.08 | 11732713_at | 10468 |
| LEF1 | Lymphoid enhancer-binding factor 1 | 2.03 | 11726333_s_at | 51176 |
| S100A2 | S100 calcium binding protein A2 | 2.04 | 11718779_at | 6273 |
| Downregulated genes | ||||
| DEK | DEK oncogene | −2.00 | 11743653_x_at | 7913 |
| Gene expression | ||||
| Upregulated genes | ||||
| CDKN1C | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | 9.07 | 11763717_a_at | 1028 |
| HEY1 | Hairy/enhancer-of-split related with YRPW motif 1 | 4.14 | 11718201_at | 23462 |
| SOX8 | SRY (sex determining region Y)-box 8 | 4.00 | 11721911_at | 30812 |
| IL11 | interleukin 11 | 3.92 | 11727690_at | 3589 |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | 3.74 | 11717994_a_at | 3164 |
| ID2 | Inhibitor of DNA binding 2, dominant negative helix-loop-helix protein | 3.68 | 11715994_x_at | 3398 |
| TGM2 | Transglutaminase 2 (C polypeptide, protein-glutamine-gamma-glutamyltransferase) | 3.29 | 11718399_s_at | 7052 |
| IER3 | Immediate early response 3 | 2.99 | 11720062_s_at | 8870 |
| ID4 | Inhibitor of DNA binding 4, dominant negative helix-loop-helix protein | 2.62 | 11721687_at | 3400 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 2.62 | 11715360_x_at | 7076 |
| VEGFA | Vascular endothelial growth factor A | 2.51 | 11738414_a_at | 7422 |
| HES1 | Hairy and enhancer of split 1, (Drosophila) | 2.42 | 11716551_s_at | 3280 |
| HEYL | Hairy/enhancer-of-split related with YRPW motif-like | 2.28 | 11722074_s_at | 26508 |
| PDGFA | Platelet-derived growth factor alpha polypeptide | 2.19 | 11729062_a_at | 5154 |
| DDIT3 | DNA-damage-inducible transcript 3 | 2.17 | 11758198_s_at | 1649 |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | 2.15 | 11734659_a_at | 2353 |
| LIF | Leukemia inhibitory factor (cholinergic differentiation factor) | 2.10 | 11758619_s_at | 3976 |
| FST | follistatin | 2.08 | 11732713_at | 10468 |
| LEF1 | Lymphoid enhancer-binding factor 1 | 2.03 | 11726333_s_at | 51176 |
| Downregulated genes | ||||
| PRKDC | Protein kinase, DNA-activated, catalytic polypeptide | −2.97 | 11719391_a_at | 5591 |
| TOP1 | Topoisomerase (DNA) I | −2.51 | 11717437_x_at | 7150 |
| ACTR2 | ARP2 actin-related protein 2 homolog (yeast) | −2.08 | 11739033_a_at | 10097 |
| DEK | DEK oncogene | −2.00 | 11743653_x_at | 7913 |
| CENPF | Centromere protein F, 350/400kDa (mitosin) | −2.00 | 11743295_a_at | 1063 |
EGR1 regulates cellular movement
Microarray results suggested that EGR1 may be involved in cellular movement and cell migration (Table 1 and Supplementary Table S5) by the following mechanisms: (1) GDF15, MMP9, ITGB8, and PDGFA alter tumor microenvironment interaction by modifying its cell adhesion ability; and (2) IL11, LIF, PDGFA, and VEGFA have cytokine-to-cytokine interaction ability that modify the epithelial–mesenchymal transition (EMT) in tumor cells. In the study, the cell migration assay results showed that EGR1 markedly arrested H1299 cell mobility (Fig. 2). The phenomenon proofed the genes involved mobility or EMT played their roles in the EGR1 induced NSCLC cells.
EGR1 directly or indirectly regulates the expression of many genes
EGR1 is an important transcription factor that directly or indirectly regulates the expression of specific genes (Table 1 and Supplementary Table S6). Some of the genes regulated by EGR1 are (1) chromatin remodeling-associated genes (ID4, ID2, SOX8, HEYL, DEK, and C14orf106); (2) transcription regulation genes (RASD1, FOS, LEF1, NR4A1, and NKX2-2); and (3) translation-associated genes (SERTAD1 and SON).
KRT18 is regulated by EGR1 in NSCLC
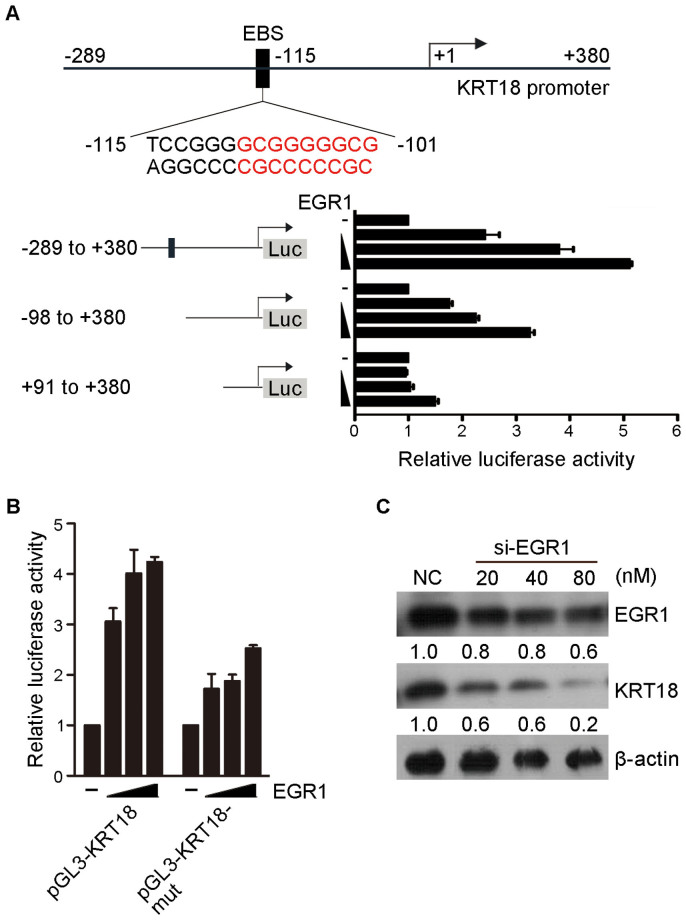
Interestingly, the promoter of KRT18 (cytokeratin 18, also named CK18) was predicted to contain the EGR1 binding sites (EBS), at positions −115 to −101 (UCSC). The microarray results showed that KRT18 was upregulated approximately 3.5-fold by EGR1 induction. KRT18, together with KRT8 (CK8), is an extremely important tumor marker in clinical diagnosis that is expressed in single-layer epithelial tissues of the body and is involved in carcinogenesis18,19. The relationship between EGR1 and KRT18 was confirmed by dual luciferase assay. A series of deletion mutants of the KRT18 proximal promoter (−289 to +380) in the luciferase reporter vector were generated. Sequence analysis revealed that cells carried EBS demonstrated 2- to 5-fold luciferase activation in a dose-dependent manner, whereas the partial deletion of the EBS presented lower activity (Fig. 4A). The complete deletion of EBS resulted in the mere induction of EGR1. To demonstrate that the EBS was indeed responsible for transactivation of the KRT18 promoter, the EBS were mutated (changed from GCGGGGGCG to TCGGTGGAT) in such a way that it no longer conformed with the consensus sequence for EGR1 binding. The reporter constructs became less transactivated in response to EGR1 transfection, confirming that the integrity of the EBS in the KRT18 promoter was essential for its expression by EGR1 (Fig. 4B). The siRNAs that target EGR1 were used to knock down EGR1 in H1299 cells to further confirm the observed relationship between EGR1 and KRT18. After EGR1 was knocked down, the level of KRT18 notably decreased, indicating that the decreased in EGR1 decreased the KRT18 level (Fig. 4C).
Figure 4. KRT18 is a downstream target of EGR1.
(A) Luciferase reporter assays of sequential deletions of the KRT18 promoter, cotransfected with increasing quantities of EGR1. The predicted positions of the EBS (black boxes) were indicated on the schematic. Nucleotides with red colors presented consensus EBS. Values are expressed as mean fold-activation to the promoter cotransfected with empty vector. (B) Luciferase reporter assays of point mutant of the KRT18 promoter. The consensus EBS in pGL3-KRT18 was mutated, creating pGL3-KRT18-mut. Relative reporter activity in response to EGR1 transfection was compared as in (A). (C) EGR1 and KRT18 protein levels after treatment with different si-EGR1 doses or NC (negative control). β-actin was used as loading control. Values are presented as fold change relative to NC. The full-length blot of Fig. 4C is presented in Supplementary Fig. S5.
KRT18 is able to decrease the malignancy of NSCLC
As previously mentioned that EGR1 decreased tumorigenicity of NSCLC, and directly regulated KRT18, we supposed that KRT18 might also function as a tumor suppressor gene. Thus, we examined the characteristics of KRT18-overexpressed H1299 and A549 cells to test this possibility. We observed significant differences in the characteristics of the KRT18-overexpressed cells and control cells. The overexpression of KRT18 decreased the proliferation and migration (Fig. 5A, 5B and 5C) of H1299 and A549 cells. Western blot showed that CDK6 expression was lower in KRT18-ovexpressed H1299 cells (Supplementary Fig. S2B). Moreover, we tested increased activity of cleaved-caspase-3 and -7 in KRT18-overexpressed H1299 cells (Fig. 5D). These results suggested that KRT18 acted as a tumor suppressor and decreased the tumorigenicity of NSCLC cells.
Figure 5. KRT18 is able to decrease the malignancy of NSCLC.
(A) The growth of H1299 and A549 cells infected with CD513B-1-KRT18 was analyzed by CCK-8 assay. The results are expressed as mean ± SD of three independent experiments. (B) The effect of EGR1 on cell migration was determined by wound healing assay. (C) Representative images showing the transfected cells that migrated through the cell. The number of migrated tumor cells was quantified as in Fig. 2B. (D) Western blot for apoptotic caspase-3 and -7 in H1299 cells with different KRT18 transfected dose as compared with control cells. β-actin was used as loading control. The full-length blot of Figure 5D is presented in Supplementary Fig. S6. (E) Expression level of EGR1 and KRT18 in 2 primary NSCLC cases. (F) Correlation of the EGR1 (+) expression with age, lymph node metastasis and KRT18 in 36 NSCLC cases. (G) Correlation of the KRT18 (+) expression with lymph node metastasis in 36 NSCLC cases.
Correlations of the EGR1 and KRT18-positive expression with clinicopathological features were also studied in 36 NSCLC. Fig. 5E confirmed the relationship between EGR1 and KRT18 in primary NSCLC cases. NSCLC tissues with EGR1 (+) were also KRT18 (+) in 54.5% of primary NSCLC cases. KRT18 (−) NSCLC cases accounted for 80.0% of EGR1 (−) tissues (P = 0.0485, Fisher's exact test; Fig. 5F, Supplementary Table S2). We found that EGR1 (+) NSCLC was significantly associated with age (P = 0.0294, Fisher's exact test; Fig. 5F, Supplementary Table S2) and lymph node metastasis (P = 0.0265, Fisher's exact test; Fig. 5F, Supplementary Table S2). Furthermore, KRT18 (+) NSCLC was also significantly associated with lymph node metastasis (P = 0.0042, Fisher's exact test; Fig. 5G, Supplementary Table S2). The clinicalpathological results conformed our previous data that EGR1 was able to decrease NSCLC malignancy through KRT18.
Discussion
The function of EGR1 in tumor growth and development has been recently widely investigated. EGR1 was downregulated in neoplasia and various tumor cell lines20. Conversely, the constitutive expression of EGR1 resulted in the suppression of tumor cell growth and transformation21. In our present study, the function of EGR1 and its regulated genes were investigated in NSCLC cells. Two lung cancer cell lines H1299 and A549 decreased in cell mobility and migration with the overexpression of EGR1; EGR1 also induced H1299 NSCLC cell apoptosis.
Several differentially expressed genes affected cell growth and proliferation. Particularly, the cell cycle-related gene CDKN1C, also named p57 Kip222, was upregulated by almost up to 9-fold in the EGR1-overexpressing H1299 cells. CDKN1C is a potent, tight-binding inhibitor of several G1 cyclin/CDK complexes and, to a lesser extent, of the mitotic cyclin B-CDC223. CDKN1C is a negative regulator of cell proliferation that may be involved in the maintenance of the non-proliferative state of the cell throughout its life cycle and consequently may also function as a tumor repressor24. CDC27 is another cell cycle-related gene that encodes for a cell cycle-regulated E3 ubiquitin ligase, which controls the progression of a cell throughout mitosis and the G1 phase of the cell cycle25; this gene also decreased in expression in the EGR1-overexpressing H1299 cells. GADD45B is a tumor suppressor gene that belongs to a group of genes previously shown to have increased transcript levels with stressful cell growth arrest26,27. In the present study, GADD45B demonstrated increased mRNA levels in the EGR1-overexpressing H1299 cells. Several anti-apoptotic proteins, such as SON28, PRKDC29, and TOP130, were also downregulated in the EGR1-overexpressing cells, and several apoptotic proteins, including KRT1831, NR4A132, and TGM233, were upregulated in the same group of cells.
The current paper is the first to report that EGR1 directly regulates KRT18 expression. KRT18 is a very important tumor marker for clinical diagnosis; it encodes for the type I intermediate filament chain keratin 18, modulates intracellular signaling, and operates in conjunction with various related proteins18. KRT18 may affect carcinogenesis through several signaling pathways, such as PI3K/Akt, Wnt, and ERK MAPK. KRT18 but not KRT8 was demonstrated to act as a substrate for caspase digestion at two distinct sites (Asp238 and Asp396) during the intermediate stage of apoptosis, thereby serving as a marker for apoptosis, mainly by the activation of caspase-3, -6, and -734,35,36.
In the present study, we only established EGR1 directly regulated KRT1 expression; however, other 99 genes were also identified to have modified expression levels with EGR1 induction. These findings strongly support that EGR1 functions as an indirect tumor repressor through the targeting of anti-oncogenes and may contribute in the development of a target in NSCLC therapy.
Methods
Cell lines and NSCLC tumor specimens
Lung cancer cell lines (H1299, H358, A549, and 95D) and human fetal lung fibroblast cell line MRC5 were purchased from the American Type Culture Collection (Manassas, VA). Cells were maintained in 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 10 mg/ml streptomycin. A total of 36 formalin-fixed and paraffin-embedded human NSCLC specimen slices were provided by Guangzhou Medical University Cancer Institute and Hospital (Guangzhou, China). Clinical data of patients included in this study are detailed in Supplementary Table S1. No patients recruited in this study received any preoperative treatment.
Construction of recombinant vectors
The proximal promoter of KRT18 was amplified by PCR and cloned into pGL3-basic vector (Promega, USA). The EGR1 and KRT18 sequence was amplified, and the products were cloned into pcDNA3.1 (Invitrogen, USA) at the EcoRI and HindIII restriction sites. The competitive EGR1 domain dnEGR1 (dominant negative EGR1) sequence was cloned into pCMV-3xFLAG (Sigma–Aldrich, USA) through the assistance of Professor Mingtao Li of Sun Yat-Sen University. Lentiviral vector CD513B-1 was kindly supported by Professor Xinyuan Guan of The University of Hong Kong. EGR1, dnEGR1 and KRT18 were cloned into CD513B-1 at the EcoRI and NotI restriction sites. Transient recombinant cells were obtained by the transfection of the recombinant vector or empty vector through the use of Lipofectamine 2000 reagent (Invitrogen, USA) according to the manufacturer's instructions Stably recombinant cells were obtained according to the common lentiviral packing and infection instructions. The total amount of DNA was normalized using an empty vector. Cell transfection assays were performed in duplicate and repeated at least three times.
RNA extraction and real-time quantitative PCR
Cells were sub-cultured in a six-well plate (5 × 105/well), transfected with empty or recombinant vectors after 24 h, and cultured for 48 h. Total RNA was isolated using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer's instructions. First-strand cDNA was synthesized using a first-strand reverse transcription kit (TOYOBO, Japan), and quantitative PCR (qPCR) reaction was performed on a Rotor-Gene 6000 (Corbertt, USA) with SYBR Green Dye mix (TAKARA, Japan). PCR amplification parameters were programmed as follows: 40 cycles of 94°C for 15 s, 60°C for 15 s, and 72°C for 15 s. The results were normalized to the relative amounts of 18s, and calculation was done by the comparative CT method (ΔΔ CT method).
Immunohistochemical staining
Immunohistochemical staining was performed using the standard streptavidin–biotin–peroxidase complex method. Briefly, paraffin block sections of squamous carcinomas were deparaffinized and rehydrated, and endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 20 min. Antigen retrieval was performed by heating the slides in a microwave oven for 10 min in 10 mmol/L citrate buffer at pH 6.0. Non-specific binding was blocked with 10% normal rabbit serum for 10 min at room temperature. Sections were incubated with rabbit anti-human EGR1 polyclonal antibody (Abgent, USA) at a dilution of 1∶500 at 4°C overnight. Bovine serum albumin (1%) diluted in PBS was included as a negative control. The slides were briefly washed, applied with biotinylated goat anti-rabbit immunoglobulin at 1∶100 concentration, and then incubated at 37°C for 30 min. The slides were subsequently incubated with streptavidin–peroxidase conjugate for 45 min at room temperature and developed with diaminobenzidine tetrahydrochloride. The status of the cytoplasmic and nuclear expression of EGR1 was assessed by three independent investigators without previous knowledge of clinicopathological data. A positive result was defined in the present study as the detection of three or more EGR1-positive cells in a microscopy field (200× in magnification). EGR1- and KRT18-positive (+) NSCLC cells were defined by the observation of three or more signs (+++) in a 200× microscopy field, whereas the observation of less than three signs (++/+/−) defined EGR1- and KRT18-negative (−) NSCLC cells.
Cell proliferation assay
The effect of EGR1 on cell growth rate was evaluated by seeding A549 cells into 96-well plates at a density of 1 × 104 per well. Cells were incubated for 24 h and then transfected with different vectors. Cell growth rate was determined by WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium) assay using a cell counting kit-8 (Dojindo Molecular Technologies, Inc., JAPAN) according to the manufacturer's instructions. Triplicate independent experiments were conducted.
Cell migration assay
Cell mobility was assessed by a scratch wound-healing assay. Briefly, six-well plates were seeded with H1299 or A549 cells, which were transfected with an empty vector or a vector containing EGR1 or dnEGR1 sequence, respectively. Transfected cells were grown to form a confluent monolayer for 24 h and then were mechanically scratched with a sterile pipette tip. The cells were rinsed with PBS and maintained with serum-free medium. Images of the cells were captured after 72 h of culture. The assays were performed in triplicate and repeated at least three times.In migration assay, cells (5 × 104) were suspended in a 500 μl serum-free medium and then seeded in the upper chambers of a Transwell plate (BD Biosciences, USA), whereas the lower chambers were filled with DMEM containing 10% FBS. The cells were incubated for 48 h, after which the plates were fixed and stained using a DiffQuick staining kit (Fisher Scientific Inc.). The cells that migrated toward the bottom surface were counted. Migration rate was determined by counting the number of cells in four high-power fields. The experiments were conducted in triplicate and repeated at least three times.
Western blot
Western blot was performed on 2 × 106 transient recombinant cells according to the general protocol. The primary antibodies for detecting EGR1, caspase-3, and caspase-7 were all purchased from Cell Signaling Technology and diluted at 1∶3000. The antibody for the housekeeping protein β-actin was obtained from Sigma and was diluted at 1∶5000. Chemiluminescence was detected using ECL western blot detection reagents (Pierce, USA).
DNA microarray
DNA microarray analysis of gene expression was performed by Gene Tech Company Ltd., Shanghai, China. Cells transfected with an empty vector or with pcDNA-EGR1 plasmid were solubilized and homogenized in TRIzol (Invitrogen, Carlsbad, CA). Total RNA was isolated according to the manufacturer's instructions, and integrity was tested. Once isolated, mRNA was used as a template for cDNA generation using reverse transcriptase. Affymetrix GeneChip PrimeView™ GeneChip (Affymetrix) covering 48937 transcripts and variants was used to identify differentially expressed genes between H1299 cells transfected with empty vectors and with pcDNA-EGR1 plasmids. Microarray reaction was completed according to the manufacturer's instructions. Briefly, 2 μg of pooled total RNA was reversed-transcribed, labeled, and hybridized to the chip at 42°C for 16 h. The chip was washed, scanned, and then visualized with a GeneArray scanner (Hewlett-Packard). Probe set intensities were calculated using the Microarray Analysis Suite (MAS v5.0, Affymetrix) software and normalized against 100 housekeeping genes to a mean intensity of 36,000 in mask files of PrimeView™ GeneChip before further statistical analysis. Normalized expression values were then compared. Fold-change differences were calculated to identify the upregulated and downregulated genes. Transcripts with more than a 2.0-fold difference in expression level were defined as differentially expressed. Specifically designed online tools, including David bioinformatics resource37 and Ingenuity system analysis (IPA)38, were used to classify the functions of the identified differentially expressed genes.
Statistical analysis
Statistical analysis was performed using SPSS and GraphPad Prism 5 software. Data were expressed as mean ± SD from at least three independent determinations. Significance of data was analyzed by Student's t-test. The correlation between EGR1 or KRT18 and clinicopathological characteristics was analyzed by Fisher's exact test. Bio-function enrichment profiles from the IPA analysis were used for Fisher's exact test. Differences were considered significant at P < 0.05.
Ethics statement
The study proposal has been reviewed by Human subject Research Ethics committee of Cancer Center of Guangzhou Medical University and found to conform to the guidelines set forth by this committee. The study did not constitute harm and potential risks to donors. The recruitment of donors was entirely on a voluntary basis and informed consent. All samples were obtained with written informed consent from all participants.
Author Contributions
X.C. and Y.G. designed the study and has primary responsibility for final content; H.Z. and X.C. wrote this manuscript; J.W. provided the clinical sample and performed the IHC assay. H.Z. and W.G. performed statistical analysis and IPA analysis. H.Z., W.G., W.H., H.Z., and X.T. performed the experiments like as cell culture and transfection assay, western blotting, QPCR, etc. All authors reviewed and approved the manuscript. H.Z., X.C. and J.W. contributed equally to this work.
Supplementary Material
Supporting information
Acknowledgments
The plasmid containing the dnEGR1 sequence was provided by Prof. Mingtao Li of Sun Yat-Sen University. The lentiviral vector CD513B-1 was kindly supported by Xinyuan Guan of The University of Hong Kong. This work was supported by the National Science Foundation 973 program of China (#2010CB945600 and #2011CB965100) and Science and Technology Planning Project of Guangdong Province, China (#20120501). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Ngiam N., Post M. & Kavanagh B. P. Early growth response factor-1 in acute lung injury. AM J PHYSIOL-LUNG C 293, L1089–1091 (2007). [DOI] [PubMed] [Google Scholar]
- Liu C., Rangnekar V. M., Adamson E. & Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther 5, 3–28 (1998). [PubMed] [Google Scholar]
- O'Donovan K. J., Tourtellotte W. G., Millbrandt J. & Baraban J. M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends NeurosciI 22, 167–173 (1999). [DOI] [PubMed] [Google Scholar]
- Gitenay D. & Baron V. T. Is EGR1 a potential target for prostate cancer therapy? Future Oncol 5, 993–1003 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff A. H. et al. Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Mol Cell Biol 18, 512–524 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber M. U. et al. Modulation of early growth response (EGR) transcription factor-dependent gene expression by using recombinant adenovirus. Gene 258, 63–69 (2000). [DOI] [PubMed] [Google Scholar]
- Thiel G. & Cibelli G. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol 193, 287–292 (2002). [DOI] [PubMed] [Google Scholar]
- Adamson E. D. & Mercola D. Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumor Biol 23, 93–102 (2002). [DOI] [PubMed] [Google Scholar]
- Whitmarsh A. J., Shore P., Sharrocks A. D. & Davis R. J. Integration of MAP kinase signal transduction pathways at the serum response element. Science 269, 403–407 (1995). [DOI] [PubMed] [Google Scholar]
- Silverman E. S. & Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. AM J Pathol 154, 665–670 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson E. et al. Egr1 signaling in prostate cancer. Cancer Biol Ther 2, 617–622 (2003). [PubMed] [Google Scholar]
- Boone D. N., Qi Y., Li Z. & Hann S. R. Egr1 mediates p53-independent c-Myc-induced apoptosis via a noncanonical ARF-dependent transcriptional mechanism. P Natl Acad Sci USA 108, 632–637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J 28, 21–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron V., Adamson E. D., Calogero A., Ragona G. & Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther 13, 115–124 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro B., Bepler G., Sharma S., Cantor A. & Haura E. B. EGR1 predicts PTEN and survival in patients with non-small-cell lung cancer. J Clin Oncol 23, 1921–1926 (2005). [DOI] [PubMed] [Google Scholar]
- Yechiel Levkovitz J. M. B. A Dominant Negative Inhibitor of the Egr Family of Transcription Regulatory Factors Suppresses Cerebellar Granule Cell Apoptosis by Blocking c-Jun Activation. J Neurosci 21, 5893–5910 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg J. & Fraizer G. Transcriptional Regulation of EGR1 by EGF and the ERK Signaling Pathway in Prostate Cancer Cells. Genes Cancer 2, 900–909 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y. R., Cui Y. & Fang J. Y. Biological functions of cytokeratin 18 in cancer. Mol Cancer Res 10, 485–493 (2012). [DOI] [PubMed] [Google Scholar]
- Saberi, et al. A Potential Association between Helicobacter pylori CagA EPIYA and Multimerization Motifs with Cytokeratin 18 Cleavage Rate during Early Apoptosis. Helicobacter 17, 350–357 (2012). [DOI] [PubMed] [Google Scholar]
- Huang R. P. et al. Decreased Egr-1 expression in human, mouse and rat mammary cells and tissues correlates with tumor formation. Int J Cancer 72, 102–109 (1997). [DOI] [PubMed] [Google Scholar]
- RP H., Liu C., Y F., Mercola D. & Adamson E. Egr-1 negatively regulates human tumor cell growth via the DNA-binding domain. Cancer Res 55, 5054–5062 (1995). [PubMed] [Google Scholar]
- Potikha T., Kassem S., Haber E. P., Ariel I. & Glaser B. p57Kip2 (cdkn1c): sequence, splice variants and unique temporal and spatial expression pattern in the rat pancreas. Lab Invest 85, 364–375 (2005). [DOI] [PubMed] [Google Scholar]
- Matsuoka S. et al. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Gene Dev 9, 650–662 (1995). [DOI] [PubMed] [Google Scholar]
- Xu X. Y. et al. Clinical implications of p57 KIP2 expression in breast cancer. Asian Pac J Cancer 13, 5033–5036 (2012). [DOI] [PubMed] [Google Scholar]
- Jin L., Williamson A., Banerjee S., Philipp I. & Rape M. Mechanism of ubiquitin-chain formation by the human anaphase-promoting complex. Cell 133, 653–665 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue M., Adachi H. & Jetten A. M. Post-transcriptional regulation of MyD118 and GADD45 in human lung carcinoma cells during 6-[3-(1-adamantyl)-4-hydroxyphenyl]-2- naphthalene carboxylic acid-induced apoptosis. Mol Pharmacol 55, 668–676 (1999). [PubMed] [Google Scholar]
- Takekawa M. & Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95, 521–530 (1998). [DOI] [PubMed] [Google Scholar]
- Greenhalf W., Lee J. & Chaudhuri B. A selection system for human apoptosis inhibitors using yeast. Yeast 15, 1307–1321 (1999). [DOI] [PubMed] [Google Scholar]
- Okazawa S. et al. Inactivation of DNA-dependent protein kinase promotes heat-induced apoptosis independently of heat-shock protein induction in human cancer cell lines. Plos one 8, e58325 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve F. A. et al. Inhibition of human topoisomerase I and activation of caspase-3 by aza-angucyclinones and arylaminopyrimido[4,5-c]isoquinoline-7,10-quinones. Int J Mol Med 30, 151–156 (2012). [DOI] [PubMed] [Google Scholar]
- Fortier A. M., Asselin E. & Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem 288, 11555–11571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. X., Ke N., Sundaram R. & Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol 21, 533–540 (2006). [DOI] [PubMed] [Google Scholar]
- Ai L. et al. The transglutaminase 2 gene (TGM2), a potential molecular marker for chemotherapeutic drug sensitivity, is epigenetically silenced in breast cancer. Carcinogenesis 29, 510–518 (2008). [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Apoptosis and keratin intermediate filaments. Cell Death Differ 9, 486–492 (2002). [DOI] [PubMed] [Google Scholar]
- Caulin C., Salvesen G. S. & Oshima R. G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138, 1379–1394 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Y. Systematic review: caspase-cleaved fragments of cytokeratin 18 - the promises and challenges of a biomarker for chronic liver disease. Aliment Pharm Therap 30, 1103–1109 (2009). [DOI] [PubMed] [Google Scholar]
- Huang da W. et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35, W169–175 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H. et al. Ingenuity pathways analysis of urine metabonomics phenotypes toxicity of gentamicin in multiple organs. Mol Biosyst 6, 2056–2067 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information