Abstract
During the last decade Martinique experienced four dengue epidemics, each characterized by the predominance of 1 or 2 serotypes. In this retrospective database analysis, we investigated the relationship between dengue serotype and disease severity. Data on dengue were collected from 715 patients (male/female ratio 0.87), 14 to 91 years of age (median 35 years) examined in the adult emergency department between 2005 and 2010. In this series, DENV-4 infections more frequently had a milder clinical presentation. The DENV-2 infections were most often secondary infections admitted at the critical phase of dengue illness with signs of plasma leakage. The DENV-1 infections were disabling, particularly in females, and most often led to disease of intermediate severity, without overt plasma leakage. These data were consistent with there being differences in virulence between serotypes, regardless of the host's immune status. However, secondary DENV-2 infections showed an increased risk of plasma leakage.
Introduction
Martinique is an island of the Lesser Antilles with a population of about 400,000. In this country, according to epidemiological reports, dengue is endemoepidemic. The circulating dengue serotypes (DENV-1 to -4) have been closely monitored since January 1995.1 The DENV-2 infections were recorded continuously over time, whereas infections by the other serotypes have had the characteristic to emerge periodically, such as DENV-4 in 1995, 2005, and 2010, DENV-3 in 2001 and 2005, and DENV-1 in 1998, 2001, and 2010. Between 2001 and 2010 the island experienced four dengue epidemics resulting in about 100,000 symptomatic cases.1 Each epidemic was characterized by the predominance of one or two serotypes: DENV-3 in 2001 (26,000 cases), co-epidemic DENV-2 and -4 in 2005 (14,500 cases), DENV-2 in 2007 (18,000 cases) and co-epidemic DENV-1 and -4 in 2010 (42,000 cases).
It is recognized that, whatever the serotype, primary dengue infection is most often asymptomatic or only mildly symptomatic, and provides immunity against re-infection by that serotype.2 Subsequent infection by another serotype (secondary infection) can lead to more severe clinical forms. This observation led to the “antibody-dependent enhancement” (ADE) hypothesis, whereby non-neutralizing anti-dengue antibodies elicited by primary infection can bind to other serotypes, thus facilitating intracellular penetration of live virus and promoting viral replication. However, the mechanisms that lead to the serious forms of the disease are poorly understood and remain mostly hypothetical. In addition to the host's immune status, mechanisms involve complex relationships between the virulence of the infecting strain, and factors such as the host's genetic capital.2
During epidemics in Martinique, most patients who develop acute febrile illness suggestive of dengue are cared for at home by general practitioners. However, many patients present to hospital emergency departments, either at their own initiative or after referral by their general practitioner. In the epidemics mentioned previously, ∼140 cases per 10,000 symptomatic cases were hospitalized (i.e., for > 24 hours), and 3 of 10,000 were fatal.1 The objective of this study was to investigate whether the various dengue serotypes circulating in Martinique in the second half of the 2000s, were associated with specific clinical forms of severity. In addition to potentially providing insight into dengue pathogenesis, identifying any serotype-specific differences is of interest in light of the Sanofi Pasteur Phase II vaccine trials, which suggest different levels of protection against the four serotypes.3,4
Methods
Study site and population.
This study is based on the retrospective analysis of computerized data. The methods used for the creation of the clinical database have been published elsewhere5; briefly, a prospective observational study was performed from 2005 to 2010 in the emergency department for adults at the University Hospital of Fort-de-France, Martinique. The study was approved by the Clinical Research Committee of the University Hospital of Fort de France and by the Regional Research Ethical Committee. Patients provided consent to the anonymous use of clinical data. Clinical data were collected at the patient's bedside in case report forms dedicated to acute febrile diseases and recorded in a database. Emergency physicians did not know the results of the laboratory diagnosis of dengue at the time of data entry. Dengue cases were confirmed and serotyped by a hemi-nested reverse transcription-polymerase chain reaction (RT-PCR) with DENV generic and serotype-specific primers, as described elsewhere.6 Dengue-specific antibodies were detected by using immunoglobulin M (IgM) capture, immunoglobulin G (IgG) capture, and IgG indirect enzyme-linked immunosorbent assay (ELISA) kits (Panbio, Brisbane, Australia). A positive IgG capture test result for a serum sample obtained within 6 days of fever onset indicated a secondary dengue infection. Serum samples with negative results by IgG capture and IgG ELISA indicated a primary infection.
Classification of clinical forms.
The diagnosis of clinical forms was retrospective, with knowledge of all the clinical and laboratory data recorded during the emergency department stay and the final progression of the disease.5 The classification of cases was based on World Health Organization (WHO) criteria.7,8 Using the clinical presentation at admission time, each case was classified into one of the three phases of dengue illness (acute febrile phase, critical phase, and recovery phase).5,8 Plasma leakage was diagnosed if ultrasonography revealed ascites or pleural effusion or if hemoconcentration was shown according to the measurement of hematocrit at admission.7 The hematocrit threshold for hemoconcentration was set at 45% in female patients and 47% in male patients, corresponding to a 20% increase of the normal levels recorded in Martinique.
The final classification was blinded in terms of the infective serotype and the year of observation. Cases were classed based on the presence or the absence of plasma leakage, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) cases diagnosed according to WHO criteria,7 and on the presence of encephalopathy, severe acute hepatitis, myocarditis, acute renal or respiratory failure, or the existence of a hemorrhage requiring blood transfusion. Patients with signs of dehydration or fatigue (syncopes or presyncopes, orthostatic hypotension inhibiting an upright position, complete food intolerance or hypochloronatremia) who improved quickly with saline serum infusion, and who did not develop signs of plasma leakage, was included in an intermediate severity group.5
Based on disease severity and the patient care methods, we distinguished three groups of clinical forms: severe forms (plasma leakage and/or acute visceral injuries and/or serious hemorrhages) that most often warranted hospitalization for longer than one day; intermediate severity forms (fatigue and dehydration syndromes) warranting intravenous rehydration in the emergency department for several hours and followed-up under ambulatory conditions; and uncomplicated forms treated at home with simple monitoring.5
Analysis of fatal cases.
The health authorities identified the dengue-associated deaths that occurred during epidemics. The circumstances and causes of death were analyzed retrospectively during a multidisciplinary discussion.
Statistical methods.
Continuous variables were expressed as median, first (Q1) and third quartiles (Q3) and compared with Wilcoxon rank-sum test (two samples) or Kruskal-Wallis rank-sum test (more than two samples). Qualitative variables were expressed as a number and a proportion and compared using Fischer's exact test and the calculation of the odds ratio (OR). Logistic regression was used to identify factors associated with clinical forms and to estimate ORs and 95% confidence intervals (CIs) for the associations between exposure variables and clinical forms. We used four models with four dependent variables: plasma leakage or not, severe form or not, intermediate form or not, and uncomplicated form or not. We used the same explicative variables in the four models: sex (male or female), age (continuous in years), immune status (primary, secondary or undetermined), serotype (DENV-1, DENV-2, DENV-4). A difference was considered to be significant for P < 0.05 or when the value 1 did not fall inside the 95% CI of the OR. The statistical analysis was conducted with the Stata 12 application from StataCorp, College Station, TX.
Results
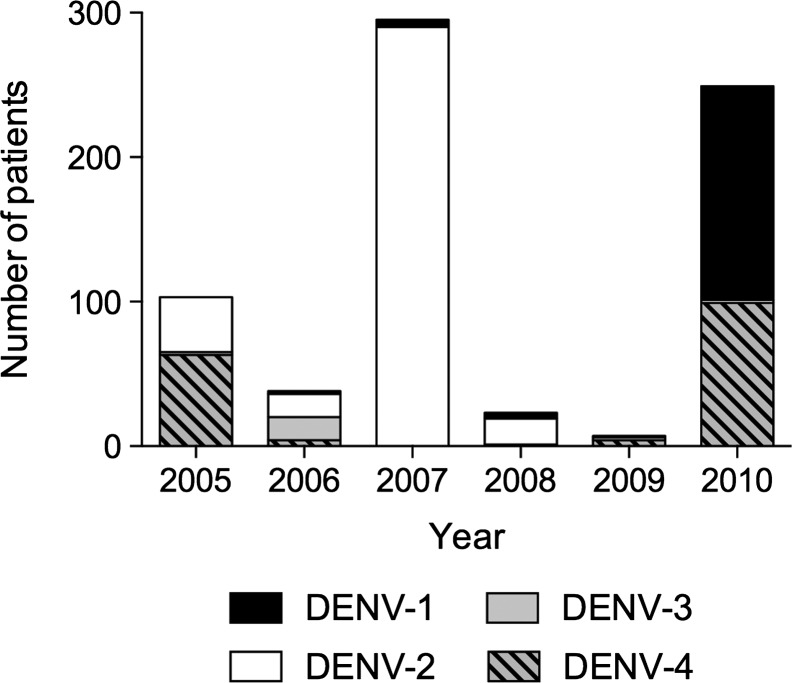
Between 2005 and 2010, 715 patients (0.87 male/female sex ratio) with dengue confirmed by RT-PCR visited the emergency department for adults at Fort-de-France CHU (Centre Hospitalier Universitaire [University Hospital Center]). Age varied between 14 years and 91 years, with a median of 35 years (Q1–Q3: 23–50 years). The DENV serotype was identified in all cases, with DENV-2 predominating in 366 (51.2%) of the cases, followed by DENV-4 in 171 (23.9%), DENV-1 in 160 (22.4%), and DENV-3 in 18 (2.5%) (Figure 1).
Figure 1.
Number of dengue cases by serotype and year.
Clinical aspects by serotype.
The patients infected by DENV-3 included 12 males and 6 females, 16 to 91 years of age (median 25 years). Seven patients presented with typical uncomplicated dengue fever, two patients developed overt plasma leakage, and two others had forms of intermediate severity. The clinical presentation was confounded by a severe chronic associated pathology in seven patients. All patients diagnosed with DENV-3 infections recovered from dengue illness.
The age and gender distributions of the patients infected by the other serotypes were comparable between serotypes (Table 1). At consultation, the time elapsed since the appearance of fever was significantly lower in patients infected by DENV-4 (P < 0.001) (Table 1). Each serotype included a large range of clinical forms and biological variations. Most DENV-4 cases had clinical pictures that were significantly symptomatic and that were very suggestive of dengue in an epidemic period (fever, cephalia, arthromyalgia, or lumbago). In addition, patients infected by DENV-1 and DENV-2 were more likely to have gastrointestinal signs (nausea, right hypochondrial or epigastric pain, vomiting, sometimes diarrhea). Patients infected by DENV-2 were more likely to have signs and symptoms associated with the critical phase of dengue illness (a rapid decrease in platelet counts with concomitant elevation of hematocrit and hypovolemia suggesting the development of plasma leakage and the progression to DHF/DSS)8 and more frequently developed severe forms. Patients infected by DENV-4 were more likely to have uncomplicated forms. Patients infected by DENV-1 were more likely to have forms of intermediate severity.
Table 1.
Clinical presentation of DENV-infected patients by main serotypes*
| Patients characteristics | Total population N = 697 | DENV-1 N = 160 | DENV-2 N = 366 | DENV-4 N = 171 |
|---|---|---|---|---|
| Age, median years (Q1–Q3) | 35 (23–50) | 31 (21–46) | 36 (24–50) | 36 (24–53) |
| Sex ratio (M/F) | 0.85 | 0.84 | 0.89 | 0.76 |
| Clinical and biological manifestations | ||||
| Time of presentation, median days (Q1–Q3) | 3 (2–5) | 4 (3–5) | 4 (2–5) | 2 (1–4) |
| Admitted during the critical phase, n (%) | 204 (29.3) | 35 (21.9) | 144 (39.3) | 25 (14.6) |
| OR [95% CI] | 1 | 2.3 [1.5–3.6] | 0.6 [0.3–1.1] | |
| Gastrointestinal signs, n (%) | 416 (60.6) | 103 (65.2) | 238 (65.8) | 75 (44.9) |
| OR [95% CI] | 1 | 1.0 [0.7–1.5] | 0.4 [0.3–0.7] | |
| Mucosal bleeding, n (%) | 112 (16.1) | 23 (14.4) | 68 (18.6) | 21(12.3) |
| OR [95% CI] | 1 | 1.3 [0.8–2.3] | 0.8 [0.4–1.6] | |
| Plasma leakage, n (%) | 100 (14.4) | 16 (10.0) | 73 (20.0) | 11 (6.4) |
| OR [95% CI] | 1 | 2.2 [1.3–4.0] | 0.6 [0.3–1.4] | |
| Platelet counts, median × 1,000/mL (Q1–Q3) | 118 (64–175) | 116 (86–164) | 104 (38–163) | 150 (110–190) |
| Hematocrit, median % (Q1–Q3) | 40 (38–44) | 42 (39–44) | 40 (37–43) | 40 (38–43) |
| ASAT, median IU/L (Q1–Q3) | 56 (30–137) | 57 (34–139) | 67 (32–157) | 39 (24–81) |
| Immune status | ||||
| Secondary infections, n (%) | 387 (55.6) | 75 (46.9) | 224 (61.2) | 88 (51.46) |
| OR [95% CI] | 1 | 1.8 [1.2–2.6] | 1.2 [0.8–1.19] | |
| Clinical forms | ||||
| Uncomplicated dengue, n (%) | 376 (54.0) | 87 (54.4) | 174 (47.6) | 115 (67.3) |
| OR [95% CI] | 1 | 0.8 [0.2–1.1] | 1.7 [1.1–2.7] | |
| Intermediate severity, n (%) | 169 (24.3) | 48 (30.0) | 92 (25.1) | 29 (17.1) |
| OR [95% CI] | 1 | 0.8 [0.5–1.2] | 0.5 [0.3–0.8] | |
| Severe dengue, n (%) | 152 (21.8) | 25 (15.6) | 100 (27.3) | 27 (15.8) |
| OR [95% CI] | 1 | 2.0 [1.3–3.3] | 1.0 [0.6–1.8] | |
| Clinical management | ||||
| Hospital stays > 1 day, n (%) | 181 (26) | 34 (21.3) | 112 (30.6) | 35 (20.5) |
| OR [95% CI] | 1 | 1.6 [1.1–2.5] | 0.9 [0.6–1.6] | |
OR = odds ratio; CI = confidence interval.
Immune status.
Immune status was determined in 596 patients (83.4%), and indicated secondary infections in 388 of 715 (54.3%) cases. The DENV-3 infections were almost primary infection (10 primary, 1 secondary, and 7 undetermined). A higher proportion of DENV-2 infections were secondary infections, compared with DENV-1 and -4 (Table 1). Patients with secondary infections visited the emergency department later than those with primary infections: median delay 4 days (Q1–Q3: 2–5 days) versus 3 days (Q1–Q3: 2–4 days) (P < 0.001). This phenomenon was especially observed with DENV-4, median 3 days (Q1–Q3: 2–5 days) versus 1 day (Q1–Q3: 1–3 days) (P < 0.001), respectively. Patients with secondary infections were older than those with primary dengue, 37 years (Q1–Q3: 24–51 years) versus 30 years (Q1–Q3: 21–46 years), (P < 0.01), respectively, and were more likely to have clinical forms with plasma leakage, 18.3% versus 7.7% (P < 0.01), respectively; serious forms, 26.8% versus 16.4% (P < 0.01), respectively; more marked thrombocytopenia, 96 × 1,000/mL (Q1–Q3: 42–158 × 1,000/mL) versus 147 × 1,000/mL (Q1–Q3: 110–190 × 1,000/mL), (P < 0.001), respectively; and elevated aspartate aminotransferase, 85 IU/L (Q1–Q3: 35–184 IU/L) versus 39 IU/L (Q1–Q3: 26–64 IU/L), (P < 0.001), respectively. However, only DENV-2 infections showed more frequent development of plasma leakage in secondary dengue compared with primary dengue, 24.1% versus 7.9% (P < 0.01), respectively, compared with 10.7% versus 11.3% and 9.1% versus 4.7%, for DENV-1 and -4, respectively.
Evolution of clinical forms between 2005 and 2010.
The DENV-3 isolates were rare and disappeared completely after September 2006. During the 2005 epidemic, out of 123 patients, 67 (54.5%) were infected by DENV-4, 54 (43.9%) by DENV-2, and 2 (1.6%) by DENV-1. During the 2007 epidemic, out of 317 patients, 308 (97.2%) were infected with DENV-2 and 9 others (2.8%) with DENV-1. During the 2010 epidemic, out of 256 patients, 149 (58.2%) were infected with DENV-1, 103 (40.2%) with DENV-4, and 4 others (1.6%) with DENV-2. Thus, we were able to compare the evolution of clinical forms for DENV-2 between 2005 and 2007 and for DENV-4 between 2005 and 2010. Table 2 shows the clinical profile of patients infected by serotypes 2 and 4 in each epidemic. There were significant changes in the immune status over this period. Between 2005 and 2007, the proportion of secondary infections went from 55–81% for DENV-2 infections, and between 2005 and 2010 it went from 12–87% for DENV-4 infections. Despite the significant increase of secondary dengue over time, the distribution of clinical forms observed over the course of the successive epidemics was not significantly different.
Table 2.
Clinical presentation of DENV-2 and DENV-4 infected patients by epidemics
| Patients characteristics | DENV-2 | DENV-4 | ||
|---|---|---|---|---|
| 2005 N = 54 | 2007 N = 308 | 2005 N = 67 | 2010 N = 103 | |
| Age, median years (Q1–Q3) | 33 (23–46) | 36.5 (24–50) | 31 (23–47) | 38 (24–54) |
| Sex ratio (M/F) | 0.93 | 0.89 | 0.56 | 0.91 |
| Clinical and biological manifestations | ||||
| Time of presentation, median days (Q1–Q3) | 3 (2–4) | 4 (2–5)* | 1 (1–3) | 3 (2–5)* |
| Admitted during the critical phase, n (%) | 16 (29.6) | 128 (41.5) | 6 (9.0) | 18 (17.5) |
| OR [95% CI] | 1 | 1.6 [0.9–3.2] | 1 | 2.2 [0.8–5.7] |
| Gastrointestinal signs, n (%) | 36 (66.7) | 200 (65.8) | 29 (43.2) | 45 (45.5) |
| OR [95% CI] | 1 | 0.9 [0.5–1.8] | 1 | 1.1 [0.6–2.0] |
| Mucosal bleeding, n (%) | 10 (18.5) | 58 (18.8) | 7 (10.4) | 14 (13.7) |
| OR [95% CI] | 1 | 1 [0.5–2.2] | 1 | 1.4 [0.5–3.6] |
| Platelet counts, median × 1000/mL (Q1–Q3) | 117 (76–181) | 97 (34–157)* | 162 (123–217) | 134 (86–181)* |
| Hematocrit, median % (Q1–Q3) | 41 (38–44) | 40 (37–43) | 38 (36–42) | 41 (38–44)* |
| ASAT, median IU/L (Q1–Q3) | 61 (32–146) | 71 (31–159) | 31 (22–54) | 41 (26–117)* |
| Plasma leakage, n (%) | 9 (16.7) | 64 (20.8) | 3 (4.5) | 8 (7.8) |
| OR [95% CI] | 1 | 1.3 [0.6–2.8] | 1 | 1.8 [0.5–7.0] |
| Immune status | ||||
| Secondary infections, n (%) | 21 (38.9) | 201 (65.3)* | 7 (10.6) | 81 (78.6)* |
| OR [95% CI] | 1 | 3.0 [1.6–5.4] | 1 | 32 [12.6–78.7] |
| Clinical forms | ||||
| Uncomplicated dengue, n (%) | 28 (51.9) | 144 (46.8) | 48 (71.6) | 67 (65.1) |
| OR [95% CI] | 1 | 0.8 [0.5–1.5] | 1 | 0.7 [0.4–1.4] |
| Intermediate severity, n (%) | 9 (16.7) | 81 (26.3) | 10 (14.9) | 18 (17.5) |
| OR [95% CI] | 1 | 1.7 [0.8–3.8] | 1 | 1.2 [0.5–2.8] |
| Severe dengue, n (%) | 17 (31.5) | 83 (26.9) | 9 (13.4) | 18 (17.5) |
| OR [95% CI] | 1 | 0.8 [0.4–1.5] | 1 | 1.4 [0.6–3.2] |
| Clinical management | ||||
| Hospital stay > 1 day, n (%) | 18 (33.3) | 93 (30.2) | 14 (20.9) | 21 (20.4) |
| OR [95% CI] | 1 | 0.9 [0.5–1.6] | 1 | 1.0 [0.5–2.1] |
P < 0.05, when compared with the previous epidemic.
OR = odds ratio; CI = confidence interval.
Factors associated with the clinical forms.
The relationships between the age, sex, immune status (primary or secondary infections), dengue serotype, and the development of principal clinical forms are presented in Table 3; the risk of developing a plasma leakage increased with age, male gender, a secondary infection, and the DENV-2 serotype. The intermediate-severity forms were more likely in the female gender, and with serotypes DENV-1 or DENV-2, regardless of age and immune status.
Table 3.
Factors associated with clinical forms (multivariate, odds ratio-adjusted analysis [CI 95%])
| Covariates | Plasma leakage (N = 100) | Severe dengue (N = 152) | Intermediate severity (N = 169) | Uncomplicated dengue (N = 376) |
|---|---|---|---|---|
| Age | 1.02 [1.01–1.03] | 1.02 [1.01–1.03] | 1.00 [0.99–1.01] | 0.98 [0.97–0.99] |
| Sex | ||||
| Male | 4.97 [3.01–8.25] | 3.18 [2.14–4.72] | 0.49 [0.34–0.71] | 0.79 [0.56–1.08] |
| Female | 1 | 1 | 1 | 1 |
| Immune status | ||||
| Primary | 1 | 1 | 1 | 1 |
| Secondary | 2.08 [1.13–3.85] | 1.78 [1,09–2.89] | 1.22 [0.80–1.87] | 0.61 [0.42–0.89] |
| Undetermined | 0.95 [0.42–2.17] | 0.83 [0.43–1.65] | 0.83 [0.45–1.52] | 1.30 [0.78–2.18] |
| Serotype | ||||
| DENV-1 | 1 | 1 | 1 | 1 |
| DENV-2 | 2.14 [1.15–3.98] | 1.95 [1.16–3.29] | 0.77 [0.50–1.19] | 0.81 [0.55–1.22] |
| DENV-4 | 0.58 [0.25–1.34] | 0.96 [0.52–1.80] | 0.45 [0.26–0.76] | 1.92 [1.21–3.05] |
Deaths.
Twenty-one patients of our cohort died. In the meantime, the epidemiological monitoring carried out by the Martinique health authorities reported four deaths linked to dengue among 14,500 symptomatic cases (2.7 deaths/10,000) during the 2005 epidemic; 4 deaths out of 17,900 cases (2.2 deaths/10,000) during the 2007 epidemic; and 17 deaths out of 41,970 cases (4 deaths/10,000) during the 2010 epidemic.1 Table 4 shows the principal clinical characteristics of the deaths associated with a serotype identified in 21 of the 25 recorded deaths in Martinique during the study period. The 2010 epidemic was characterized by the appearance of deaths associated with DENV-4 infections, which had not been observed during the 2005 epidemic in which DENV-4 primary infections were predominant. The majority of patients had signs of plasma leakage. However, in half of the cases, the cause of death could be attributed to associated pathology rather than the clinical severity of the dengue itself. Sudden death was identified in three cases, including one autopsied case revealing the existence of multifocal hepatic and myocardial necrosis.
Table 4.
Fatal cases by serotype and year
| DENV-1 | |||||
|---|---|---|---|---|---|
| Year | Sex, age | Immunity | Clinical presentation | Immediate cause of death | Initial cause of death |
| 2005 | M, 63 | Secondary | DHF | Fulminating hepatitis | Dengue |
| 2010 | M, 66 | ND | Acute renal failure, diabetes | MOF | Associated pathology |
| 2010 | M, 15* | ND | DHF/DSS | Ventilator associated pneumonia | Associated pathology |
| 2010 | F, 6* | Secondary | DHF/DSS, HbSC Acute thoracic syndrome | MOF | Associated pathology |
| 2010 | F, 62 | Secondary | DHF/DSS, HbSC | MOF | Associated pathology |
| 2010 | M, 90 | ND | Heart failure | Cardiogenic shock | Associated pathology |
| 2010 | F, 42 | ND | DHF/DSS, HbSC | Sudden death | Dengue |
| 2010 | M, 80 | Secondary | Acute renal failure, diabetes | Acute renal failure | Associated pathology |
| 2010 | F, 37 | Secondary | DHF/DSS | Sudden death | Dengue |
| DENV-2 | |||||
| 2005 | F, 62 | Secondary | DHF | Head trauma, cerebral hemorrhage | Associated pathology |
| 2005 | M, 53 | Secondary | DHF | Fulminating hepatitis | Dengue |
| 2007 | M, 59 | ND | DHF/DSS | Status epilepticus, ventilator associated pneumonia | Associated pathology |
| 2007 | M, 65 | Primary | DHF/DSS | Encephalitis | Dengue† |
| 2007 | F, 41 | Secondary | DHF/DSS | Alithiasic gangrenous cholecystitis, septic shock | Dengue |
| DENV-4 | |||||
| 2010 | F, 49 | Secondary | DHF/DSS | MOF | Dengue |
| 2010 | M, 89 | ND | Heart failure | Pneumonia, cardiogenic shock | Associated pathology |
| 2010 | M, 1* | ND | DHF/DSS | MOF | Dengue |
| 2010 | F, 35 | Secondary | DHF/DSS | Sudden death, multifocal myocardial infarction and hepatic necrosis | Dengue† |
| 2010 | F, 10* | Secondary | DHF/DSS, HbSC | Shock | Dengue† |
| 2010 | F, 49 | Primary | DSS | Shock | Dengue |
| 2010 | F, 43 | Secondary | DHF/DSS | Diabetes, lactic acidosis | Associated pathology |
DHF/DSS = dengue hemorrhagic fever/dengue shock syndrome according to WHO definition6; ND = not determined; MOF = multiple organ failure; HbSC = sickle cell disease (SC type).
Not included in the study cohort.
Autopsy case.
Discussion
This observational study reports on patients with a confirmed diagnosis of dengue admitted in the emergency department of a tertiary care hospital in Martinique. The study is relevant regarding the duration of observation and the number of patients included, allowing the comparison of the clinical presentation of the different serotypes involved during following epidemics. As these epidemics repeated with different serotypes, an increasing proportion of the population became immunized against one or more dengue serotypes. Given the “captive” character of Martinique's population, the recruitment bias was probably limited. However, conducting clinical studies in the emergency department during dengue epidemics is difficult because of the large number of patients that present to care facilities day and night, and because of the sometimes misleading clinical pictures. This may lead to a significant loss of clinical data.
The evolving character of the signs and symptoms of dengue infection is another difficulty. Most often, the warning signs and other signs of severity do not appear until the 3rd or 4th day after the start of the disease.7,8 Consequently, the number of days passed between the beginning of the disease and the day of the consultation is a confounding factor that could have been involved in the observation of signs and symptoms of patients infected by DENV-4 who, in case of primary infection, presented earlier to the emergency department. However, we do not think that this factor could have skewed our results because we reviewed the clinical evolution of all of our patients after going to the emergency department. Among the patients who were followed in an outpatient setting and who were seen again in a visit, very few progressed toward a more severe form.5 In most cases, the clinical data recorded in the emergency department allowed for a diagnosis of the phase of progression of the dengue (acute febrile phase, critical phase, or recovery phase). The appearance of signs and symptoms suggesting the progression to a critical phase prompted patients to be kept under observation.8 In certain cases, the presence of an associated chronic disease that was decompensate by dengue made attributing the observed signs and symptoms to dengue difficult.5 Likewise, increased age, associated illness, or unfavorable social conditions could have justified a decision to hospitalize, although the dengue illness itself may not have been very severe.
Another difficulty is the absence of international consensus regarding key points such as the value of warning signs and the classification of cases.9–11 This issue confounds the ability to compare our results with those that used different case classifications or that were conducted in a pediatric setting.12–16 Our study in adolescents and adults showed that, in this series, specific clinical aspects existed for each serotype. The DENV-4 infections most often had a milder clinical profile, regardless of the immune status and the year of observation. The DENV-2 infections were most often secondary infections admitted at the critical phase of the disease with signs of plasma leakage, thrombocytopenia, and elevated transaminases, regardless of the year of observation. The DENV-1 infections were disabling, particularly in the female gender, and most often led to intermediate forms (orthostatic malaise or dehydration), without plasma leakage, regardless of the immune status. Overall, secondary infections were associated with older age and a higher risk of severe forms with plasma leakage. However, in terms of differences observed between primary and secondary dengue, an important finding in this study was to that the increased risk of plasma leakage in cases of secondary dengue really only concerned DENV-2. The comparison of clinical forms developed by the DENV-4 infections that occurred in 2005 and 2010 did not show significant differences despite the very large predominance of primary dengue in 2005 and secondary dengue in 2010. However, for various technical reasons, the immune status could not be determined in 16.1% of patients, which could have skewed the results of this study. The addition of an “undetermined” category of immune status did not significantly alter the results of the multivariate analysis presented in Table 3 and we see no reason to suspect a selection bias for any serotype.
Pathogenicity differences between the different serotypes and within the same serotype raise numerous questions. Despite a significant amount of work, the relationships between dengue immunity and the mechanisms leading to severe forms of dengue remain hypothetical and there is a lack of experimental model.17 In a seroprevalence study conducted in Thai children in Bangkok, it was shown that most seroconversions to dengue were clinically unapparent and that most DHF cases were secondary DENV-2 infections.18 In another study, it was shown that dengue could occur in the presence of cross-neutralizing anti-dengue antibodies, and that there was a titer below which higher viremia levels and more severe clinical forms of the disease were observed.19 In that study, however, the influence of preexisting immunity varied according to the serotype and did not concern DENV-2, whose severe forms could develop despite the presence of high levels of neutralizing homologous antibodies. These data are consistent with prior data from Bangkok and with recent dengue modeling studies suggesting that the ADE phenomenon may preferentially involve certain strains of dengue, especially serotype 2. Such an interplay with certain serotypes and this ADE mechanism could explain the results of the recent Phase IIb CYD dengue vaccine trial in Ratchaburi, Thailand (Burke D, personal communication, Fondation Meyrieux, Veyrier-du-Lac, France, July 10, 2013),20 which showed a lack of protection against DENV-2 despite obtaining neutralizing levels of specific antibodies after vaccination.3 However, in this trial, there was no clinical difference between cases among vaccinees and control in terms of severity and duration of infection. Specifically, regarding the patients who developed virologically confirmed dengue, the proportion of patients with plasma leakage was 5.3% in vaccinees and 4.8% in control and no DSS case was observed in vaccinees as compared with two cases in control3; interestingly, in another Phase II trial, the Sanofi Pasteur vaccine showed an anecdotal breakthrough case protection against DENV-2.4
Another mechanism could be the acquisition of specific virulence by certain viral strains. The problem is not well documented. It is all the more complex in that preexisting immunity and viral genetics could interact to determine the severity of the dengue. A recent publication showed that secondary DENV-2 infections could result from more virulent clades than others, depending on the serotype of the primary infecting serotype, DENV-1 or DENV-3.21 Under these conditions, the development dynamics of epidemics, the function of the implicated genotypes, and the intervals between the successive epidemics,22 the immune and genetic status of the population,23 and other environmental factors such as the population density,24 the climate and density of mosquitoes of the Aedes genus,25 could create specific situations in each endemic region and thus create many confounding factors. This could explain the differences in results shown in various publications on the theme of clinical presentation by serotype. However, the highest frequency of severe forms in DENV-2 infections was observed in Southeast Asia and in Latin America.13,15,21,22,26 The DENV-2 was recently found to be significantly associated with DSS in several countries.27
However, all serotypes could lead to severe forms and death1; among the deaths observed during the study period, one notes that half were attributed to cardiovascular-associated disease, diabetes, sickle cell disease, or nosocomial infections contracted in intensive care. The existence of a chronic disease that has been decompensated by viral infection increases the risk of death in dengue.28 However, whether there was an associated disease, these patients had most often developed a severe clinical form of DHF/DSS type with plasma leakage. Death could be related to massive visceral injuries in four cases: fulminant hepatitis (two cases), encephalitis (one case), and gangrenous cholecystitis (one case). Of note is the existence of three cases of sudden death. The autopsy carried out in one of these cases revealed multifocal injuries from hepatic and especially myocardial necrosis without coronary obstruction, suggesting that the sudden death could have followed ventricular fibrillation.
Overall, the severe dengue cases observed in Martinique were frequently associated with the plasma leakage in the case of secondary DENV-2 infections. The 2005 and 2007 DENV-2 epidemics were of genotype III, named Asian-American (Dussart P, personal communication). The DENV-4 isolates of the 2005 epidemic were genotype II29; the deaths associated with DENV-4 infections occurred in 2010, although no death associated with this serotype had been observed during the 2005 epidemic. Consequently, sequencing of DENV-4 strains from the 2010 epidemic is required to compare them to the strains isolated in 2005.
In summary, this study performed in Martinique shows that DENV-2 infections were more often secondary infections admitted at the critical phase with signs of plasma leakage than other serotypes. The DENV-1 infections were disabling, particularly in females, and most often led to disease of intermediate severity. The DENV-4 infections were admitted more frequently during the acute febrile phase and featured milder clinical forms, regardless of the host's immune status. However, DENV-4 was occasionally responsible for deaths during the last outbreak. These data are consistent with there being differences in virulence between serotypes and/or the genotypes. Consequently, this study does not predict what might be observed in children and adults in the next epidemic, notably with DENV-3, which has rarely circulated in Martinique since 2001.
ACKNOWLEDGMENTS
We thank Grenville Marsh at Sanofi Pasteur for editorial assistance and Jean Lang at Sanofi Pasteur for the critical review of the draft manuscript.
Working Group on Dengue: Sylvie Abel, Department of Infectious and Tropical Diseases and CIC-EC (Inserm CIE 802), University Hospital, Fort-de-France, Martinique; Guillaume Avenin, CIC-EC (Inserm CIE 802), University Hospital, Fort-de-France, Martinique; Pierre Brihier, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Yannick Brouste, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Christophe Deligny, Department of Internal Medicine, University Hospital, Fort-de-France, Martinique; Jean-Marc Dueyme, Department of Nephrology and Hemodialysis, General Hospital, Lamentin, Martinique; Gisèle Elana, Department of Pediatrics, General Hospital, Lamentin, Martinique; Christiane Fonteau, Laboratory of Biochemistry, University Hospital, Fort-de-France, Martinique; Yves Hatchuel, Department of Pediatrics, University Hospital, Fort-de-France, Martinique; Patrick Hochedez, Department of Infectious and Tropical Diseases, University Hospital, Fort-de-France, Martinique; Guillaume Hurtrel, Department of Infectious and Tropical Diseases, University Hospital, Fort-de-France, Martinique; Janick Jean-Marie, CIC-EC (Inserm CIE 802), University Hospital, Fort-de-France, Martinique; Christian Léonard, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Sophie Mainguy, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Stéphane Malbranque, Forensic Unit, University Hospital, Fort-de-France, Jenny Martial, Laboratory of Virology and Immunology, University Hospital, Fort-de-France, Martinique; Hossein Mehdaoui, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Harold Merle, Department of Ophthalmology, University Hospital, Fort-de- France, Martinique; Yves Plumelle, Laboratory of Hematology and Coagulation, University Hospital, Fort-de-France, Martinique; Dabor Résières, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique; Vincent Ronin, Department of Infectious and Tropical Diseases, University Hospital, Fort-de- France, Martinique.
Footnotes
Financial support: The study was supported by the University Hospital of Fort-de-France, Martinique and the Regional office (Cire) of the French Institute for Public Health Surveillance, Fort-de-France, Martinique.
Authors' addresses: Laurent Thomas, François Besnier, and Ruddy Valentino, Emergency and Intensive Care Department, University Hospital, Fort-de-France, Martinique, E-mails: laurent.thomas@chu-fortdefrance.fr, francois.besnier@chu-fortdefrance.fr, and ruddy.valentino@chu-fortdefrance.fr. Fatiha Najioullah, Laboratory of Virology and Immunology, University Hospital, Fort-de-France, Martinique, E-mail: fatiha.najioullah@chu-fortdefrance.fr. Jacques Rosine, Regional office (Cire) of the French Institute for Public Health Surveillance, Fort-de-France, Martinique, E-mail: jacques.rosine@ars.sante.fr. Raymond Césaire, Laboratory of Virology and Immunology and CIC-EC (Inserm CIE 802), University Hospital, Fort-de-France, Martinique, E-mail: raymond.cesaire@chu-fortdefrance.fr. André Cabié, Department of Infectious and Tropical Diseases and CIC-EC (Inserm CIE 802), University Hospital, Fort-de- France, Martinique, E-mail: andre.cabie@chu-fortdefrance.fr.
References
- 1.Quénel P, Rosine J, Cassadou S, Ardillon V, Blateau A, Mattheus S, Chappert JL, Flamand C, Carvalho L, Cardoso T, Chaud P, Dussart P, Ledrans M. Épidémiologie de la dengue dans les Départements français d'Amérique. Bulletin épidémiologique hebdomadaire, 20 Septembre. 2011;2011:358–362. http://www.invs.sante.fr/Publications-et-outils/BEH-Bulletin-epidemiologique-hebdomadaire/Archives/2011/BEH-n-33-34-2011 Available at. Accessed September 20, 2011. [Google Scholar]
- 2.Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med. 2012;366:1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 3.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 4.Villar LA, Rivera-Medina DM, Arredondo-García JL, Boaz M, Starr-Spires L, Thakur M, Zambrano B, Miranda MC, Rivas E, Dayan GH. Safety and immunogenicity of a recombinant tetravalent dengue vaccine in 9–16 years old: a randomized, controlled, Phase II trial in Latin America. Pediatr Infect Dis J. 2013;32:1102–1109. doi: 10.1097/INF.0b013e31829b8022. [DOI] [PubMed] [Google Scholar]
- 5.Thomas L, Moravie V, Besnier F, Valentino R, Kaidomar S, Villain Coquet L, Najioullah F, Lengellé F, Césaire R, Cabié A. Working Group on Dengue Clinical presentation of dengue among patients admitted to the adult emergency department of a tertiary care hospital in Martinique: implications for triage, management, and reporting. Ann Emerg Med. 2012;59:42–50. doi: 10.1016/j.annemergmed.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Najioullah F, Combet E, Paturel L, Martial J, Koulmann L, Thomas L, Hatchuel Y, Cabié A, Cesaire R. Prospective evaluation of nonstructural 1 enzyme-linked immunosorbent assay and rapid immunochromatographic tests to detect dengue virus in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2011;69:172–178. doi: 10.1016/j.diagmicrobio.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Dengue Hemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. http://www.who.int/csr/resources/publications/dengue/Denguepublication/en Available at. Accessed January 20, 2010. [Google Scholar]
- 8.World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. 2009. http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf WHO/HTM/NTD/DEN/2009.1. Available at. Accessed May 17, 2010. [PubMed]
- 9.Srikiatkhachorn A, Rothman AL, Gibbons RV, Sittisombut N, Malasit P, Ennis FA, Mannitya S, Kalayanarooj S. Dengue–how best to classify it. Clin Infect Dis. 2011;53:563–567. doi: 10.1093/cid/cir451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead SB. Dengue: the syndromic basis to pathogenesis research. Inutility of the 2009 WHO case definition. Am J Trop Med Hyg. 2013;88:212–215. doi: 10.4269/ajtmh.12-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrar JJ, Hien TT, Horstick O, Hung NT, Jaenisch T, Junghanns T, Kroeger A, Laksono IS, Lum L, Martinez E, Simmons CP, Tami A, Tomashek KM, Wills BA. Dogma in classifying dengue disease. Am J Trop Med Hyg. 2013;89:198–201. doi: 10.4269/ajtmh.13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murgue B, Deparis X, Chungue E, Cassar O, Roche C. Dengue: an evaluation of dengue severity in French Polynesia based on an analysis of 403 laboratory-confirmed cases. Trop Med Int Health. 1999;4:765–773. doi: 10.1046/j.1365-3156.1999.00478.x. [DOI] [PubMed] [Google Scholar]
- 13.Balmaseda A, Hammond SN, Pérez L, Tellez Y, Saborío SI, Mercado JC, Cuadra R, Rocha J, Pérez MA, Silva S, Rocha C, Harris E. Serotype-specific differences in clinical manifestations of dengue. Am J Trop Med Hyg. 2006;74:449–456. [PubMed] [Google Scholar]
- 14.Tsai JJ, Chan KS, Chang JS, Chang K, Lin CC, Huang JH, Lin WR, Chen TC, Hsieh HC, Lin SH, Lin JC, Lu PL, Chen YH, Lin CY. Effect of serotypes on clinical manifestations of dengue fever in adults. J Microbiol Immunol Infect. 2009;42:471–478. [PubMed] [Google Scholar]
- 15.Fried JR, Gibbons RV, Kalayanarooj S, Thomas SJ, Srikiatkhachorn A, Yoon IK, Jarman RG, Green S, Rothman AL, Cummings DA. Serotype-specific differences in the risk of dengue hemorrhagic fever: an analysis of data collected in Bangkok, Thailand from 1994 to 2006. PLoS Negl Trop Dis. 2010;4:e617. doi: 10.1371/journal.pntd.0000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halsey ES, Marks MA, Gotuzzo E, Fiestas V, Suarez L, Vargas J, Aguayo N, Madrid C, Vimos C, Kochel TJ, Laguna-Torres VA. Correlation of serotype-specific dengue virus infection with clinical manifestations. PLoS Negl Trop Dis. 2012;6:e1638. doi: 10.1371/journal.pntd.0001638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.St John AL, Abraham SN, Gubler DJ. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat Rev Microbiol. 2013;11:420–426. doi: 10.1038/nrmicro3030. [DOI] [PubMed] [Google Scholar]
- 18.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38:172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 19.Endy TP, Nisalak A, Chunsuttitwat S, Vaughn DW, Green S, Ennis FA, Rothman AL, Libraty DH. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189:990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Barraquer I, Mier-Y-Teran-Romero L, Burke DS, Cummings DA. Challenges in the interpretation of dengue vaccine trial results. PLoS Negl Trop Dis. 2013;7:e2126. doi: 10.1371/journal.pntd.0002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.OhAinle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborío S, Nuñez A, Lennon NJ, Birren BW, Gordon A, Henn MR, Harris E. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med. 2011;3:114–128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 23.Halstead SB, Streit TG, Lafontant JG, Putvatana R, Russell K, Sun W, Kanesa-Thasan N, Hayes CG, Watts DM. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65:180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt WP, Suzuki M, Thiem VD, White RG, Tsuzuki A, Yoshida LM, Yanai H, Haque U, Tho le H, Anh DD, Ariyoshi K. Population density, water supply, and the risk of dengue fever in Vietnam: cohort study and spatial analysis. PLoS Med. 2011;8:e1001082. doi: 10.1371/journal.pmed.1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Descloux E, Mangeas M, Menkes CE, Lengaigne M, Leroy A, Tehei T, Guillaumot L, Teurlai M, Gourinat AC, Benzler J, Pfannstiel A, Grangeon JP, Degallier N, De Lamballerie X. Climate-based models for understanding and forecasting dengue epidemics. PLoS Negl Trop Dis. 2012;6:e1470. doi: 10.1371/journal.pntd.0001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loroño-Pino MA, Farfán-Ale JA, Zapata-Peraza AL, Rosado-Paredes EP, Flores-Flores LF, García-Rejón JE, Díaz FJ, Blitvich BJ, Andrade-Narváez M, Jiménez-Ríos E, Blair CD, Olson KE, Black W, 4th, Beaty BJ. Introduction of the American/Asian genotype of dengue 2 virus into the Yucatan State of Mexico. Am J Trop Med Hyg. 2004;71:485–492. [PubMed] [Google Scholar]
- 27.Huy NT, Van Giang T, Thuy DH, Kikuchi M, Hien TT, Zamora J, Hirayama K. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2412. doi: 10.1371/journal.pntd.0002412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigau-Pérez JG, Laufer MK. Dengue-related deaths in Puerto Rico, 1992–1996: diagnosis and clinical alarm signals. Clin Infect Dis. 2006;42:1241–1246. doi: 10.1086/501355. [DOI] [PubMed] [Google Scholar]
- 29.Dussart P, Lavergne A, Lagathu G, Lacoste V, Martial J, Morvan J, Césaire R. Reemergence of dengue virus type 4, French Antilles and French Guiana, 2004–2005. Emerg Infect Dis. 2006;12:1748–1751. doi: 10.3201/eid1211.060339. [DOI] [PMC free article] [PubMed] [Google Scholar]