Abstract
Although malnutrition and malaria co-occur among individuals and populations globally, effects of nutritional status on risk for parasitemia and clinical illness remain poorly understood. We investigated associations between Plasmodium falciparum infection, nutrition, and food security in a cross-sectional survey of 365 Batwa pygmies in Kanungu District, Uganda in January of 2013. We identified 4.1% parasite prevalence among individuals over 5 years old. Severe food insecurity was associated with increased risk for positive rapid immunochromatographic test outcome (adjusted relative risk [ARR] = 13.09; 95% confidence interval [95% CI] = 2.23–76.79). High age/sex-adjusted mid-upper arm circumference was associated with decreased risk for positive test among individuals who were not severely food-insecure (ARR = 0.37; 95% CI = 0.19–0.69). Within Batwa pygmy communities, where malnutrition and food insecurity are common, individuals who are particularly undernourished or severely food-insecure may have elevated risk for P. falciparum parasitemia. This finding may motivate integrated control of malaria and malnutrition in low-transmission settings.
Introduction
Plasmodium falciparum malaria and malnutrition are co-occurring health risks within populations globally; however, nutritional modulation of individual risk for P. falciparum parasitemia is complex and poorly understood.1 Although poverty and host-level biological pathways contribute to the dual burden of malnutrition and malaria within resource-poor populations, relative contributions of such distal and proximate factors remain unclear.1–4 Low-transmission tropical areas at high altitudes, including the east African highlands, are forecasted to account for a large share of malaria resurgence under projected changes in temperature and precipitation regimes associated with global climatic change.5–12 Because vector exposure is heterogeneous in these settings and prevalence of naturally acquired immunity tends to be low in resident populations,13 host-level behavioral and immunological factors are of particular importance to individual risk for infection.14–18 However, low- and variable-transmission settings are underrepresented within present literature relating malaria risk to nutrition. We thus lack an evidence base to inform integrated management of malaria and nutrition in areas at risk for becoming hyper- or holoendemic as climate change alters vector habitat suitability.
Presently hypothesized associations between malaria risk and nutrition suggest that resolving a population's chronic and acute nutrient deficiencies may lead to any of several scenarios for malaria control. Generally, improved individual immune function with better nutrition may limit health burdens from malaria resurgence3,19–21 by reducing morbidity and mortality associated with parasitemia.22 Alternatively, host-level protective effects of iron and folic acid deficiencies against Plasmodia may indicate that micronutrient supplementation could increase incidence of clinical malaria within an endemic population23–26 and heighten severity of disease episodes.3,4,26–31 In the event that such antagonistic effects are self-canceling,32 reducing prevalence of malnutrition may serve more distally as a poverty-reduction tool to facilitate malaria control (for instance, by alleviating economic and life-year losses from incapacitation).33–36 Observational evidence for all three scenarios indicates demand for better understanding of factors driving local variation in how malnutrition and malaria relate epidemiologically and interventions tailored to local circumstances, which may differ with respect to transmission intensity, prevalence of acquired immunity, and nutritional or food security risks.
Household food insecurity, defined here as constrained social, economic, and physical access to sufficient and nutritionally adequate foods for maintaining a healthy and active lifestyle, is a determinant for malnutrition and associated comorbid conditions globally37,38 and specifically, within sub-Saharan Africa.39 Although food insecurity is often a perennial state, it may additionally occur intermittently when conditions surrounding food production or acquisition become adverse.40–42 Because individuals can be inclined to reduce dietary quality or variety as a coping mechanism in such events,37,43 transitory food insecurity may cause acute micronutrient deficiency, even in individuals who are typically well-nourished.44–46
Relationships among food insecurity, malnutrition, and risk for malaria have been sparsely investigated and are difficult to observe given the multifactorial host-level biology of malnutrition and immune function, the need of P. falciparum for labile micronutrients within a human host, reverse-causal effects of malarial anemia on anthropometric indicators for nutritional status, and external socioenvironmental factors predisposing individuals to risk for all three conditions. For instance, as a cause for poor mental health outcomes, including psychological stress, food insecurity may disrupt normal host endocrine function and increase susceptibility to P. falciparum or other infections.47–51 More proximately, food insecurity serves as a determinant for malnutrition, which may hamper immune responses to Plasmodium infection while also limiting nutrient availability for parasite reproduction and pathogenesis within the human host.31 Heterogeneous prevalence of acquired immunity among settings with different transmission patterns may likewise contribute to variation in observed nutrition and food security associations with risk for parasitemia or clinical illness.13 Of the two contemporary studies assessing food insecurity associations with clinical malaria, one study conducted within Haiti identified food insecurity as a risk factor among children under 5 years old,19 whereas one study in Colombia identified no significant association among children under 15 years old.52 Both regions have low transmission, respectively, of P. falciparum and P. vivax, with parasite prevalence between 3% and 4% and high adult to children under 5 years case ratios (> 1) characteristic of unstable malaria epidemiology.1,53–55 Presently, no studies have assessed food security and risk for parasitemia.
As a marginalized, semisubsistent Indigenous population with pre-existing burden from these climate-sensitive health risks, the Batwa pygmies of Uganda are expected to be vulnerable to increasing malaria, food insecurity, and malnutrition amidst climate change.56–59 Together with monetary poverty, coerced migration from their ancestral forest home to agrarian settlements has constrained the Batwa's coping options during frequent episodes of famine and has not reduced health gaps relative to other Ugandans.56 Average life expectancy at birth is only 28 years compared with the Ugandan average of 53 years; the Batwa child mortality ratio of 41% is high relative to the southwestern Ugandan average of 18.1% and the national average of 13.7%.56 Although we know that P. falciparum transmission is generally low and volatile in Uganda's southwestern highlands,60,61 prevalence and causes for individual infection among the Batwa, whose settlements are geographically separated from the settlements of the ethnic majority population, have not been assessed.
Here, we present the outcomes of a malariometic, anthropometric, and food security census among Batwa pygmies residing in Kanungu District, Uganda. Our objectives were to (1) characterize the distribution of P. falciparum infection among Batwa pygmies and (2) contribute observational evidence of whether risk for current P. falciparum infection covaries with food insecurity or nutritional status in a low-transmission setting.
Materials and Methods
Ethics.
The study was approved in accordance with the requirements of the McGill University Policy on the Ethical Conduct of Research Involving Human Subjects and the Canadian Tri-Council Policy Statement on Ethical Conduct for Research Involving Human Subjects; the latter affords particular attention to issues among Indigenous and disadvantaged peoples. We, moreover, followed best practice frameworks for working with Indigenous communities in climate change vulnerability research.59,62,63 We obtained informed consent from adult participants for each component of the clinical, household, and anthropometric surveys and from parents for children under 18 years old. Parents or guardians answered on behalf of children under 12 years old.
Design.
During the biannual transmission peak from January to February of 2013,60 we administered a standardized questionnaire addressing issues in environment and health identified by Batwa communities during a pilot research phase.56 We pre-tested the survey instrument during reduced seasonal administrations preceding the January–February 2013 census. We obtained finger-prick blood samples from all consenting participants for in-field rapid P. falciparum diagnostic tests. Respondents were interviewed in the Rukiga language by paid interviewers from Kanungu District. We sought to achieve census-level representation across all ages within Kanungu District's 10 Batwa settlements (mapped in Figure 1) as identified by partners from the Batwa Development Program and Bwindi Community Hospital.64 Children who attended residential school were in their home communities during the surveying because of the Ugandan school holiday, maximizing survey response. We constructed a sampling frame in field by verifying with village chiefs the number and composition of households within their communities. We asked household heads to identify co-occupants and if co-occupants were not present at the interview, indicate their whereabouts. We visited individuals who did not report to interview sites at their homes and worksites.
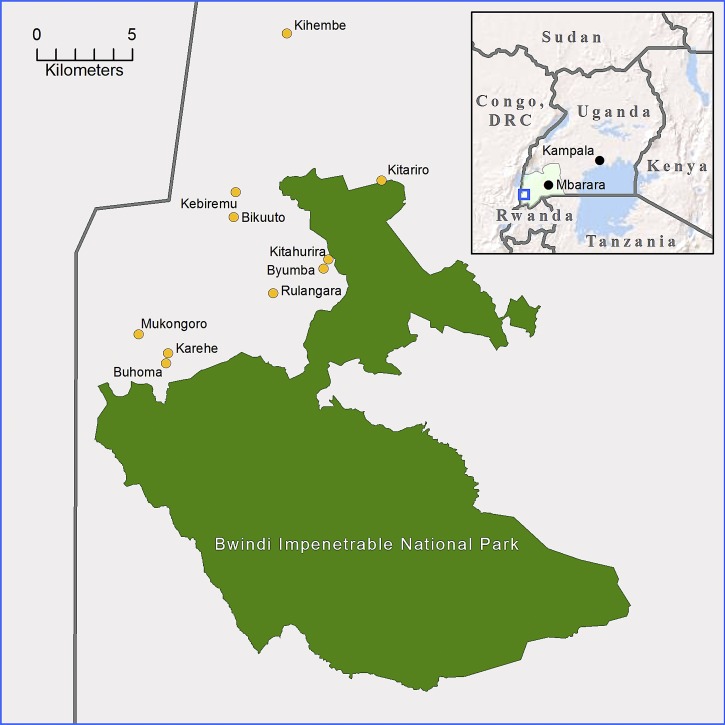
Figure 1.
Map of the study region. The locations of 10 Batwa settlements within Kanungu District, Western Region, Uganda (Inset) are shown. The study communities are situated between the Bwindi Impenetrable National Park and the border with the Democratic Republic of the Congo (DRC).
Food security assessment.
We assessed food insecurity using a modified Rukiga-language translation of the US Department of Agriculture (USDA) Food Security Supplement to the Current Population Survey (CPS).65 We used a 3-month recall period to understand participants' access to food during the preceding rainy season. Adapting the CPS survey as a metric for international settings is common practice and has been successful in developed as well as developing countries.66,67 Outcomes comprised an ordinal scale, including four levels ranging from severe food insecurity to food security.
Anthropometric assessment.
We assessed variation in acute nutritional status using individuals' relative standing within the observed distribution of age- and sex-specific mid-upper arm circumference (MUAC), which was measured on an individual's left arm halfway between the tip of the shoulder and the tip of the elbow. In undernourished populations, MUAC outperforms body mass index (BMI) as an anthropometric measure for acute nutritional status among children and adults and as a prognostic indicator for malnutrition-related outcomes.68 It has been used frequently within pygmy populations,69–71 among whom BMI and its prognostic indications have not been validated. Our analysis used relative MUAC within the study population, a technique validated in prior work with children and adults,72–75 because there is debate concerning optimal MUAC cutoff points for signifying acute malnutrition, how such cutoffs differ between women and men,76–80 and the fact that there is no evidence that non-pygmy–derived MUAC cutoffs are valid within pygmy populations. For age- and sex adjustment, we calculated mean MUAC and SDs within 3-year age- and sex-specific groupings between ages 6 and 17 years and among individuals of age 18+ years. We centered MUAC measurements on mean for age–sex class and scaled by SD within each class to produce normal (z) scores for the analysis.
Rapid diagnostic testing.
Parasitemia was inferred from positive outcome to an SD-BIOLINE rapid immunochromatographic assay detecting Histidine-rich protein II (HRP-II) antigen from P. falciparum (Standard Diagnostics, Inc., Yongin-si, Gyeonggi-do, Republic of Korea). The test additionally bound Plasmodium lactate dehydrogenase (pLDH) antigen to indicate infection by non-falciparum Plasmodia (sensitivity of 95.5%). Previous work has validated rapid diagnostic tests (RDTs) in low-prevalence settings within east Africa, including in Uganda's southwestern highlands, indicating that RDT sensitivity, specificity, and predictive values do not differ appreciably from gold standard polymerase chain reaction (PCR) procedures.81–83 Here, HRP-II RDTs offered the additional advantage of detecting low-density infections potentially missed by conventional microscopy,84,85 and their in-field implementation allowed immediate notification to parasite-positive subjects. We referred all individuals with a positive test outcome to staff nurses from Bwindi Community Hospital.
Statistical analysis.
We estimated parasitemia prevalence by dividing the number of positive test outcomes by the number of tests administered, assuming that all individuals to be at risk for infection. To examine associations between relative risk for positive RDT outcome and individual- and household-level factors within the study population, we built a multivariable log-binomial generalized linear model accounting for demographics (age, sex, and household characteristics), relative nutritional status and food security, behavioral protective measures, knowledge of malaria, and vector exposure through a modified best subsets variable-entry procedure.86 This procedure entailed an exhaustive evaluation of combinations of covariables without stepwise selection. The algorithm identified optimal model configurations for set numbers of parameters through information criterion scores.87 Tables 1 and 2 indicate the individual- and household-level variables assessed. To prevent overfitting given the small sample size (N = 365), we opted a priori to consider models with k < 10 parameters only. Before model-building, we ruled out collinearity among main effects by Spearman's rank correlation coefficient (ρ) with a cutoff of 10%. We evaluated component plus residual plots to determine appropriate functional forms of continuous and factor variables. After identifying optimal main effect terms, we evaluated potential interactions through a model-based recursive partitioning algorithm testing candidate main effect terms for joint significance.88 To avoid model overspecification and compare among fitted models with k < 10 parameters, we assessed whether incorporating additional significant and non-significant terms resulted in global model improvement through likelihood ratio tests. Because individuals' risks for infection were stratified by heterogeneous exposure to parasite-positive vectors domestically and within communities, we calculated Huber–White SEs robust to multilevel clustering by household and settlement.89,90 We considered outcomes with a type I error rate P < 0.05 to be statistically significant. We conducted analyses within the R environment (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Characteristics of the study population (individuals)
| Variables | Total n (%) | Males n (%) | Females n (%) |
|---|---|---|---|
| Age (years) | 365 (100) | 167 (100) | 198 (100) |
| 6–12 | 103 (28) | 53 (32) | 50 (25) |
| 13–23 | 92 (25) | 42 (25) | 50 (25) |
| 24–35 | 81 (22) | 30 (18) | 51 (26) |
| 36–47 | 40 (11) | 20 (12) | 20 (10) |
| 48–59 | 29 (8) | 13 (8) | 16 (8) |
| 60+ | 20 (5) | 9 (5) | 11 (6) |
| Malaria infection | 15 (4) | 11 (7) | 4 (2) |
| Protective measures | |||
| Sleeping under ITNs last night | 174 (48) | 82 (49) | 92 (47) |
| Environment (clearing brush near home, draining stagnant water) | 143 (39) | 69 (41) | 74 (37) |
| Closing windows and doors at night | 125 (34) | 57 (34) | 68 (34) |
| Daily forest exposure | |||
| Spends no time in forest during the day | 189 (53) | 89 (53) | 106 (54) |
| Spends less than one-half of the day in forest | 150 (41) | 64 (38) | 86 (43) |
| Spends about one-half of the day in forest | 14 (4) | 6 (4) | 8 (4) |
| Spends more than one-half of the day in forest | 3 (1) | 3 (2) | 0 (0) |
| Spends all day in forest | 6 (2) | 6 (4) | 0 (0) |
| Spends time at night in forest (not excluding other categories) | 4 (1) | 0 (0) | 4 (2) |
| Other daily environmental exposures | |||
| Any time in fields | 265 (73) | 112 (67) | 153 (77) |
| Any time near rivers or lakes | 236 (65) | 104 (62) | 132 (67) |
| Any time in or near animal sheds | 58 (16) | 26 (16) | 32 (16) |
| MUAC | |||
| ≤ 1 SD below mean for age and sex | 46 (13) | 22 (13) | 24 (12) |
| −0.99–0 SD below mean for age and sex | 152 (42) | 67 (40) | 85 (43) |
| 0.01–1 SD above mean for age and sex | 116 (32) | 51 (31) | 65 (33) |
| > 1 SD above mean for age and sex | 51 (14) | 27 (16) | 24 (12) |
| Knowledge of malaria | |||
| Identifying mosquito as vector | 290 (79) | 140 (84) | 150 (76) |
| Identifying ITNs as a preventative measure | 155 (43) | 73 (44) | 82 (41) |
| Identifying insecticide sprays as a preventative measure | 88 (24) | 38 (23) | 50 (25) |
Table 2.
Characteristics of the study population (households)
| Variables | Households n (%) | Individuals n (%) |
|---|---|---|
| 120 (100) | 365 (100) | |
| Malaria infection | 13 (11) | 15 (4) |
| Household food insecurity | ||
| Food security | 1 (< 1) | 1 (< 1) |
| Mild food insecurity | 8 (7) | 19 (5) |
| Moderate food insecurity | 44 (37) | 117 (32) |
| Severe food insecurity | 67 (56) | 228 (62) |
| Protective measures | ||
| Living in a household with indoor residual spraying in past 12 months | 4 (3) | 18 (5) |
| Household wealth indicators | ||
| Household owns any livestock | 27 (23) | 87 (24) |
| Household receives money from family/friends outside the community | 23 (19) | 84 (23) |
| Either household wealth indicator | 45 (38) | 146 (40) |
| Number of children living in household | ||
| 0–1 | 40 (33) | 79 (22) |
| 2–3 | 37 (31) | 97 (27) |
| 4–5 | 35 (29) | 137 (38) |
| 6–7 | 8 (7) | 52 (14) |
We limited analysis to cases among older children (> 5 years) and adults to avoid confounding caused by two factors. First, cases among individuals over 5 years old are atypical in hyper- and holoendemic areas relative to cases among children under 5 years old.55 Lacking longitudinal data on parasitemia within Kanungu in the absence of environmental interventions60 or any prior data on malaria among the Batwa elsewhere, we could not assess whether observed prevalence among children 5 years and under during January of 2013 represented typical transmission levels or was affected by exogenous factors of interest to our model; such factors could, however, be assumed to cause a high proportion of infections among individuals over 5 years old in any setting.1,91 Second, evidence exists for differential treatment-seeking behavior among individuals under and above 5 years of age within east Africa.92,93 Varied probability for prior malarial diagnosis and antimalarial receipt across ages could bias parasite prevalence estimation in a malariometric survey.
Results
Response rate.
Our sampling frame consisted of 630 individuals, among whom 475 individuals were over 5 years old. Of these individuals, we reached 434 individuals, among whom 407 individuals (94%) consented to clinical evaluation, including anthropometric measurement and finger prick. Complete household-level, individual-level, and clinical data were obtained for 365 individuals, representing a 77% overall response rate. Parasite prevalence did not differ significantly between participants who dropped out or completed the full survey (F1,409 = 0.01; P > 0.9). By Little's test for missingness completely at random, we identified no pattern to data missingness in the variables under consideration (χ2 = 59.6; degree of freedom [df] = 53; P = 0.25) and expected complete case analysis to yield unbiased results.
Outcome measures.
Parasite prevalence was 4.1% among participants over 5 years of age and 3.9% among participants of all ages; all parasite-positive cases were attributed to P. falciparum caused by HRP-II detection. The adult to child clinical ratio of 3.0 indicated unstable local transmission and generally, an absence of acquired immunity.55 We observed a mean MUAC of 24.2 cm (SD = 2.7 cm) among adult males and 23.2 cm (SD = 3.1 cm) among adult females; details of MUAC values by age and sex are available in Table 3. In the 3 months preceding the survey, most (62%) of the sample individuals lived in households experiencing severe food insecurity, whereas fewer individuals experienced moderate food insecurity (32%), mild food insecurity (5%), and food security (< 1%). Age seemed uncorrelated with food insecurity classes (ρ = −0.07). Normal score distributions for MUAC by age–sex class did not differ across food security levels (ρ = −0.08) or between severely and non-severely food-insecure individuals (ρ = −0.06), motivating separate consideration of nutrition status and food security variables. We identified −0.1 < ρ < 0.1 in pairwise comparisons across all candidate covariables presented in Tables 1 and 2, indicating that collinearity among predictors was unlikely.
Table 3.
MUAC observations
| Age/sex | Percentile | MUAC (cm) | MUAC normal score |
|---|---|---|---|
| 6–8 years | |||
| Male | 25 | 15 | −0.89 |
| Male | 50 | 17 | 0.08 |
| Male | 75 | 18 | 0.57 |
| Female | 25 | 16 | −0.55 |
| Female | 50 | 16 | −0.55 |
| Female | 75 | 18 | 0.33 |
| 9–11 years | |||
| Male | 25 | 16 | −0.78 |
| Male | 50 | 17 | −0.25 |
| Male | 75 | 18 | 0.28 |
| Female | 25 | 16.3 | −0.50 |
| Female | 50 | 17 | −0.24 |
| Female | 75 | 18.8 | 0.38 |
| 12–14 years | |||
| Male | 25 | 17 | −0.69 |
| Male | 50 | 18 | −0.27 |
| Male | 75 | 20 | 0.56 |
| Female | 25 | 20 | −0.53 |
| Female | 50 | 20.5 | −0.38 |
| Female | 75 | 22.8 | 0.33 |
| 15–17 years | |||
| Male | 25 | 19.3 | −0.85 |
| Male | 50 | 22.5 | 0.29 |
| Male | 75 | 23 | 0.46 |
| Female | 25 | 22.8 | −0.57 |
| Female | 50 | 23.5 | −0.32 |
| Female | 75 | 27.3 | 0.89 |
| 18 + years | |||
| Male | 25 | 22 | −0.83 |
| Male | 50 | 24.3 | −0.08 |
| Male | 75 | 26 | 0.68 |
| Female | 25 | 21 | −0.71 |
| Female | 50 | 23 | −0.07 |
| Female | 75 | 25 | 0.57 |
Statistical model.
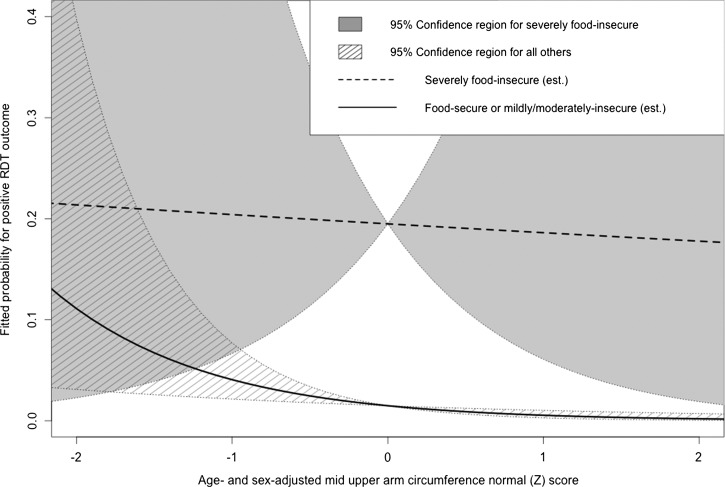
Table 4 presents the adjusted relative risk (ARR) fitted by the multivariable model as well as the unadjusted relative risk attributed to each factor in univariable regression. Being male (ARR = 3.35; 95% confidence interval [95% CI] = 1.96–5.74), having daily forest exposure (ARR = 1.80; 95% CI = 1.01–3.21), and having severe food insecurity (ARR = 13.09; 95% CI = 2.23–76.79) were significantly associated with elevated odds for positive RDT outcomes. Asset ownership, defined as living in a household that owned an animal or received financial support from family or friends outside the community, was significantly associated with decreased odds for positive RDT (ARR = 0.11; 95% CI = 0.01–0.97). Relative nutritional status, as indicated by within-sample MUAC normal score for age and sex, was significant conditional on household status as food-secure or mildly or moderately insecure. Although the relationship between positive RDT outcome and relative nutritional status was non-significant for severely food-insecure participants (ARR = 2.61, 95% CI = 0.85–7.99), participants who were not severely food-insecure likewise had decreasing odds for positive RDT outcomes with increasing relative nutritional status (ARR = 0.37; 95% CI = 0.19–0.69). Figure 2 depicts this relationship graphically, indicating that the 95% confidence bounds of the fitted probability for parasitemia were non-overlapping between severely food-insecure individuals at most MUAC normal scores.
Table 4.
Results of the log-binomial generalized linear model
| RR (95% CI) | ||
|---|---|---|
| Univariable (unadjusted) RR | Multivariable ARR | |
| Age (years) | 1.01 (0.97–1.05) | 1.01 (0.97–1.04) |
| Male sex | 3.26* (2.02–5.27) | 3.35* (1.96–5.74) |
| Daily forest exposure | 2.20 (0.87–5.53) | 1.80† (1.01–3.21) |
| Asset ownership | 0.11† (0.01–0.98) | 0.11† (0.01–0.97) |
| Severe food insecurity | 8.41‡ (1.77–39.98) | 13.09‡ (2.23–76.79) |
| Age/sex-adjusted MUAC z score | 0.83 (0.43–1.61) | |
| Not severely food-insecure | 0.37‡ (0.19–0.69) | |
| Severely food-insecure | 2.61 (0.85–7.99) | |
P < 0.001.
P < 0.05.
P < 0.01.
Figure 2.
Conditional effects of relative undernourishment by status as severely food-insecure. The fitted probability of a positive RDT outcome is shown for a 25-year-old man who enters the forest regularly and does not own animals or receive outside financial support with respect to relative nutritional status under the scenarios where the man is or is not severely food-insecure. The 95% confidence regions are distinct at z scores greater than −1 SD from the mean. Est = estimated.
Variables rejected because of statistical non-significance of additive main effects included sleeping under an insecticide-treated bed net (ITN) the night before the survey, knowledge about malaria and its prevention, time spent in environments other than the forest, knowledge of or reported engagement in domestic and peridomestic malaria prevention efforts, recent travel outside home settlements, and household crowding (measured as the number of children occupying a house). Because only four households reported indoor residual spraying with insecticide within the last 12 months, this variable was not considered. Despite statistical non-significance, retaining age served to reduce noise within component plus residual plots, decrease global deviation (thus, improving goodness of fit), and reduce the largest studentized residuals. Consequently, we force-included age as a continuous variable in candidate models. We identified no significant outlying observations in the model controlling for age (Bonferroni-adjusted P > 0.1). An overall χ2 deviance test comparing the fitted model with a saturated model with k = n parameters indicated that deviance for the fitted model was not significantly larger than for the saturated model. Accordingly, we expected a maximally fitted model to offer no significant improvement relative to the present model in accounting for variation in RDT outcomes.
Discussion
Severe food insecurity may be a risk factor for P. falciparum parasitemia among older children and adult Batwa pygmies in Kanungu, Uganda during the biannual high-transmission season. Poor relative acute nutritional status, which was indicated by MUAC z score for age and sex, was associated with risk for parasitemia among individuals living in a household that did not experience severe food insecurity in the preceding season. Because we controlled for a protective effect from asset ownership, it is unlikely that severe food insecurity effects owed solely to socioeconomic confounding. Relative undernourishment was not associated with elevated risk for parasitemia among individuals in severely food-insecure households.
The observed 4.1% parasite prevalence in individuals over 5 years old falls within range of prior epidemiologic studies in southwestern Uganda61 and above the lower measurement reliability limit of malariometric surveys.91,94 Reference MUAC measurements among ethnically similar Twa group pygmies are available from surveys conducted in the early 1980s among forest-dwellers in the Democratic Republic of the Congo; these measurements indicated 23.3 cm mean MUAC among adult males in the Kivu region bordering Kanungu during an unspecified season95 and 22.6 cm mean MUAC among adult females after the rainy season, similar to the timing of our survey.96 These historical measurements fell roughly within the second quartiles of observed distributions in our survey (Table 3). Such marginal gains in MUAC among the Batwa evidenced little progress in nutritional success, despite rising life expectancies in the Ugandan and Congolese general populations over the intervening decades.97
Normal scores from observed MUAC distributions within the sample here allowed us to assess how risk for positive RDT outcome varied among Batwa individuals of differing acute nutritional status. Using international standards derived from non-pygmy populations for MUAC, cutoffs would likely have led to overestimating malnutrition prevalence within our sample, because growth trajectories differ between pygmies and non-pygmies98; among pygmies, average MUAC is lower than international standards across sexes and all ages greater than 5 years old.70,71 Without knowing how Batwa ethnicity and inadequate nutrient intake respectively contributed to individuals' MUACs, we lacked a basis for interpreting observed MUAC measurements in the context of international cutoff values and accordingly, identifying wasting in an absolute sense.
In previous parasitological surveys, associations between malnutrition and risk for parasitemia seemed to differ from those associations involving risk for clinical illness. A study from a P. falciparum-endemic region of Colombia suggested elevated risk for parasitemia in acutely undernourished individuals, showing log-linear increases in parasite density with decreasing BMI.99 Cross-sectional surveys in Kenya identified associations between parasitemia and chronic undernutrition among children under 36 months but did not identify significant associations between parasitemia and wasting.100 Clinical trials in Uganda have additionally shown elevated risk for recurrent parasitemia after clearance in chronically undernourished children (however, without corresponding risk elevation among the acutely undernourished).101
As measured here, a household's experience of food insecurity involved reducing portion sizes or skipping meals, reducing dietary quality and diversity, and worrying about running out of food in the 3 months before the survey.65 The metric differentiated severely food-insecure from other food-insecure households according to experienced hunger rather than reduced dietary quality and diversity, which had greater relevance for distinguishing between food-secure and -insecure households. Experiencing acute hunger indicated that individuals in severely food-insecure households likely lacked sufficient caloric intake and may have been at risk for protein-energy malnutrition102; given the observed prevalence of lesser food-insecure states, nearly all individuals surveyed were likely at risk for micronutrient deficiencies associated with low-quality, low-variety diet.103
Elsewhere in Uganda, household- and individual-level risk factors associated with low MUAC have been shown to differ from those factors associated with other malnutrition outcomes.98 Accordingly, an interaction effect between MUAC and food insecurity status may indicate that living in a severely food-insecure household and having low MUAC here acted as a proxy for differing nutritional risks and physiological outcomes and had distinct causal implications for risk for parasitemia. Because of its sensitivity to decreasing fat or muscle stores, low MUAC may have acted as a measure of near-term inadequate nutritional intake, conveying effects of acute undernutrition on susceptibility to P. falciparum.37,80,99,104,105 We do not know if households' food-insecurity experiences recalled during the 3 months before surveying were exceptional, seasonally cyclic, or chronic in nature. In the event that reported experiences were not atypical for Batwa households, the severe food-insecurity variable may have captured risk for parasitemia effects associated with chronic undernutrition. Conditional significance may indicate that increased risk for parasitemia associated with chronic undernutrition (for instance, because of severe food insecurity) outweighed effects owing to acute undernutrition, which was suggested in other cross-sectional parasitological studies.100,101 Alternatively, low MUAC for age and sex may have been attributed to wasting from chronic medical conditions, including malarial anemia or undiagnosed human immunodeficiency virus (HIV) infection106; although a cohort study among non-Batwa persons in the region indicated 7.7% HIV-1 seroprevalence in 2005,107 only one person within our survey self-reported HIV-positive serostatus and receipt of antiretroviral treatment. Contributions of unobserved factors, including HIV, to MUAC reductions would presumably be less statistically apparent in individuals from severely food-insecure households than the rest of the sample, because food shortages would likely cause a high relative proportion of all-cause wasting in the most severely food-insecure households.
In the event that downstream physiological effects of severely constrained food access may have been similar to those effects seen among persons with low MUAC,108,109 the binary severe food-insecurity variable may have served as a catchall variable representing such immune effects. Small sample size and rareness of positive RDT outcomes indicate that our study may be at risk for a spurious finding of this nature. In such an event, variation in MUAC may have achieved conditional significance, because it contributed meaningfully to the model only among individuals in households for which the severe food insecurity indicator did not apply.
Potential protective effects of iron and folic acid deficiencies against clinical illness confound implications of a malnutrition–parasitemia risk association for reducing malaria morbidity and mortality. A recent meta-analysis31 indicated that iron and iron plus folic acid supplementation may be unsafe in areas of intense malaria transmission unless accompanied by expansion of disease surveillance and treatment services. Likewise, the World Health Organization has advised against implementing such supplementation in malaria-endemic settings.110 Certain micronutrient combinations seem in vitro to bolster the cytokine-mediated immune response to Plasmodia,111 and supplementation with zinc, vitamin A, or both has proven varyingly efficacious against clinical illness in field trials.112–118 These trials have nonetheless produced minimal evidence that supplementation protects against parasitemia.
Individual exposure-related variables may have had particular significance in the present study because of malaria ecology and transmission dynamics in Kanungu. Low parasite prevalence, a lack of parasitological evidence for premunition, and a high adult to child case ratio here suggested that naturally acquired immunity was negligible within the population.13 In hyper- and holoendemic settings, where exposures are ubiquitous and most often indoors, young children have elevated risk for P. falciparum infection because of their lack of acquired immunity, whereas men and non-pregnant women typically do not differ in risk for infection.119 Elevated risk among men and among individuals who regularly entered the forest here suggested outdoor vector exposures, particularly in recently deforested landscapes, where microclimatic factors may have led to elevated mosquito vectorial capacity.120 Elevated risk among men and persons with outdoor exposures typically indicates that malaria is an occupational disease in populations lacking premunition, where risk relates to sexual division of labor.16,17 Non-significant protection from sleeping under ITNs (relative risk [RR]= 0.66; 95% CI = 0.34–1.25), moreover, suggested that transmission was unlikely to occur indoors.121 A significant effect from crowding, which was indicated by the number of children within a household, would likewise typically suggest domestic or peridomestic exposure18; here, this variable was non-significant (univariable RR = 1.13; 95% CI = 0.82–1.55). Although unknown vector distribution in the study region prevented us from interpreting these outcomes in the context of Anopheles ecology, recent work elsewhere within the east African highlands suggests a shift to exophagic and exophilic species compositions relative to historical observations.122,123 Throughout Africa, such changes to vector ecology have challenged domicile-based interventions, such as ITN distribution and indoor residual spraying.18,124
Our cross-sectional study design did not facilitate inference into the causal nature of observed nutrition and food security associations with P. falciparum parasitemia. Reductions in MUAC associated with acute malnutrition, for instance, may have occurred subsequent to infection, particularly if coaffected individuals suffered from chronic parasitemia or malarial anemia. In the latter event, reverse causality could dictate that infection, in fact, contributed to low MUAC. Although associations with food insecurity and relative undernourishment may suggest host nutrition as a factor in risk for parasitemia, our lack of information about associations with specific nutrient deficiencies limited mechanistic inference in this relationship. Also, our sample was small and included only 15 positive RDT outcomes. We lacked statistical power to reliably estimate small effect sizes, for instance, from sleeping under ITNs, and such findings may have been subject to type II error. High prevalence of severe food insecurity likewise indicated that our outcomes may have lacked specificity; although psychometric properties of the instrument are unlikely to reduce its validity in underdeveloped and resource-poor contexts,67,125,126 the predominance of severe food insecurity among observed states indicated the population's food-insecurity experiences were among the most severe that the USDA survey metric could identify. Taken together with the Batwa's exceptionally low life expectancy and historic socioeconomic marginality, they may indicate that outcomes from the present study are not sufficiently generalizable for informing regional intervention priorities.
Our findings motivate future longitudinal studies suited to identifying temporal and causal associations among the variables assessed here, which may account for immunological factors underlying nutritional associations with parasitemia. Two important objectives for understanding present outcomes will be (1) characterizing whether risk for food insecurity varies in phase with risk for P. falciparum infection, particularly in settings where both oscillate intra-annually, and (2) establishing whether malnutrition-associated wasting precedes or follows infection among individuals who are not severely food-secure. Characterizing livelihoods among individuals who are severely food-insecure may likewise identify behaviors or exposures associated with risk for P. falciparum, accounting for non-nutritional factors explaining why persons in severely food-insecure households here had elevated risk for infection. Such research could extend current evidence regarding the safety or suitability of food security and nutritional interventions in P. falciparum-endemic settings and address differential implications of supplementation for parasitemia and malaria morbidity.
ACKNOWLEDGMENTS
The authors thank Sabastian Twesigomwe and the Batwa Development Programme; Hubert Nkabura and the Bwindi Community Hospital; Emmanuel Eloku and the District of Kanungu; Sam Okware and the Uganda National Health Research Organization; research assistants Jamen Kasumba, Martin Kigozi, and Fortunate Twembabaze; and our Batwa and non-Batwa surveyors for their assistance in conducting this work.
Footnotes
Indigenous Health Adaptation to Climate Change Research Team 2013: James D. Ford (McGill University); Victoria L. Edge (University of Guelph); Alejandro Llanos (Universidad Peruana Cayetano Heredia).
Financial support: As part of the Indigenous Health Adaptation to Climate Change Project (http://www.ihacc.ca), we received funding from the International Development Research Centre, the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Social Sciences and Humanities Research Council of Canada, the Public Health Agency of Canada (SSHRC), and McGill University. S.L. H. received financial support from the Vanier Graduate Scholarship (CIHR).
Authors' addresses: Joseph A. Lewnard, Lea Berrang-Ford, Kaitlin A. Patterson, and Blánaid Donnelly, Department of Geography, McGill University, Montreal, Quebec, Canada, E-mails: joseph.lewnard@mail.mcgill.ca, lea.berrangford@mcgill.ca, kaitlin.patterson@mail.mcgill.ca, and blanaid.donelly@mail.mcgill.ca. Shuaib Lwasa, Department of Geography, Makerere University, Kampala, Uganda, E-mail: shuaiblwasa@gmail.com. Didacus Bambaiha Namanya, Ugandan Ministry of Health, Kampala, Uganda, E-mail: didamanya@yahoo.com. Manisha A. Kulkarni, Department of Epidemiology and Community Medicine, University of Ottawa, Ontario, Canada, E-mail: manisha.kulkarniwoodstock@gmail.com. Sherilee L. Harper, Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada, E-mail: harpers@uoguelph.ca. Nicholas H. Ogden, Zoonoses Division, Public Health Agency of Canada, Saint-Hyacinthe, Quebec, Canada, E-mail: Nicholas.ogden@phac-aspc.gc.ca. Cesar P. Carcamo, School of Public Health and Administration, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mail: cesarcarcamo@yahoo.es.
References
- 1.Shankar AH. Nutrition and Health in Developing Countries. Totowa, NJ: Springer; 2008. Malaria and nutrition; pp. 229–274. [Google Scholar]
- 2.Crawley J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: from evidence to action. Am J Trop Med Hyg. 2004;71:25–34. [PubMed] [Google Scholar]
- 3.Shankar AH. Nutritional modulation of malaria morbidity and mortality. J Infect Dis. 2000;182:S37–S53. doi: 10.1086/315906. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S–635S. doi: 10.1093/jn/131.2.616S. [DOI] [PubMed] [Google Scholar]
- 5.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay S, Martens W. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76:33. [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual M, Ahumada J, Chaves L, Rodo X, Bouma M. Malaria resurgence in the East African highlands: temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small J, Goetz SJ, Hay SI. Climatic suitability for malaria transmission in Africa, 1911–1995. Proc Natl Acad Sci USA. 2003;100:15341–15345. doi: 10.1073/pnas.2236969100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves PM, Osgood DE, Thomson MC, Sereke K, Araia A, Zerom M, Ceccato P, Bell M, Del Corral J, Ghebreselassie S, Brantly EP, Ghebremeskel T. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998–2003. Trop Med Int Health. 2008;13:218–228. doi: 10.1111/j.1365-3156.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 10.Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Myers MF, Snow RW. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Lieshout M, Kovats R, Livermore M, Martens P. Climate change and malaria: analysis of the SRES climate and socio-economic scenarios. Glob Environ Change. 2004;14:87–99. [Google Scholar]
- 12.Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Trop Med Int Health. 2000;5:263–274. doi: 10.1046/j.1365-3156.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 13.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drakeley C, Corran P, Coleman P, Tongren J, McDonald S, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya W. Estimating medium-and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 16.Valle D, Clark JS, Zhao K. Enhanced understanding of infectious diseases by fusing multiple datasets: a case study on malaria in the Western Brazilian Amazon region. PLoS ONE. 2011;6:e27462. doi: 10.1371/journal.pone.0027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsuragawa TH, Gil LHS, Tada MS, Silva Ad A, Costa JDAN, da Silva Araújo M, Escobar AL, da Silva LHP. The dynamics of transmission and spatial distribution of malaria in riverside areas of Porto Velho, Rondônia, in the Amazon region of Brazil. PLoS ONE. 2010;5:e9245. doi: 10.1371/journal.pone.0009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pates H, Curtis C. Mosquito behavior and vector control. Annu Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Escamilla R, Dessalines M, Finnigan M, Pachón H, Hromi-Fiedler A, Gupta N. Household food insecurity is associated with childhood malaria in rural Haiti. J Nutr. 2009;139:2132–2138. doi: 10.3945/jn.109.108852. [DOI] [PubMed] [Google Scholar]
- 20.Tshikuka JG, Gray DK, Scott M, Olela KN. Relationship of childhood protein—energy malnutrition and parasite infections in an urban African setting. Trop Med Int Health. 1997;2:374–382. doi: 10.1111/j.1365-3156.1997.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 21.El Samani FZ, Willett W, Ware J. Nutritional and socio-demographic risk indicators of malaria in children under five: a cross-sectional study in a Sudanese rural community. J Trop Med Hyg. 1987;90:69–78. [PubMed] [Google Scholar]
- 22.Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, Williams TN, Mohammed S, Osier F, Kinyanjui S, Fegan G. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis. 2009;49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gera T, Sachdev H. Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ. 2002;325:1142. doi: 10.1136/bmj.325.7373.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Bäck R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–444. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 25.Jonker FA, Calis JC, van Hensbroek MB, Phiri K, Geskus RB, Brabin BJ, Leenstra T. Iron status predicts malaria risk in malawian preschool children. PLoS ONE. 2012;7:e42670. doi: 10.1371/journal.pone.0042670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 27.Kiszewski AE, Teklehaimanot A. A review of the clinical and epidemiologic burdens of epidemic malaria. Am J Trop Med Hyg. 2004;71:128–135. [PubMed] [Google Scholar]
- 28.Awah NW, Kaneko A. Iron deficiency and severe Plasmodium falciparum malaria. Clin Infect Dis. 2012;54:1145–1147. doi: 10.1093/cid/cis020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis. 2012;54:1137–1144. doi: 10.1093/cid/cis010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth DE, Black RE, Ojukwu JU, Okebe JU, Yahav D, Paul M. Commentary on ‘Oral iron supplementation for preventing or treating anaemia among children in malaria-endemic areas’ with a response from the review authors. Evid Based Child Health. 2010;5:1186–1188. [Google Scholar]
- 31.Okebe JU, Yahav D, Shbita R, Paul M. Oral iron supplements for children in malaria-endemic areas. Cochrane Database Syst Rev. 2011:10. doi: 10.1002/14651858.CD006589.pub3. [DOI] [PubMed] [Google Scholar]
- 32.Berger J, Dyck JL, Galan P, Aplogan A, Schneider D, Traissac P, Hercberg S. Effect of daily iron supplementation on iron status, cell-mediated immunity, and incidence of infections in 6–36 month old Togolese children. Eur J Clin Nutr. 2000;54:29–35. doi: 10.1038/sj.ejcn.1600888. [DOI] [PubMed] [Google Scholar]
- 33.Hunt JM. The potential impact of reducing global malnutrition on poverty reduction and economic development. Asia Pac J Clin Nutr. 2005;14:10–38. [Google Scholar]
- 34.Randolph T, Schelling E, Grace D, Nicholson CF, Leroy J, Cole D, Demment M, Omore A, Zinsstag J, Ruel M. Invited review: role of livestock in human nutrition and health for poverty reduction in developing countries. J Anim Sci. 2007;85:2788–2800. doi: 10.2527/jas.2007-0467. [DOI] [PubMed] [Google Scholar]
- 35.Atinmo T, Mirmiran P, Oyewole OE, Belahsen R, Serra‐Majem L. Breaking the poverty/malnutrition cycle in Africa and the Middle East. Nutr Rev. 2009;67:S40–S46. doi: 10.1111/j.1753-4887.2009.00158.x. [DOI] [PubMed] [Google Scholar]
- 36.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 37.Barrett CB. Measuring food insecurity. Science. 2010;327:825–828. doi: 10.1126/science.1182768. [DOI] [PubMed] [Google Scholar]
- 38.Thompson B, Cohen MJ, Meerman J. World Food Insecurity and Malnutrition: Scope, Trends, Causes and Consequences. The Impact of Climate Change and Bioenergy on Nutrition. New York, NY: Springer; 2012. pp. 21–41. [Google Scholar]
- 39.Pinstrup-Andersen P. The African Food System and Its Interaction with Human Health and Nutrition. Ithaca, NY: Cornell University Press; 2010. [Google Scholar]
- 40.Washington R, Downing TE. Seasonal forecasting of African rainfall: prediction, responses and household food security. Geogr J. 1999;165:255–274. [Google Scholar]
- 41.Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- 42.Nord M, Romig K. Hunger in the summer: seasonal food insecurity and the National School Lunch and Summer Food Service programs. J Child Poverty. 2006;12:141–158. [Google Scholar]
- 43.Maxwell DG. Measuring food insecurity: the frequency and severity of “coping strategies.”. Food Policy. 1996;21:291–303. [Google Scholar]
- 44.Skalicky A, Meyers AF, Adams WG, Yang Z, Frank DA. Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern Child Health J. 2006;10:177–185. doi: 10.1007/s10995-005-0036-0. [DOI] [PubMed] [Google Scholar]
- 45.Tontisirin K, Nantel G, Bhattacharjee L. Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. Proc Nutr Soc. 2002;61:243–250. doi: 10.1079/PNS2002155. [DOI] [PubMed] [Google Scholar]
- 46.Oldewage-Theron WH, Dicks EG, Napier CE. Poverty, household food insecurity and nutrition: coping strategies in an informal settlement in the Vaal Triangle, South Africa. Public Health. 2006;120:795–804. doi: 10.1016/j.puhe.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Sorsdahl K, Slopen N, Siefert K, Seedat S, Stein DJ, Williams DR. Household food insufficiency and mental health in South Africa. J Epidemiol Community Health. 2011;65:426–431. doi: 10.1136/jech.2009.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadley C, Tegegn A, Tessema F, Asefa M, Galea S. Parental symptoms of common mental disorders and children's social, motor, and language development in sub-Saharan Africa. Ann Hum Biol. 2008;35:259–275. doi: 10.1080/03014460802043624. [DOI] [PubMed] [Google Scholar]
- 49.Weinreb L, Wehler C, Perloff J, Scott R, Hosmer D, Sagor L, Gundersen C. Hunger: its impact on children's health and mental health. Pediatrics. 2002;110:e41. doi: 10.1542/peds.110.4.e41. [DOI] [PubMed] [Google Scholar]
- 50.Borghetti P, Saleri R, Mocchegiani E, Corradi A, Martelli P. Infection, immunity and the neuroendocrine response. Vet Immunol Immunopathol. 2009;130:141–162. doi: 10.1016/j.vetimm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDade TW. The ecologies of human immune function. Annu Rev Anthropol. 2005;34:495–521. [Google Scholar]
- 52.Uscátegui Peñuela RM, Pérez Tamayo EM, Corrales Agudelo LV, Correa Botero A, Estrada Restrepo A, Carmona Fonseca J. Relationship between malaria, malnutrition, food insecurity and low socio-economical conditions in children of Turbo, Colombia. Perspectivas en Nutrición Humana. 2009;11:153–164. [Google Scholar]
- 53.Rodríguez JCP, Uribe GÁ, Araújo RM, Narváez PC, Valencia SH. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106:114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisele TP, Keating J, Bennett A, Londono B, Johnson D, Lafontant C, Krogstad DJ. Prevalence of Plasmodium falciparum infection in rainy season, Artibonite Valley, Haiti, 2006. Emerg Infect Dis. 2007;13:1494. doi: 10.3201/eid1310.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, Snow RW, Hay S, Noor A, Simba M. Clinical epidemiology of malaria in the highlands of western Kenya. Emerg Infect Dis. 2002;8:543. doi: 10.3201/eid0806.010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berrang-Ford L, Dingle K, Ford JD, Lee C, Lwasa S, Namanya DB, Henderson J, Llanos A, Carcamo C, Edge V. Vulnerability of indigenous health to climate change: a case study of Uganda's Batwa Pygmies. Soc Sci Med. 2012;75:1067–1077. doi: 10.1016/j.socscimed.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Ford JD, Vanderbilt W, Berrang-Ford L. Authorship in IPCC AR5 and its implications for content: climate change and Indigenous populations in WGII. Clim Change. 2012;113:201–213. doi: 10.1007/s10584-011-0350-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ford JD. Indigenous health and climate change. Am J Public Health. 2012;102:1260–1266. doi: 10.2105/AJPH.2012.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearce T, Ford JD, Caron A, Kudlak BP. Climate change adaptation planning in remote, resource-dependent communities: an Arctic example. Reg Environ Change. 2012;12:825–837. [Google Scholar]
- 60.Bukirwa H, Yau V, Kigozi R, Filler S, Quick L, Lugemwa M, Dissanayake G, Kamya M, Wabwire-Mangen F, Dorsey G. Short report: assessing the impact of indoor residual spraying on malaria morbidity using a sentinel site surveillance system in western Uganda. Am J Trop Med Hyg. 2009;81:611–614. doi: 10.4269/ajtmh.2009.09-0126. [DOI] [PubMed] [Google Scholar]
- 61.Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–195. doi: 10.1016/j.actatropica.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherman M, Berrang-Ford L, Ford J, Lardeau M-P, Hofmeijer I, Cortijo CZ. Balancing indigenous principles and institutional research guidelines for informed consent: a case study from the Peruvian Amazon. Am J Bioeth Primary Res. 2012;3:53–68. [Google Scholar]
- 63.Pearce TD, Ford JD, Laidler GJ, Smit B, Duerden F, Allarut M, Andrachuk M, Baryluk S, Dialla A, Elee P. Community collaboration and climate change research in the Canadian Arctic. Polar Res. 2009;28:10–27. [Google Scholar]
- 64.Bwindi Community Hospital Batwa and Byumba. 2013. http://www.bwindihospital.com Available at. Accessed May 15, 2013.
- 65.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security. Alexandria, VA: Department of Agriculture Food and Nutrition Service; 2000. [Google Scholar]
- 66.Radimer KL. Measurement of household food security in the USA and other industrialised countries. Public Health Nutr. 2002;5:859–864. doi: 10.1079/PHN2002385. [DOI] [PubMed] [Google Scholar]
- 67.Melgar-Quinonez HR, Nord M, Perez-Escamilla R, Segall-Correa AM. Psychometric properties of a modified US-household food security survey module in Campinas, Brazil. Eur J Clin Nutr. 2007;62:665–673. doi: 10.1038/sj.ejcn.1602760. [DOI] [PubMed] [Google Scholar]
- 68.Powell-Tuck J, Hennessy EM. A comparison of mid upper arm circumference, body mass index and weight loss as indices of undernutrition in acutely hospitalized patients. Clin Nutr. 2003;22:307–312. doi: 10.1016/s0261-5614(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 69.Jenike MR. Variation in body fat and muscle mass: responses to seasonal hunger among tropical horticulturists, Zaire. Ecol Food Nutr. 1995;34:227–249. [Google Scholar]
- 70.De Souza RG. Body size and growth: the significance of chronic malnutrition among the Casiguran Agta. Ann Hum Biol. 2006;33:604–619. doi: 10.1080/03014460601062759. [DOI] [PubMed] [Google Scholar]
- 71.Adhikari A, Sen A, Brumbaugh RC, Schwartz J. Altered growth patterns of a mountain Ok population of Papua New Guinea over 25 years of change. Am J Hum Biol. 2011;23:325–332. doi: 10.1002/ajhb.21134. [DOI] [PubMed] [Google Scholar]
- 72.Bisai S, Bose K. Undernutrition in the Kora Mudi tribal population, West Bengal, India: a comparison of body mass index and mid-upper-arm circumference. Food Nutr Bull. 2009;30:63–67. doi: 10.1177/156482650903000106. [DOI] [PubMed] [Google Scholar]
- 73.Benefice E, Malina R. Body size, body composition and motor performances of mild-to-moderately undernourished Senegalese children. Ann Hum Biol. 1996;23:307–321. doi: 10.1080/03014469600004542. [DOI] [PubMed] [Google Scholar]
- 74.Muthayya S, Kurpad AV, Duggan CP, Bosch RJ, Dwarkanath P, Mhaskar A, Mhaskar R, Thomas A, Vaz M, Bhat S. Low maternal vitamin B12 status is associated with intrauterine growth retardation in urban South Indians. Eur J Clin Nutr. 2006;60:791–801. doi: 10.1038/sj.ejcn.1602383. [DOI] [PubMed] [Google Scholar]
- 75.Gernaat HB, Dechering WH, Voorhoeve HW. Absolute values or Z scores of mid-upper arm circumference to identify wasting? Evaluation in a community as well as a clinical sample of under fives from Nchelenge, Zambia. J Trop Pediatr. 1996;42:27–33. doi: 10.1093/tropej/42.1.27. [DOI] [PubMed] [Google Scholar]
- 76.Rodrigues VC, Rao RS, Lena A. Utility of arm circumference as a screening instrument to identify women at nutritional risk. Trop Doct. 1994;24:164. doi: 10.1177/004947559402400408. [DOI] [PubMed] [Google Scholar]
- 77.Hakewill PA, Moren A. Monitoring and evaluation of relief programmes. Trop Doct. 1991;21:24–28. doi: 10.1177/00494755910210S106. [DOI] [PubMed] [Google Scholar]
- 78.Olukoya AA. Identification of underweight women by measurement of the arm circumference. Int J Gynaecol Obstet. 1990;31:231–235. doi: 10.1016/0020-7292(90)91016-j. [DOI] [PubMed] [Google Scholar]
- 79.Collins S. Using middle upper arm circumference to assess severe adult malnutrition during famine. JAMA. 1996;276:391–395. doi: 10.1001/jama.1996.03540050051023. [DOI] [PubMed] [Google Scholar]
- 80.James WP, Mascie-Taylor GC, Norgan NG, Bistrian BR, Shetty PS, Ferro-Luzzi A. The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. Eur J Clin Nutr. 1994;48:883. [PubMed] [Google Scholar]
- 81.Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, Rapuoda B, Greenwood B, Cox J. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202. doi: 10.1186/1475-2875-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–518. doi: 10.1086/526502. [DOI] [PubMed] [Google Scholar]
- 83.Nicastri E, Bevilacqua N, Schepisi MS, Paglia MG, Meschi S, Ame SM, Mohamed JA, Mangi S, Fumakule R, Di Caro A. Accuracy of malaria diagnosis by microscopy, rapid diagnostic test, and PCR methods and evidence of antimalarial overprescription in non-severe febrile patients in two Tanzanian hospitals. Am J Trop Med Hyg. 2009;80:712–717. [PubMed] [Google Scholar]
- 84.Bell DR, Wilson DW, Martin LB. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am J Trop Med Hyg. 2005;73:199–203. [PubMed] [Google Scholar]
- 85.Harris I, Sharrock WW, Bain LM, Gray K-A, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson M-L. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:29. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berk KN. Comparing subset regression procedures. Technometrics. 1978;20:1–6. [Google Scholar]
- 87.Calcagno V, de Mazancourt C. glmulti: an R package for easy automated model selection with (generalized) linear models. J Stat Softw. 2010;34:29. [Google Scholar]
- 88.Zeileis A, Hothorn T, Hornik K. Model-based recursive partitioning. J Comput Graph Stat. 2008;17:492–514. [Google Scholar]
- 89.Maas CJ, Hox JJ. Robustness issues in multilevel regression analysis. Stat Neerl. 2004;58:127–137. [Google Scholar]
- 90.Petersen MA. Estimating standard errors in finance panel data sets: Comparing approaches. Rev Financ Stud. 2009;22:435–480. [Google Scholar]
- 91.Hay SI, Smith DL, Snow RW. Measuring malaria endemicity from intense to interrupted transmission. Lancet Infect Dis. 2008;8:369–378. doi: 10.1016/S1473-3099(08)70069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guyatt HL, Snow RW. The management of fevers in Kenyan children and adults in an area of seasonal malaria transmission. Trans R Soc Trop Med Hyg. 2004;98:111–115. doi: 10.1016/s0035-9203(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 93.Filmer D. Fever and its treatment among the more and less poor in sub-Saharan Africa. Health Policy Plan. 2005;20:337–346. doi: 10.1093/heapol/czi043. [DOI] [PubMed] [Google Scholar]
- 94.Yekutiel P. Problems of epidemiology in malaria eradication. Bull World Health Organ. 1960;22:669. [PMC free article] [PubMed] [Google Scholar]
- 95.Ghesquiere J, Karvonen M. Some anthropometric and functional dimensions of the pygmy (Kivu Twa) Ann Hum Biol. 1981;8:119–134. doi: 10.1080/03014468100004861. [DOI] [PubMed] [Google Scholar]
- 96.Pagezy H. Seasonal hunger as experienced by the Oto and the Twa women of a Ntomba village in the equatorial forest (Lake Tumba, Zaire) Ecol Food Nutr. 1984;15:13–27. [Google Scholar]
- 97.Mathers CD, Sadana R, Salomon JA, Murray CJ, Lopez AD. Healthy life expectancy in 191 countries, 1999. Lancet. 2001;357:1685–1691. doi: 10.1016/S0140-6736(00)04824-8. [DOI] [PubMed] [Google Scholar]
- 98.Kikafunda JK, Walker AF, Collett D, Tumwine JK. Risk factors for early childhood malnutrition in Uganda. Pediatrics. 1998;104:e45. doi: 10.1542/peds.102.4.e45. [DOI] [PubMed] [Google Scholar]
- 99.Caicedo O, Villamor E, Forero Y, Ziade J, Perez P, Quinones F, Arevalo-Herrera M, Herrera S. Relation between vitamin B12 and folate status, and hemoglobin concentration and parasitemia during acute malaria infections in Colombia. Acta Trop. 2010;114:17–21. doi: 10.1016/j.actatropica.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedman JF, Kwena AM, Mirel LB, Kariuki SK, Terlouw DJ, Phillips-Howard PA, Hawley WA, Nahlen BL, Ping Shi Y, Ter Kuile FO. Malaria and nutritional status among pre-school children: results from cross-sectional surveys in western Kenya. Am J Trop Med Hyg. 2005;73:698–704. [PubMed] [Google Scholar]
- 101.Verret WJ, Arinaitwe E, Wanzira H, Bigira V, Kakuru A, Kamya M, Tappero JW, Sandison T, Dorsey G. Effect of nutritional status on response to treatment with artemisinin-based combination therapy in young Ugandan children with malaria. Antimicrob Agents Chemother. 2011;55:2629–2635. doi: 10.1128/AAC.01727-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davidenko O, Darcel N, Fromentin G, Tomé D. Control of protein and energy intake—brain mechanisms. Eur J Clin Nutr. 2013;67:455–461. doi: 10.1038/ejcn.2013.73. [DOI] [PubMed] [Google Scholar]
- 103.Steyn NP, Nel JH, Nantel G, Kennedy G, Labadarios D. Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 2006;9:644–650. doi: 10.1079/phn2005912. [DOI] [PubMed] [Google Scholar]
- 104.Schaible UE, Stefan HE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garenne M, Willie D, Maire B, Fontaine O, Eeckels R, Briend A, van den Broeck J. Incidence and duration of severe wasting in two African populations. Public Health Nutr. 2009;12:1974–1982. doi: 10.1017/S1368980009004972. [DOI] [PubMed] [Google Scholar]
- 106.Villamor E, Misegades L, Fataki MR, Mbise RL, Fawzi WW. Child mortality in relation to HIV infection, nutritional status, and socio-economic background. Int J Epidemiol. 2005;34:61–68. doi: 10.1093/ije/dyh378. [DOI] [PubMed] [Google Scholar]
- 107.Shafer LA, Biraro S, Nakiyingi-Miiro J, Kamali A, Ssematimba D, Ouma J, Ojwiya A, Hughes P, Van der Paal L, Whitworth J, Opio A, Grosskurth H. HIV prevalence and incidence are no longer falling in southwest Uganda: evidence from a rural population cohort 1989–2005. AIDS. 2008;22:1641–1649. doi: 10.1097/QAD.0b013e32830a7502. [DOI] [PubMed] [Google Scholar]
- 108.Cook JT, Frank DA, Berkowitz C, Black MM, Casey PH, Cutts DB, Meyers AF, Zaldivar N, Skalicky A, Levenson S, Heeren T, Nord M. Food insecurity is associated with adverse health outcomes among human infants and toddlers. J Nutr. 2004;134:1432–1438. doi: 10.1093/jn/134.6.##. [DOI] [PubMed] [Google Scholar]
- 109.Stuff JE, Casey PH, Szeto KL, Gossett JM, Robbins JM, Simpson PM, Connell C, Bogle ML. Household food insecurity is associated with adult health status. J Nutr. 2004;134:2330–2335. doi: 10.1093/jn/134.9.2330. [DOI] [PubMed] [Google Scholar]
- 110.World Health Organization Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria-endemic areas. Food Nutr Bull. 2007;28:S621–S627. doi: 10.1177/15648265070284s414. [DOI] [PubMed] [Google Scholar]
- 111.Mbugi EV, Meijerink M, Veenemans J, Jeurink PV, McCall M, Olomi RM, Shao JF, Verhoef H, Savelkoul HF. Alterations in early cytokine-mediated immune responses to Plasmodium falciparum infection in Tanzanian children with mineral element deficiencies: a cross-sectional survey. Malar J. 2010;9:130. doi: 10.1186/1475-2875-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Veenemans J, Milligan P, Prentice AM, Schouten LR, Inja N, van der Heijden AC, de Boer LC, Jansen EJ, Koopmans AE, Enthoven WT. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8e1001125 doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeba AN, Sorgho H, Rouamba N, Zongo I, Rouamba J, Guiguemde RT, Hamer DH, Mokhtar N, Ouedraogo JB. Major reduction of malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial. Nutr J. 2008;7:7. doi: 10.1186/1475-2891-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:126–132. doi: 10.4269/ajtmh.2006.75.1.0750126. [DOI] [PubMed] [Google Scholar]
- 115.Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Bannon D, Tielsch JM, West KP, Alpers MP. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomized trial in preschool children in Papua New Guinea. Am J Trop Med Hyg. 2000;62:663–669. doi: 10.4269/ajtmh.2000.62.663. [DOI] [PubMed] [Google Scholar]
- 116.Shankar AH, Genton B, Semba RD, Baisor M, Paino J, Tamja S, Adiguma T, Wu L, Rare L, Tielsch JM, Alpers MP, West KP. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomised trial. Lancet. 1999;354:203–209. doi: 10.1016/S0140-6736(98)08293-2. [DOI] [PubMed] [Google Scholar]
- 117.Cusick SE, Tielsch JM, Ramsan M, Jape JK, Sazawal S, Black RE, Stoltzfus RJ. Short-term effects of vitamin A and antimalarial treatment on erythropoiesis in severely anemic Zanzibari preschool children. Am J Clin Nutr. 2005;82:406–412. doi: 10.1093/ajcn.82.2.406. [DOI] [PubMed] [Google Scholar]
- 118.Binka F, Ross D, Morris S. Vitamin A supplementation and chilhood malaria in northern Ghana. Am J Clin Nutr. 1995;61:853–859. doi: 10.1093/ajcn/61.4.853. [DOI] [PubMed] [Google Scholar]
- 119.Reuben R. Women and malaria—special risks and appropriate control strategy. Soc Sci Med. 1993;37:473–480. doi: 10.1016/0277-9536(93)90282-9. [DOI] [PubMed] [Google Scholar]
- 120.Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100:189. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, Mtasiwa D, Mshinda H, Fillinger U, Lindsay SW. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stevenson J, Laurent BS, Lobo NF, Cooke MK, Kahindi SC, Oriango RM, Harbach RE, Cox J, Drakeley C. Novel vectors of malaria parasite in the western highlands of Kenya. Emerg Infect Dis. 2012;18:1547. doi: 10.3201/eid1809.120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White G. Comparative studies on sibling species of Anopheles gambiae Giles complex (Dipt. Culicidae). III. The distribution, ecology, behaviour and vectorial importance of species D in Bwamba County, Uganda, with analysis of biological, ecological, morphological and cytogenetical relationships of Ugandan species D. Bull Entomol Res. 1973;63:65–97. [Google Scholar]
- 124.Okumu FO, Moore SJ, Okumu F, Moore S. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J. 2011;10:208. doi: 10.1186/1475-2875-10-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perez-Escamilla R, Segall-Correa AM, Kurdian Maranha L, Sampaio Md Mde F, Marin-Leon L, Panigassi G. An adapted version of the U.S. Department of Agriculture Food Insecurity module is a valid tool for assessing household food insecurity in Campinas, Brazil. J Nutr. 2004;134:1923–1928. doi: 10.1093/jn/134.8.1923. [DOI] [PubMed] [Google Scholar]
- 126.Perez-Escamilla R, Segall-Correa AM. Food security measurement and indicators. Rev Nutr. 2008;21:S15–S26. [Google Scholar]