Abstract
We report a case of Ancylostoma ceylanicum infection detected by endoscopy. It was diagnosed and confirmed using polymerase chain reaction (PCR) and DNA sequencing. The patient is a 58-year-old Malaysian woman who lives in a rural area, where uncontrolled populations of stray and semidomesticated dogs live in close proximity with humans.
Hookworms are one of the most common parasitic nematodes that infect both humans and animals. The two primary species of hookworm infecting humans are Ancylostoma duodenale and Necator americanus.1 The public health impact of human hookworm infections is extensive, infecting an estimated 600 million people worldwide and resulting in up to 135,000 deaths annually.2 Other than the two anthropophilic species of human hookworms, cat and dog hookworms, such as A. ceylanicum, A. braziliense, and A. caninum, are also able to cause zoonotic disease in humans. The symptoms caused by zoonotic hookworms include creeping eruption, eosinophilic enteritis (EE), and less frequently, symptoms such as localized myositis, erythema multiforme, and ophthalmological manifestations.3 A. ceylanicum, however, is the only species of animal hookworm known to produce patent infection in humans.
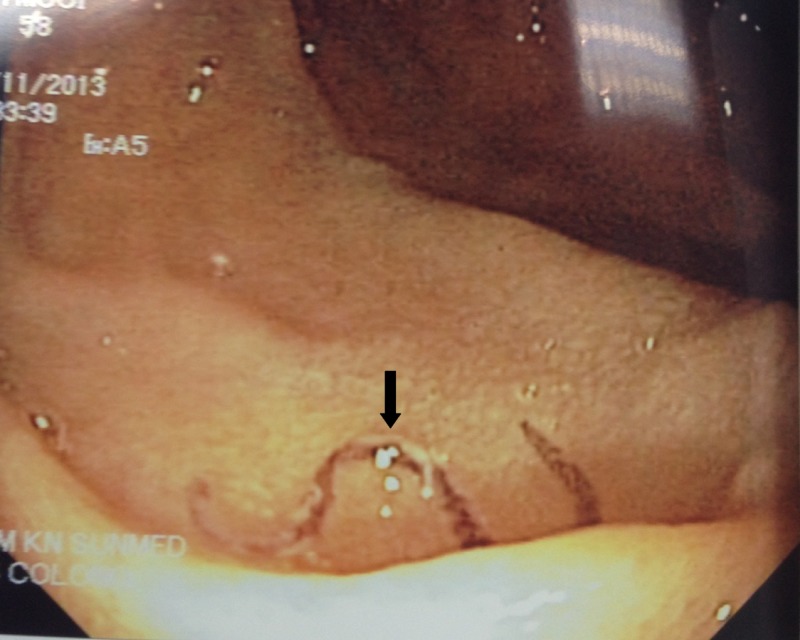
On November 11, 2013, a 58-year-old Chinese woman who presented with upper gastrointestinal (GI) bleeding (melena) was admitted to a private hospital for additional management. She was passing out black-colored stool one to two times per day for 1 week in early November of 2013. She was admitted to a local hospital for a similar problem before this admission. She had a few episodes of dizziness, tightness of chest, and cold sweats. There was no history of fever or weight loss. Laboratory investigations showed a hemoglobin concentration of 11.4 g/dL (11.5–16.5 g/dL), a platelet count of 169 k/μL (150–400 k/μL), a total white blood cell count of 8.0 k/μL (4.0–11.0 k/μL), neutrophils of 68% (40–75%), lymphocytes of 24% (20–45%), monocytes of 6% (2–10%), eosinophils of 1% (0–6%), and basophils of 1% (0–2%). Liver and renal functions were normal. The patient underwent oesophagogastroduodenoscopy (OGDS) and colonoscopy. Colonoscopy showed three polyps that were removed by polypectomy. Histopathological examination (HPE) showed them to be malignant. OGDS showed a gastric ulcer with signs of bleeding. Biopsy of the ulcer edge proved it to be malignant. Incidentally, a single adult blood-filled worm that measured 8–10 mm in size was seen moving on the duodenal mucosa (Figure 1 ). The worm was removed and sent to the Parasite Southeast Asia Diagnostic (Para:SEAD) Laboratory, Department of Parasitology, Faculty of Medicine, University of Malaya for species identification. Microscopic examination of the worm, including its buccal capsule or mouthpart, showed it to be an adult female hookworm (Figure 2 ). In addition, ova that were characteristic of hookworm species were seen in the ruptured uterus. Microscopic examination of the mouthpart was not clear and detailed enough to allow specific species identification. For specific species characterization, the worm was, therefore, subjected to polymerase chain reaction (PCR) targeted to the internal transcribed spacer-2 (ITS-2) ribosomal RNA gene as described previously.4 Briefly, the adult worm was ground with a sterile scalpel using mechanical vortex, and the homogenate was then digested with proteinase K followed by genomic DNA extraction using a commercial kit (Macherey-Nagel, Neumann-Neander, Duren, Germany). DNA amplification was performed, and the positive amplicon was subjected to DNA sequencing. Homology search using the National Center for Biotechnology Information (NCBI) reference sequences with Basic Local Alignment Search Tool (BLAST) confirmed the hookworm species as A. ceylanicum.
Figure 1.
The OGDS image shows an adult blood-filled worm that measures about 8 mm in length moving on the patient's duodenal mucosa.
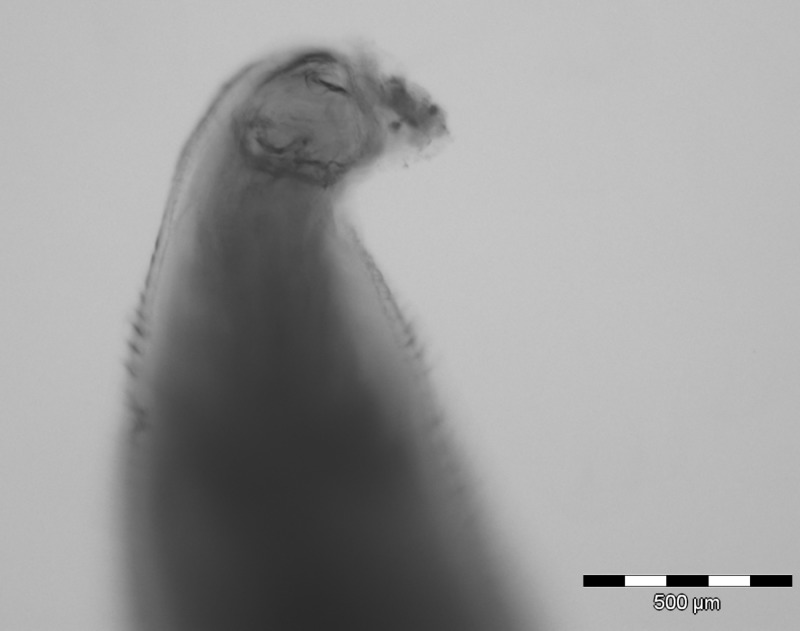
Figure 2.
The microscopic image shows the buccal capsule (mouthpart) of the nematode parasite, which is suggestive of hookworm.
A. ceylanicum is a hookworm species of dogs and cats. It is known to be endemic and widely distributed in Asia, especially southeast Asia.5 It is a neglected parasitic zoonotic nematode. Additional history revealed that the patient lives in a rural village, where uncontrolled populations of stray and semidomesticated dogs live in close proximity to human populations. In these rural settings, the close relationship shared with dogs, lack of veterinary attention, poor hygiene, and overcrowding contribute to a high risk of acquiring this zoonotic disease.6,7 A. ceylanicum is the only zoonotic species of hookworm known to infect humans, which was shown experimentally and naturally. Its contribution to human morbidity remains largely unexplored. It has always been regarded as a rare and uncommon parasitic infection. In fact, recent molecular-based surveys conducted mainly in southeast Asia, which included Malaysia,8 Thailand,9 and Laos,10 showed A. ceylanicum to be the second highest hookworm species infecting humans.
There have been several clinical manifestations of A. ceylanicum infection in humans, and they were recently reviewed and summarized by Traub.5 In experimental infection of humans, clinical symptoms mimic the descriptions produced by anthroponotic hookworm species, including ground itch, abdominal pain, and GI discomfort. Although there are increasing reports of natural infections of A. ceylanicum in humans, unfortunately, in most of these cases, clinical information is limited or not available. Much like anthroponotic hookworm infections, patent infection with A. ceylanicum is capable of producing chronic infections, such as blood loss, and in high worm burden, it could lead to iron deficiency anemia.
In recent years, the development and advancement in capsule endoscopy technologies that allow gastroenterologists to record images of the digestive tract have shown promise in shedding additional light on the clinicopathological implications of this neglected zoonotic parasite in humans. To date, at least more than 10 findings of hookworm using OGDS and colonoscopy in patients with GI symptoms have been reported. More recently, two cases in Taiwan reported worms that were removed from patients using OGDS and colonoscopy and identified as A. ceylanicum.11,12 Clinicopathological findings in both cases included eosinophilia, occult blood in stools, severe abdominal pain, nausea, and prolonged watery diarrhea. The latest study in Australia using PCR coupled with DNA sequencing in humans presenting with GI discomfort showed A. ceylanicum as the etiological agent. However, no clinical information was available.13
In our case, although the laboratory results showed that hemoglobin was slightly below the normal limit, it may not have been caused by A. ceylanicum infection, because only a single worm was observed and recovered during endoscopy. Moreover, the patient also suffered GI bleeding from a stomach ulcer. If it had not been for the ulcer-associated melena, the adult hookworm may have been overlooked because of the asymptomatic nature of low-intensity hookworm infections. This report calls attention to the fact that, in areas where A. ceylanicum occurs, this worm can reach maturity in humans and in heavy infections, may perhaps produce hookworm disease. Often, this information is forgotten by practitioners who assume that all adult hookworms reported from humans are either N. americanus or A. duodenale.
Whether A. ceylanicum is capable of producing classical hookworm disease in human remains uncertain, and this largely forgotten zoonotic species has been frequently discovered and reported in humans using several advance clinical and diagnostic tools. Because our knowledge in the clinicopathology of patent A. ceylanicum infection in humans is lacking, future investigation combining the use of modern diagnostic tools with a detailed study on pathogenicity and clinical features will shed additional light on its role as a human pathogen.
ACKNOWLEDGMENTS
The authors thank Sister Ang at Sunway Medical Hospital for giving us the permission to assess medical record of the patient. This work was supported by University of Malaya (BK007-2014, H-20001-00-E00051, and H-20001-00-E000061).
Footnotes
Authors' addresses: Romano Ngui, Yvonne A. L. Lim, Wan Hafiz Wan Ismail, and Rohela Mahmud, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia, E-mails: skyromano@gmail.com, limailian@um.edu.my, wanhafiz@ummc.edu.my, and rohela@ummc.edu.my. Kie Nyok Lim, Sunway Medical Centre, No. 5, Jalan Lagoon Selatan, Bandar Sunway, Petaling Jaya, Selangor Darul Ehsan, Malaysia, E-mail: klelim@yahoo.com.
References
- 1.Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994;109:373–387. doi: 10.1017/s0031182000078410. [DOI] [PubMed] [Google Scholar]
- 2.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26:162–167. doi: 10.1016/j.pt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Ngui R, Lee SC, Tan TK, Muhammad Aidil R, Lim YAL. Genetic characterization of human hookworm infections in rural and remote areas of Peninsular Malaysia. Am J Trop Med Hyg. 2012;86:837–842. doi: 10.4269/ajtmh.2012.11-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013;43:1009–1015. doi: 10.1016/j.ijpara.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RC. The role of dogs in transmission of gastrointestinal parasites in a remote tea-growing community in northeastern India. Am J Trop Med Hyg. 2002;67:539–545. doi: 10.4269/ajtmh.2002.67.539. [DOI] [PubMed] [Google Scholar]
- 7.Ngui R, Lim YA, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012;6:e1522. doi: 10.1371/journal.pntd.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngui R, Mahdy MA, Chua KH, Traub RJ, Lim YAL. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop. 2013;128:154–157. doi: 10.1016/j.actatropica.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naaglor T, Taamari P, Leelayoova S. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 2011;84:594–598. doi: 10.4269/ajtmh.2011.10-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, Sripa B, Blacksell SD, Fenwick S, Thompson RC. Soil-transmitted helminthiasis in Laos: a community-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg. 2012;86:624–634. doi: 10.4269/ajtmh.2012.11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CS, Lin CK, Su KE, Liu CY, Lin CC, Liang CC, Lee TH. Diagnosis of Ancylostoma ceylanicum infestation by single-balloon enteroscopy (with video) Gastrointest Endosc. 2012;76:671–672. doi: 10.1016/j.gie.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Hsu YC, Lin JT. Images in clinical medicine. Intestinal infestation with Ancylostoma ceylanicum. N Engl J Med. 2012;366:e20. doi: 10.1056/NEJMicm1101717. [DOI] [PubMed] [Google Scholar]
- 13.Koehler AV, Bradbury RS, Stevens MA, Haydon SR, Jex AR, Gasser RB. Genetic characterization of selected parasites from people with histories of gastrointestinal disorders using a mutation scanning coupled approach. Electrophoresis. 2013;34:1720–1728. doi: 10.1002/elps.201300100. [DOI] [PubMed] [Google Scholar]