Abstract
Aims—To define the relation between the quantity of silver stained nucleolar organiser regions (AgNORs) and histological grade, clinical stage, DNA content, and MIB-1 immunostaining in needle biopsy specimens of prostatic adenocarcinomas.
Methods—Histological grade was determined according to the Gleason system. AgNOR quantity, DNA content and MIB-1 immunostaining were evaluated by image cytometry on routine histological sections stained with silver, Feulgen reaction and MIB-1 antibody, respectively.
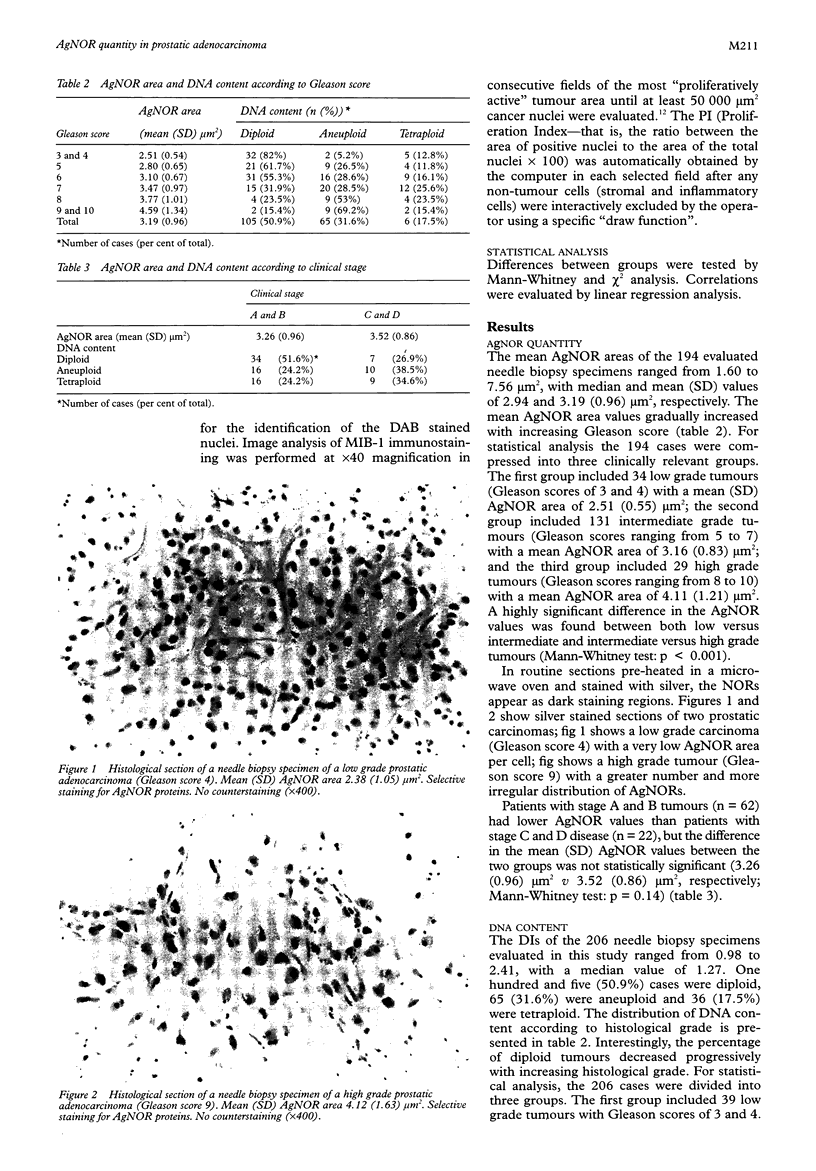
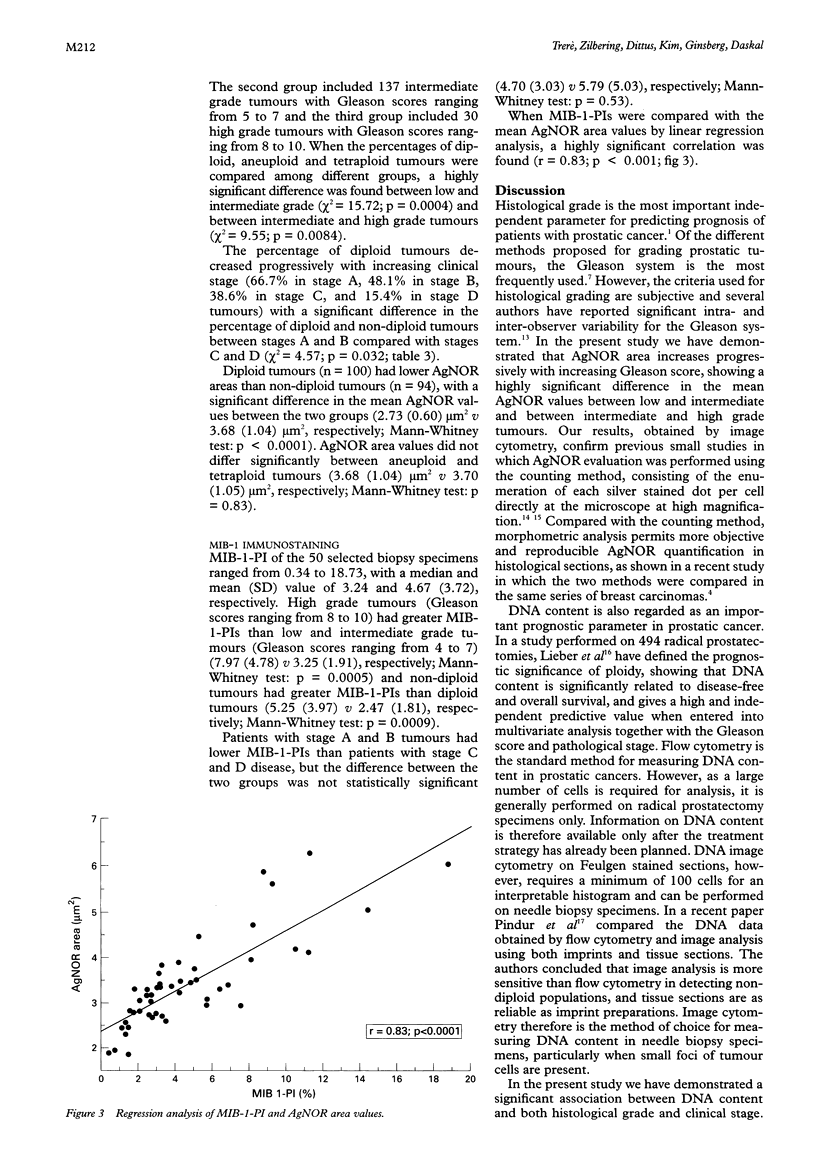
Results—The mean AgNOR area increased with increasing Gleason score. A significant difference was found in the AgNOR values between low, intermediate and high grade tumours. Patients with clinically localised tumour (stages A and B) had lower AgNOR values than patients with advanced disease (stages C and D), but the difference in the mean AgNOR values between the two groups was not statistically significant. Non-diploid tumours had a significantly higher mean (SD) AgNOR area than diploid tumours (3.68 (1.04) μm2v 2.73 (0.60) μm2, respectively), while no significant difference was observed in the mean AgNOR values between aneuploid and tetraploid tumours (3.68 (1.04) μm2v 3.70 (1.05) μm2). When AgNOR and MIB-1-PI values were compared using linear regression analysis, a highly significant correlation was found.
Conclusions—These data demonstrate that AgNOR quantity reflects the proliferative potential of prostatic adenocarcinomas, and is significantly related to histological grade and DNA content. The ease of application on routine sections, maintaining the morphological integrity of the tissue, the ability to evaluate selected histological areas of limited size and objective quantification by image cytometry make the AgNOR method particularly suitable for cell kinetic analysis in prostatic needle biopsy specimens.
Keywords: AgNORs
Keywords: Gleason grade
Keywords: DNA content
Keywords: MIB-1 immunostaining
Keywords: cell proliferation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alivizatos G., Pavlaki K., Giannopoulos A., Mitropoulos D., Tsega A., Deliveliotis C., Dimopoulos C. Nucleolar organizer regions in prostatic adenocarcinomas. Comparison with flow cytometric analysis, tumor grade, stage and serum prostate-specific antigen levels. Eur Urol. 1992;21(2):141–145. [PubMed] [Google Scholar]
- Beck T., Weller E. E., Weikel W., Brumm C., Wilkens C., Knapstein P. G. Usefulness of immunohistochemical staining for p53 in the prognosis of breast carcinomas: correlations with established prognosis parameters and with the proliferation marker, MIB-1. Gynecol Oncol. 1995 Apr;57(1):96–104. doi: 10.1006/gyno.1995.1104. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Derenzini M., Trerè D., Oliveri F., David E., Colombatto P., Bonino F., Brunetto M. R. Is high AgNOR quantity in hepatocytes associated with increased risk of hepatocellular carcinoma in chronic liver disease? J Clin Pathol. 1993 Aug;46(8):727–729. doi: 10.1136/jcp.46.8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh M., Sasaki Y., Oguro T., Aihara K. Silver staining of nucleolar organizer regions in prostatic lesions. Histopathology. 1991 Oct;19(4):369–372. doi: 10.1111/j.1365-2559.1991.tb00053.x. [DOI] [PubMed] [Google Scholar]
- Ofner D., Bankfalvi A., Riehemann K., Bier B., Böcker W., Schmid K. W. Wet autoclave pretreatment improves the visualization of silver-stained nucleolar organizer-region-associated proteins in routinely formalin-fixed and paraffin-embedded tissues. Mod Pathol. 1994 Dec;7(9):946–950. [PubMed] [Google Scholar]
- Pinder S. E., Wencyk P., Sibbering D. M., Bell J. A., Elston C. W., Nicholson R., Robertson J. F., Blamey R. W., Ellis I. O. Assessment of the new proliferation marker MIB1 in breast carcinoma using image analysis: associations with other prognostic factors and survival. Br J Cancer. 1995 Jan;71(1):146–149. doi: 10.1038/bjc.1995.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindur A., Chakraborty S., Welch D. G., Wheeler T. M. DNA ploidy measurements in prostate cancer: differences between image analysis and flow cytometry and clinical implications. Prostate. 1994 Oct;25(4):189–198. doi: 10.1002/pros.2990250404. [DOI] [PubMed] [Google Scholar]
- Ploton D., Menager M., Jeannesson P., Himber G., Pigeon F., Adnet J. J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986 Jan;18(1):5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Ploton D., Menager M., Jeannesson P., Himber G., Pigeon F., Adnet J. J. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986 Jan;18(1):5–14. doi: 10.1007/BF01676192. [DOI] [PubMed] [Google Scholar]
- Shankey T. V., Kallioniemi O. P., Koslowski J. M., Lieber M. L., Mayall B. H., Miller G., Smith G. J. Consensus review of the clinical utility of DNA content cytometry in prostate cancer. Cytometry. 1993;14(5):497–500. doi: 10.1002/cyto.990140508. [DOI] [PubMed] [Google Scholar]
- Trerè D. Critical analysis of the methods commonly employed in the assessment of cell proliferation: advantages of the NOR silver-staining technique in routine cyto-histopathology. Anal Cell Pathol. 1993 Jul;5(4):191–201. [PubMed] [Google Scholar]
- Trerè D., Migaldi M., Trentini G. P. Higher reproducibility of morphometric analysis over the counting method for interphase AgNOR quantification. Anal Cell Pathol. 1995 Jan;8(1):57–65. [PubMed] [Google Scholar]
- Veronese S. M., Gambacorta M., Gottardi O., Scanzi F., Ferrari M., Lampertico P. Proliferation index as a prognostic marker in breast cancer. Cancer. 1993 Jun 15;71(12):3926–3931. doi: 10.1002/1097-0142(19930615)71:12<3926::aid-cncr2820711221>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Whitmore W. F., Jr Natural history and staging of prostate cancer. Urol Clin North Am. 1984 May;11(2):205–220. [PubMed] [Google Scholar]





