Abstract
Merkel cell carcinoma (MCC) is an aggressive skin cancer with rising incidence. In this study, we demonstrate that mTOR activation and suppressed autophagy is common in MCCs. mTOR inhibition in two primary human MCC cell lines induces autophagy and cell death that is independent of caspase activation but can be attenuated by autophagy inhibition. This is the first study to evaluate mTOR and autophagy in MCC. Our data suggests a potential role of autophagic cell death upon mTOR inhibition and thus uncovers a previously underappreciated role of mTOR signaling and cell survival, and merits further studies for potential therapeutic targets.
Keywords: Merkel cell carcinoma, mTOR pathway, autophagy, cell death, mTOR inhibitor
1. Introduction
Merkel cell carcinoma (MCC) is an aggressive skin cancer with a rising incidence (1500 cases per year in the US). Moreover, the incidence of MCC is approximately 11-fold greater in AIDS patients and 5-fold greater in organ transplant patients [9, 34]. In addition to a higher risk for other skin cancers, MCC patients also have an increased risk for hematologic malignancies [35]. In Australia between 1986–2001, the age-adapted incidence of MCC rose at an annual increase of 8%, compared to the 3% rise in cutaneous melanoma. Furthermore, 50% of patients are metastatic at presentation with a 5-year disease-associated mortality rate of 46%, far exceeding that of melanoma [3]. Nevertheless, there is no effective treatment so far [1, 4].
Despite this grim epidemiologic data, the cellular and molecular mechanisms dictating MCC pathogenesis and metastasis remain largely unknown. Studies have led to the discovery of Merkel cell carcinoma virus (MCV) [11]; however, the pathogenic role of MCV remains a captivating question. Intense investigations show no significant contribution of the p53, PTEN, MAPK pathways to MCC pathogenesis. PI3K/AKT and MAPK pathways, the two pathways that are most commonly dysregulated in human malignancies, are less frequently mutated in MCCs. Moreover, growth factor pathways are not constitutively activated in MCC. Interestingly, activation of the MAPK pathway leads to apoptosis in human MCC cells [17]. Most recently, studies have reported Akt hyper-phosphorylation in MCC regardless of the presence of MCV [15, 33]. In addition, up-regulation of the mammalian target of the rapamycin (mTOR) pathway has been found in MCCs that are positive for MCV through the interaction of eukaryotic translation initiation factor 4E-binding protein (4E-BP1) with MCV small T antigen [38].
mTOR is a critical mediator of the canonical pathway of the PI3K and MAPK pathways [24]. It resides in at least two functional multiprotein complexes, mTOR complex1 (mTORC1) and mTOR complex2 (mTORC2), which exhibit different subunit compositions and execute distinct cellular tasks. Tuberous sclerosis complex (TSC) is the key inhibitor of this pathway and functions as a guanosine triphosphatase-activating protein for the small guanine nucleotide-binding protein Rheb, which, in its GTP-bound form, is essential for the stimulation of mTORC1 activity. Disruption of this complex, through the loss of either TSC1 or TSC2 function results in constitutive activation of mTORC1 that is exemplified in the genetic disorder tuberous sclerosis. mTOR controls many cellular processes including apoptosis, autophagy, translation, energy metabolism, and inflammation. The mTORC1 pathway is frequently activated in many human cancers [6]. Clinically, rapalogs such as rapamycin has shown some therapeutic efficacy in a subset of tumors via inhibiting mTORC1 [45]. Moreover, mTOR kinase inhibitors have been shown to halt cell growth and survival in various cancers [18, 49]. However, the role of mTOR pathway in MCC carcinogenesis has not been extensively studied.
Autophagy refers to the highly regulated and evolutionally conserved process of turnover and maintenance of cellular component that is required for cellular homeostasis [25, 29]. The connections between autophagy malfunction and human diseases have been under recent intensive scrutiny [46]. Although autophagy has been assigned a largely cytoprotective role, it does accompany cell death, and its inhibition attenuates cellular demise in certain physiologic processes [2, 43]. In tumorigenesis, however, autophagy is a double-edged sword. From the perspective of cellular energetics, autophagy is likely a pro-survival mechanism in both early and late stages of cancer development. Conversely, autophagy may also have antagonistic roles in the oncogenic process, i.e., diminishing malignant transformation and facilitating tumor progression [14]. Several studies have documented increased autophagy in cancer cell death when anti-cancer agents were used [10, 13, 23]. More specifically, a recent study has shown that oncogenic RAS induces autophagy and autophagic cell death by up-regulation of the BH3-only protein NOXA [8]. Autophagic cell death has also been detected in cells lacking Bax and Bak, two pro-apoptotic factors, and can be induced by caspases-8 inhibition [50]. Therefore, defining the context-specific role for autophagy in cancer and determining the mechanisms involved will be important to guide autophagy-based therapeutic interventions. As autophagy is a downstream target of the mTOR pathway, we chose to examine its potential role in MCC pathogenesis.
To investigate whether mTOR signaling contributed to the development of MCC, we first evaluated mTOR pathway using formalin fixed paraffin embedded (FFPE) human MCC samples. Using tissue microarray (TMA) and immunohistochemistry, we found that mTOR pathway was activated as indicated by positive staining of phosphorylation-4E-BP1 (p-4E-BP1), phosphorylation-S6K (p-S6K), and phosphorylation-mTOR (p-mTOR) regardless of MCV status. Moreover, our data suggested alternative mechanisms of mTOR activation other than through MCV small antigen in MCCs. Furthermore, there was p62 accumulation in MCCs, indicative of impaired autophagy. To facilitate in vitro mechanistic studies, we then established two primary human MCC cell lines (MCC-2 and MCC-3) from two patients with lymph node metastases. We also demonstrated mTOR pathway up-regulation and decreased autophagy in both MCC cells. Moreover, we have shown that inhibition of mTOR pathway decreased cell proliferation and induced autophagy and cell death in MCC cells. Furthermore, cell death induced by mTOR inhibitors was independent of caspase activation and attenuated by an autophagy inhibitor. Thus, our study provides a new insight into the MCC pathogenesis and a rationale for potential new therapeutic targets.
2. Materials and Methods
2.1 Sample selection and Tissue Microarray
In accordance with institutional approvals for human study protocols, 65 archival formalin fixed and paraffin embedded (FFPE) MCC tissue blocks and 9 fresh MCC tissues were employed in this study. TMAs were prepared as previously described. For each case, a representative area from the tumor was carefully selected from a hematoxylineosin stained section of a MCC block. Core cylinders (0.6mm) were punched from each FFPE tumor and deposited into a recipient paraffin block using the MTA-I manual tissue arrayer (Beecher). A total of 65 MCC tumors were employed in the TMA study. Five-micrometer sections of the resulting TMA blocks were made and used for immunohistochemistry.
2.2 Immunostaining
Immunohistochemistry was performed on 5-mm sections of TMA slides. The slides were deparaffinized and rehydrated. Antigen retrieval was performed by microwaving in 0.01 M sodium citrate for 20 min. Endogenous tissue peroxide was blocked with 1% hydrogen peroxide at room temperature (RT) for one hour following washing twice in PBS. The sections were further blocked with normal goat serum at RT for one hour following incubation with p-S6K (T389) (1:50), p-4E-BP1 (Thr 37/46) (1:200) antibodies, p-mTOR (Ser 2248) (1:100), p62 (1:1000) (Cell Signaling) and MCV antibodies (CM2B4,1:10) (Santa Cruz) at 4°C overnight, respectively [28]. Secondary goat anti-rabbit antibody (1:200) was applied to the slides for one hour at RT before developing in HRRP detection system and freshly prepared diaminobenzidine as the chromogen (brown). Sections were counterstained with hematoxylin. Staining was manually scored. Immunostained slides were viewed on an Olympus BX51 Research System Microscope by 10x and 20x UPlanApo air objective lenses (Olympus America). Images were photographed using a high-resolution interline CCD camera (CoolSNAP cf, Photometrics), and acquired with automated microscopy acquisition software (MetaMorph version 7.7, Molecular Devices). For immunofluorecent staining, slides were prepared by cytospin (Shandon Cytospin 4 Cytocentrifuge, Thermo Scientific) with MCC-2 cells in suspension, followed by fixing in methanol for 5 min. The cells were incubated with CK20 antibody (DAKO) at 4°C overnight and then incubated with secondary anti-rabbit Ig HRP antibody for 1 hour. After washing, cells were incubated with TSA fluorescein reagent (Perkin Elmer, Boston, MA) for 30 minutes, and then incubated with 1 μg ml−1 Hoechst 33342 (Invitrogen, Carlsbad, CA) for 30 minutes at room temperature. Dehydrated slides were mounted and visualized with viewed on a microscope and images were acquired using a device as described above. Excitation wavelength of 480 nm and emission wavelength of 535 nm were selected.
2.3 Establishment and characterization of human MCC cell lines
In accordance with institutional approvals for human study protocol, fresh MCC tumor tissues from a patient with lymph node metastasis were finely minced and mechanically dissociated tumor materials were passing through a nylon sieve [30]. The resultant cell suspension obtained was seeded in RPMI medium with 10% fetal bovine serum (FBS), penicillin and streptomycin. Fresh medium was added every other day and cultures were split 1:2 weekly following complete removal of the medium.
2.4 Cell lines and reagents
A human small cell lung cancer cell line (SCLC 2049, ATCC) was grown in RPMI-1640 (ATCC) with 10% FBS. 293T/17 (ATCC) was grown in DMEM medium. KU-63794, WYE-354, PP242, E64d, pepstatin A and bafilomycin A1 (Baf A1) were purchased from Sigma Aldrich CO. Staurosporine and z-VAD-FMK were obtained from ENZO Life Sciences.
2.5 Gene expression analysis
RNA was isolated from MCC cells and MCC fresh tissues with RNeasy kit (Qiagen). cDNA was generated from MCC mRNA using Reverse Transcription Kit (Applied Biosystems). RT-PCR was performed in MCC cells as described previously using specific primers for CK7, 18, 19, 20, neuron specific enolase, synaptophysin and Math-1. SYBR Green-based quantitative reverse transcription-PCR (qRT-PCR) was performed with a StepOne Plus Real-Time PCR System (Applied Biosystems). Triplicate runs of each sample were normalized to MRPS2 mRNA to determine relative expression. Primer pair sequences for RT-PCR are listed in Table S1. Primers for qRT-PCR were purchased from Applied Biosystems.
2.6 MCV detection by PCR and direct sequencing
DNA was prepared using DNeasy kit (Qiagen). DNA quality was confirmed by GAPDH. PCR was performed with 120ng of genomic DNA using the Taq DNA polymerase (Invitrogen) in a final volume of 50 μl for 30–35 cycles. Primer sets for LT3 and MCPVS1 were used [7, 21]. PCR products were submitted to automated nucleotide sequencing in an ABI genetic analyzer (3130XL Genetic Analyzer at the UAMS DNA Sequencing Core Facility). DNA sequences were compared with the reference sequence from the National Center for Biotechnology Information (NCBI), Entrez Nucleotide database gb/EU375803.1 Merkel cell polyomavirus isolate MCC350 or gb/EU375804.1 Merkel cell polyomavirus isolate MCC339, using the NCBI Blast program.
2.7 Immunoblottings
Membranes were blotted with antibodies directed against p-S6 ribosomal protein (p-S6K), p-4E-BP1, p-mTOR, cleaved caspase-3 and LC3 (all from Cell Signaling). Antibody against p62 is from MBL International (Woburn, MA), respectively. Bound antibodies were detected with horseradish peroxidase-linked antibody against mouse or antibody against rabbit (IgG; Amersham), followed by ECL (Amersherm).
2.8 Cell death detection
Cell death was detected by flow cytometry for Annexin V-FITC per the manufacturer's protocol (BD). Briefly MCC cells (1×106 cells) were plated in 6 well plates for 12 hours followed by treatment with mTOR inhibitors for 24 hours before Annexin-V and propidium iodide (PI) staining (BD Biosciences FACS Aria). Cells incubated in the binding buffer with only Annexin-V or PI served as controls. For each dye, appropriate electronic compensation of the instrument was performed to avoid overlapping of the two emission spectra.
2.9 Cell proliferation and cell cycle analysis
MCC cells (1×106 cells) were seeded in 6-well plates for 12 hours, followed by treatment with mTOR inhibitors and were labeled with 10μM BrdU for 24 hours. BrdU incorporation was detected using Alexa Fluor 488-conjugated mouse anti-BrdU antibody (BD Biosences-Pharmingen) followed by 7AAD staining (BD Biosences-Pharmingen) for cell cycle analysis per the manufacture's protocol.
2.10 Construction of GFP-LC3 expression vector
PLVUT-tTR-KRAB lentiviral vector (Addgene, Cambridge,MA) was digested with EcoRI and then ligated with synthetic oligonucleotides containing BamHI and EcoRI sites to construct PLVUT-1 vector. Fragments from pMX-GFP and pMX-GFP-LC3 digested with BamHI and EcoRI were inserted into PLVUT-1 to generate PLVUT-GFP and PLVUT-GFP-LC3, respectively.
2.11 The Lentiviral transduction
To generate recombinant lenti-viruses, 293T/17 cells were co-transfected with PLVUT-GFP-LC3, virus packaging vectors, delta-H8.2 and VSVG using TransIT-LT1 transfection reagent (Mirus) [48]. Virus supernatants were collected 48 hours after transfection. Lentiviral transduction of MCC-2 cells was performed in the presence of 8μg/ml polybrene and spinoculation at 800×g for 30 minutes at 32°C.
2.12 Flow cytometry analysis of acidic vesicular organelles (AVO)
As described previously, MCC cells (1×106 cells per well) were plated in 6-well plate for 12 hours followed by treatment with mTOR inhibitor for 24 hours. The control and treated cells were stained with Acridine Orange hydrochloride solution (Sigma Aldrich, CO) at a final concentration of 0.01μg/ml [51].
3. Results
3.1 mTOR pathway is up-regulated in MCC tissue samples
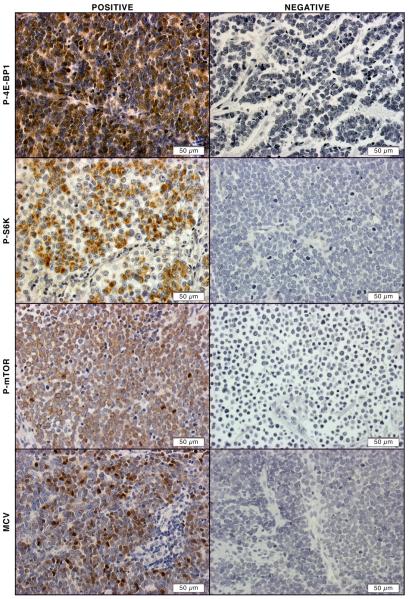
Unlike other cancers, common tumor suppressor genes and oncogenes, such as p53, PTEN, Rb, Ras, B-RAF, c-kit, and β-catenin, are less frequently mutated in MCCs. Growth receptor pathways such as c-kit, VEGF, and PDGF are also not highly activated in MCC as in other skin cancers such as melanoma, indicating that proteins and/or pathways critical for MCC carcinogenesis are yet to be identified. Recently Shuda et al described mTOR up-regulation in MCC with small T antigen [38]. Therefore, we decided to examine mTOR pathway in MCCs. First we examined 65 MCC tumor samples using tissue microarray (TMA). Consecutive sections were made from TMA and subjected to immunohistochemistry staining. As shown in Figure 1, m-TOR activation in MCCs as determined by positive staining of p-mTOR, p-4E-BP1 and p-S6K, was detected in the majority of MCCs. Fifty-seven (89%), fifty-five (86%) and 56 (86%) of the MCCs were positive for p-4E-BP1, p-S6K and p-mTOR, respectively (Figure 1). Therefore, not only did we show up-regulation of mTOR downstream molecules, but also activation of mTOR itself.
Figure 1. Activation of mTOR pathway in MCC tissues.
Representative positive immunohistochemical staining of P-4E-BP-1, P-S6K, P-mTOR, and MCV in sections from MCC TMA (left column). Representative negative immunohistochemical staining of P-4E-BP-1, P-S6K, P-mTOR, and MCV in sections from MCC TMA (right column)
3.2 mTOR pathway activation is independent of MCV status
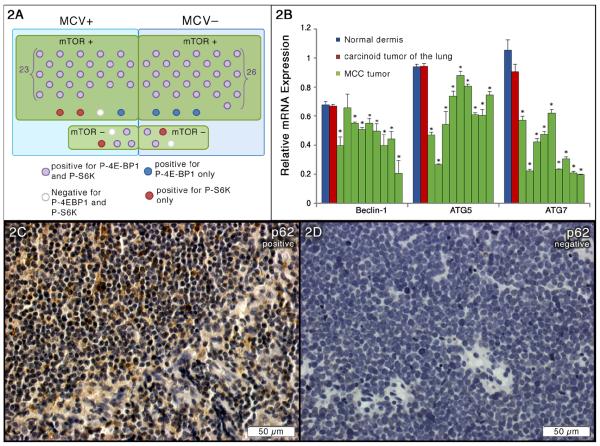
To explore the mechanism by which mTOR is activated in MCCs, we examined mTOR activation in relation to the MCV status in MCCs. MCV immunohistochemistry staining was performed on TMA samples. We found that 32/65 (48%) of our samples were positive for MCV (Figure 1). As shown in Figure 2A, the majority of both MCV positive (23/32) and MCV negative samples (26/33) had mTOR pathway activation. A total of 23/27 MCV positive and 26/29 MCV negative tumors showed hyperphosphorylation of all three markers mTOR, 4E-BP1 and S6K. Interestingly, 3/5 MCV positive tumors with undetectable p-mTOR and p-S6K activities also demonstrated 4E-BP1 hyperphosphorylation that was consistent with a previous study showing hyperphosphorylation of 4E-BP1 by small T antigen in MCC tumors harboring MCV. Furthermore, among 9 samples with undetectable mTOR activity, 5 and 2 samples showed p-4E-BP1 and p-S6K activity, respectively. However, the etiology needs further elucidation. Collectively, we have demonstrated that mTOR activation is independent of the presence of MCV. Our data also points to other possible mechanisms of mTOR activation, in addition to MCV small T antigen, such as by upstream mTOR regulators.
Figure 2. Suppressed autophagy in MCC tissues.
(A) mTOR activation in relation to MCV status in MCC TMA samples. (B) qRT-PCR analysis of autophagy-related gene expressions in 9 fresh MCC tumors. Samples were run in triplicate and normalized to MRPS2 mRNA to determine relative expression (means ± SEM). *P < 0.05 compared with controls. (C) Representative positive immunohistochemical staining of p62 in sections from MCC TMA. (D) Representative negative immunohistochemical staining of p62 in sections from MCC TMA.
3.3 Primary human MCC cell lines display increased mTOR activity and has decreased autophagosome formation
To further investigate the molecular events driving tumorigenesis in MCC, we have established two primary human MCC cell lines (MCC-2 and MCC-3) from two patients with lymph node metastases. MCC cells form clusters on tumor stromal cell feeder layers (Figure S1-A, B). Morphologically, MCC cells contained large, round to oval, vesicular nuclei with loosely textured chromatin as well as scant cytoplasm, a classic feature of MCC (Figure S1-C,D) [30]. CK20 is an intermediate filament and expressed in the epidermal Merkel cells. Characteristic paranuclear dot staining of CK20 has been applied in the clinic to secure the diagnosis of MCC [31]. Similarly, paranuclear dot staining as demonstrated by immunofluorecent staining was seen in MCC-2 cells (Figure S1-E). By RT-PCR analysis, MCC cells expressed both epithelial and neuronal markers, such as cytokeratin 18, 19, synaptophysin, neurospecific enolase and Merkel cell specific transcriptional factor Math-1(Figure S1-F) [5]. Interestingly, MCC-3 is CK20 positive in primary tumor, however, it loses CK20 positivity when it metastasized to lymph node. cDNA from a small cell lung cancer cell line SCLC 2049 was used as the control since both tumors are neuroendocrine carcinoma with similar morphology. To examine whether MCC-2 cells harbored MCV, we used primer sets for LT3 and MCPVS1 as previously described followed by direct DNA sequencing analysis [21]. We did not detect MCV by PCR and sequencing, indicating that MCC cell lines are negative for MCV (data not shown). Therefore, we have successfully established two primary human MCC cell lines.
To assess mTOR activity in MCC cells, cell lysates from MCC were subjected to immunoblottings using antibodies against p-4E-BP1, p-S6K and p-mTOR, respectively. Cell lysates from 293T/17A cells with known activated mTOR activity were used as controls. Consistent with our finding in MCC tissue samples, mTOR pathway upregulation was confirmed by hyperphosphorylation of p-4E-BP1, p-S6K and p-mTOR in both MCC-2 and MCC-3 cells (Figure S1-G). Having established the activation of mTOR in MCC tissue samples and MCC-2 cells, we sought to reveal the underlying downstream process.
3.4 Autophagy is suppressed in MCC tumors
Autophagy is increased in many tumors, however we hypothesized that autophagy could be defective in MCCs in response to chronic mTOR activation. P62 also known as Sequestosome 1 or SQSTM1, is believed to target ubiquitinated proteins and organelles to the autophagosome that are selectively degraded by autophagy [22, 41]. Impaired autophagy that is associated with persistence of p62 aggregates has been found in various human diseases. To test autophagy status we analyzed p62 accumulation by immunohistochemistry in TMA samples. As anticipated, p62 accumulation was observed in 49/64 (76%) of the MCCs (one sample was lost during the staining) that was indicative of impaired autophagy in these tumors (Figures 2C and 2D). Then, mRNAs were extracted from fresh tumors and qPCR analysis for autophagy genes was conducted. Since MCC is a neuroendocrine tumor, cDNA from a carcinoid tumor of the lung, also a neuroendocrine tumor, was used as a control. Moreover, MCCs usually occupied in dermis of the skin, thus cDNA from normal dermis of the skin was also employed as a control. Suppressed autophagy was evident by significant decreased expressions of Beclin1 (8/9), ATG5 (9/9) and ATG7 (9/9) (Figure 2B). We thus concluded that the mTOR pathway is chronically activated and endows impaired autophagy in MCCs.
3.5 Specific mTOR inhibitors induce autophagy formation and apoptosis in MCC cells
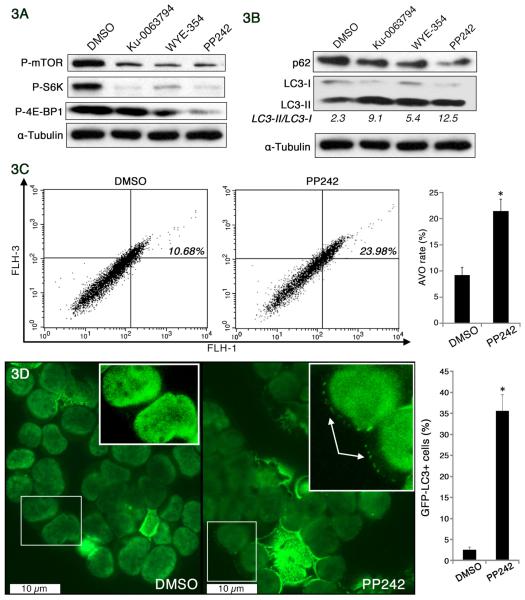
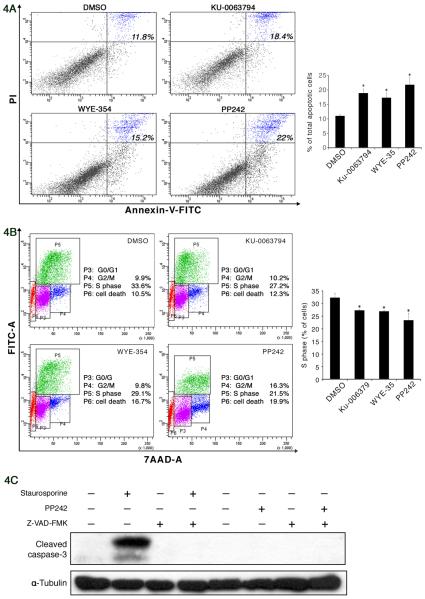
To refine the role of mTOR activation in MCCs, we next analyzed the effect of mTOR inhibition in MCC cells. Rapamycin, which partially inhibits 4E-BP1 phosphorylation, has shown limited anticancer effect clinically, and possibly due to its paradoxical activation of Akt. In contrast, a growing body of evidence suggests that small molecule inhibitors, such as WYE-354, Ku-0063794, and PP242, specifically block both mTORC1 and mTORC2 substrate phosphorylation and are more potent than rapamycin, but have no effect on PI3K [12, 18, 49]. To confirm mTOR inhibition induced autophagy, MCC-2 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24 hours, respectively. As shown in Figure 3A, decreased phosphorylation of mTOR, p-S6K and p-4E-BP1 was evident upon mTOR inhibition. Microtubule-associated protein light chain 3 (LC3-I), which is an abundant cytoplasmic protein that is cleaved and lipidated, forms LC3-II during initiation of autophagy. Ratio of LC3-II to LC3-I serves as a surrogate marker of autophagy activity [42]. However, an increased level of LC3-II could also be interpreted either as increased autophagy influx or blocked autophagosome maturation. Therefore, to investigate the magnitude of the flux, cells were treated with mTOR inhibitors in the presence of the lysosomal protease inhibitors E64d (10μm/ml) and pepstatin A (10μg/ml), an increase in the ratio of LC3-II/LC3-I further confirmed the increased autophagy upon mTOR inhibition (Figure 3B). As a consequence of increased autophagy, decreased p62 accumulation was also evident as shown in Figure 3B. Similar results were observed in MCC-3 cells treated with mTOR inhibitors (Figure S2). To further confirm increased autophagy, flow cytometry- acridine orange analysis and GFP-tagged LC3 were used to monitor autophagic activity [39, 51]. PP242 was the most effective in blocking mTOR activity, therefore it was used for further studies. The formation of autophagosomes, which then fuse with lysosomes to form mature acidified autolysosomes (acidic vesicular organelles (AVOs)) where degradation of the cargo occurs. Detection of AVOs was obtained through staining with acridine orange, which emits red light when it accumulates inside acidic vesicles. Therefore, MCC-2 cells were treated with PP242 for 24 hours following staining with acridine orange at a final concentration of 0.01ug/ml31 and the intensity of the red fluorescence was measured as described previously [20]. As expected, increased autophagy was evident by increased AVO (Figure 3C). Normally, LC3 is expressed in a diffuse pattern. During autophagy, LC3 –II is recruited to autophagosomal membranes, resulting in a more punctate distribution pattern. Thus, we explored whether GFP-tagged LC3 converted from a diffuse to punctate pattern after PP242 treatment. MCC-2 cells were transduced with lentivirus expression of GFP-LC3. MCC-2-GFP-LC3 cells were treated with PP242 for 24 hours and cytospinned on slides. A total of 100 MCC-2-GFP-LC3 cells per sample were counted, and cells with more than three GFP-LC3 dots were considered positive and counted. As seen in Figure 3D, we were able to confirm increased autophagy by punctate distribution in MCC-2-GFP-LC3 cells. Because mTOR is a target of both growth factor and nutrient signaling, its blockade is likely to activate one or more survival pathways that act to enable cells to endure periods of starvation and stress. Moreover, autophagy inhibition can lead to either increased cell death or increased cell survival, depending on tissue type, tumor grade, and any concomitant therapy used. We therefore wanted to determine whether the induction of autophagy would promote cell death in MCC-2 cells. Annexin-V and PI staining was performed on MCC-2 cells treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24 hours, respectively. Unexpectedly, increased cell death was demonstrated by Annexin-V and PI flow cytometry (Figure 4A). Similar results were also observed in MCC-3 cells treated with mTOR inhibitors (Figure S3-A, B). It has been previously shown that rapamycin inhibits the proliferation of glioma cells via autophagy induction [40]. We therefore tested if this was also the case in MCC-2 cells. MCC-2 cells treated with for 24 hours WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM), respectively, and were subjected to BrdU cell cycle analysis. We found decreased cell proliferation and increased cell death as measured by quantification of cells in S phase and the sub-G1 fraction, an indicator of cell proliferation and DNA fragmentation, respectively (Figure 4B). Therefore, we have shown that mTOR inhibition arrests cell proliferation, induces autophagy and potentiates cell death.
Figure 3. Increased autophagy upon mTOR inhibition in a primary human Merkel cell carcinoma cell line (MCC-2).
(A) Inhibition of mTOR pathway by mTOR kinase inhibitors. MCC-2 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. Lysates were prepared and subjected to immunoblotting analysis with indicated antibodies. (B) Induction of autophagy through inhibition of mTOR. MCC-2 cells were treated with mTOR kinase inhibitors in the presence of the lysosomal protease inhibitors E64d (10 μg/ml) and pepstatin A (10 μg/ml) for 24h, and cell lysates were subjected to immunoblotting against LC3-I, LC3-II and p62. The LC3-II/LC3-I ratio was calculated using ImageJ software. The increased LC3 II/LC3 I ratio and decreased p62 accumulation were indicative of increased autophagy. Tubulin was used as a loading control. (C) Induction of acidic vesicular organelles (AVOs) in PP242-treated MCC-2 cells. MCC-2 cells treated with PP242 (2.5μM) for 24h were subsequently stained with acridine orange (0.01 μg/ml) for 15 min and then subjected to flow cytometric analysis. FL1-H indicates green fluorescence intensity (cytoplasm and nucleus), while FL3-H shows red fluorescence intensity (AVOs). Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells. (D) Punctate pattern of MCC-2-GFP-LC3 upon mTOR inhibition. MCC-2 cells were transduced with lentivirus expression of GFP-LC3 and treated with DMSO and PP242 (2.5μM) for 24h, respectively. Images were captured using a fluorescence microscope. Cells were considered positive if they had more than three MCC-2-GFP-LC3 dots and a minimum of 100 GFP positive cells per sample were counted. Data are presented as a percentage of the total number of MCC-2 positive GFP positive cells visualized. Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells.
Figure 4. Increased caspase-3-independent cell death upon mTOR inhibition in MCC-2 cells.
(A) Increased cell death upon mTOR inhibition. MCC-2 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. The cells were then stained with propidium iodide (PI) and Annexin-V followed by flow analysis. Representative cytograms show percentages of total Annexin V positive cells. (B) Decreased cell proliferation upon mTOR inhibition. MCC-2 cells were treated with PP242 (2.5μM) for 24h and stained with BrdU and 7-AAD followed by flow cytometry analysis. (C) PP242 induced caspase-3-independent cell death in MCC-2 cells. MCC-2 cells were exposed to 0.1μM staurosporine (6h) or 2.5μM PP242 (24h) with or without Z VAD-FMK (20 μM). Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells.
3.6 Autophagy inhibitor Baf A1 but not pan-caspase inhibitor z-VAD-FMK blocks cell death induced by PP242 in MCC cells
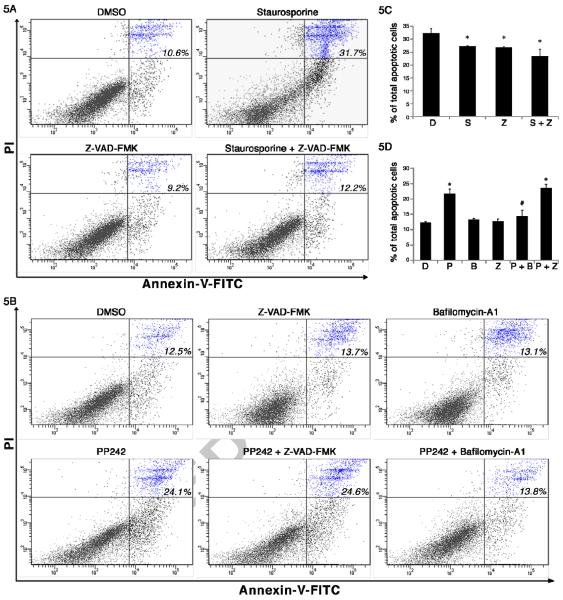
Anti-apoptotic proteins Bcl-2 and Bcl-xL have been shown to inhibit autophagy as well as apoptosis by binding to Beclin-1, an autophagy-inducing protein [26]. In order to understand the interplay of autophagy and cell death, we extended our studies to analyze the role of mTOR inhibitors in the induction of cell death. One of the key pathways through which apoptosis is regulated depends on the control of mitochondrial outer membrane permeability, which in turn regulates the release of apoptotic factors from the mitochondria that can then trigger a cascade of caspase activation. Therefore, we wondered if cell death could be attenuated by pan-caspase inhibitor z-VAD-FMK. Since PP242 was the most effective in inducing cell death and decreasing cell proliferation in MCC-2 cells, PP242 was chosen for further studies. The presence of cleaved caspase-3 subunit is an indicator of apoptosis. We first tested cleaved caspase-3 in MCC-2 cells by challenging cells with staurosporine (0.1 μM) and PP242 (2.5μM) in the absence or presence of the pan-caspase inhibitor Z-VAD-FMK (20μM). As shown in Figure 4C, cleaved caspase-3 was detected after exposure of MCC-2 cells to staurosporine for 6 hours, indicating induced apoptosis. Addition of Z-VAD-FMK (20 μM) was able to completely block caspase cleavage. However, there was no detection of cleaved caspase-3 after PP242 treatment in MCC-2 cells, suggesting possible other mechanism involved in PP242 induced MCC-2 cell death. To confirm our findings, Annexin V and PI staining was performed followed by flow cytometric analysis. MCC-2 cells were treated with PP242 (2.5μM) and pan caspase inhibitor z-VAD-FMK (20μM) for 24 hours. In keeping with our cleaved caspase-3 data, z-VAD-FMK failed to block cell death induced by PP242 (Figure 5A). Next, we asked if the PP242 induced cell death could be blocked by autophagy inhibition. MCC-2 cells were treated with PP242 (2.5μM) for 18 hours and then with Baf A1 (10nM) for additional 6 hours. Interestingly, cell death was attenuated by autophagy inhibitor Baf A1 (Figure 5B). Similar results were also observed in MCC-3 cells treated with mTOR inhibitors (Figure S3-C, D). Collectively, we have shown that cell death induced by PP242 is independent of caspase activation and potentially is related to autophagy induction.
Figure 5. mTOR inhibition-induced cell death was independent of caspase activation and blocked by autophagy induction.
(A) Apoptotic cell death induced by staurosporine was completely blocked by Z-VAD-FMK. MCC-2 cells were exposed to staurosporine (0.1μM) with or without Z-VAD-FMK (20 μM) for 6h followed by PI and Annexin V staining. (B) PP242-induced cell death was blocked by an autophagy inhibitor (bafilomycin-A1), but unaffected by a pan caspase inhibitor (Z-VAD-FMK). To confirm that PP242-induced cell death was caspase-independent, MCC-2 cells were treated with PP242 (2.5μM) and Z VAD-FMK (20μM) for 24 hours followed by PI and Annexin-V staining. To test if PP242-induced cell death could be blocked by autophagy, MCC-2 cells were treated with PP242 (2.5μM) for 18 hours and then with bafilomycin-A1(10nM) for additional 6 hours followed by PI and Annexin-V staining and flow cytometry analysis. Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells. #P < 0.05 compared with staurosporine or PP242-treated cells.
Discussion
In this study, by employing archival and fresh MCC tumors, as well as a primary human MCC cell line, we have first identified that mTOR activation, including activation of two downstream molecules 4E-BP1 and S6K and also mTOR per se, is common in MCCs. Second, we have shown that mTOR activation is independent of MCV and lead to impaired autophagy. Finally, augmentation of autophagy by mTOR inhibition potentiates cell death that is independent of caspase activation in MCC cells.
A recent study from Moore's group indicates that MCV entails mTOR activation by sustained hyperphosphorylation of 4E-BP1 via small T antigen, which further prevents 4E-BP1 from sequestering the eIF4E cap-dependent translation and thus enabling unchecked cell proliferation in MCC [38]. Along similar lines, we not only demonstrate that the mTOR pathway is activated in MCCs regardless of the presence of MCV, but also show mTOR hyperphosphorylation in both MCV positive and MCV negative MCCs. Our data suggests alternative mechanisms of mTOR activation other than sustained hyperphosphorylation of 4E-BP1 by MCV small T antigen. In addition to activation of two downstream molecules 4E-BP1 and S6K, mTOR activation per se suggests the involvement of upstream regulators in both MCV positive and negative MCCs. Moreover, MCCs with undetectable mTOR hyperphosphorylation still demonstrate activation at the level of 4E-BP1, which is consistent with accumulating evidence pointing to the deregulation of 4E-BP1 /eIF4E axis playing a central role in tumor formation [6]. The mTORC1 pathway integrates inputs from at least five major intracellular and extracellular cues- growth factor, stress, energy status, oxygen and amino acids. Both TSC1/TSC2 dependent and independent signal transmission have been reported. PI3K, Wnt and Ras pathways activate mTORC1 via negatively regulation of TSC1/TSC2. Similarly, adenosine monophosphate-activated protein kinase (AMPK) in response to low energy and oxygen levels, phosphorylates TSC2 and thus mTORC1 becomes activated. Additionally, like Akt, AMPK also communicates directly and inhibit mTORC1. Conversely, mTORC2 directly activates Akt by phosphorylating its hydrophobic motif (Ser473), a site required for its maximal activation [36]. Relevant to MCC, two studies have reported that Akt hyperphosphorylation is independent of MCV status. Interestingly, one study claims that Akt phosphorylation is undetectable in 2 of the 3 MCV positive cell lines. Furthermore, although low mutation rates of PI3K/Akt are detected in both studies, an activated PI3K mutation was identified exclusively in MCCs without the presence of MCV. Since mTOR is a critical mediator of the canonical pathway of PI3K/AKT and Ras/ extracellular signal-regulated kinase, the intimate connection of mTOR and PI3K/Akt deserves further study in our samples. Mechanistic studies of mTOR activation in MCC, especially through upstream regulators are also warranted.
Befitting a pathway that positively regulates cellular metabolism, mTOR also promotes growth by negatively regulating autophagy [16, 19]. It has been shown that decreased autophagy and p62 accumulation owing to chronic mTOR activation leads to the development of hepatocellular carcinoma in a liver specific TSC1 knockout mouse model [28]. Several studies suggest that p62 accumulation attributes to oxidative stress and sustained p62 expression can promote tumorigenesis. Likewise, autophagy suppresses tumorigenesis through elimination of p62 [27]. In line with these observations, impaired autophagy is evident by immunohistochemistry of p62 accumulation and is further confirmed by LC3 immunoblotting in MCC tumors and in two primary human MCC cell lines. Upon specific mTOR inhibition, MCC cells display cell growth arrest and increased autophagosome formation. Interestingly, the cell death accompanied with increased autophagy is not only caspase independent, but is also attenuated by an autophagy inhibitor. Therefore, in addition to the established role of mTOR activation in promoting anabolic growth and proliferation, we reveal a previously less appreciated role for up-regulated mTOR pathway in prolonging cancer cell survival.
Autophagy can protect cells, but it can also mediate cellular demise, depending on the specific circumstances. Several scenarios of autophagic cell death have been proposed. First, the degree of cellular destruction that happens during autophagy can lead to cell death. Second, autophagy develops as a primary response to stress and then triggers cell death. Cell death that is dependent on successful autophagy has been described following the inhibition of apoptosis, implying a role as a backup once classic cell death has been abrogated [10, 32, 37]. ATG proteins may have autophagy-independent functions and may even be converted from pro-autophagy to pro-death proteins by proteolytic cleavage [47]. Furthermore, activation of NRF2, a nuclear factor erythrooid 2-related factor 2 by p62 aggregation provides an alternative mechanism of autophagic cell death [44]. Interestingly, Ras-driven tumors not only induce but also depend on autophagy for tumor progression [14]. In a separate context, oncogenic Ras is also found to induce autophagic cell death, that raise the possibility of autophagic cell death described in a study by Houben et al showing that activation of MAPK pathway in MCC induces apoptosis [8, 17]. Taken together, the role of autophagy in mediating the effect of mTOR activation on cancer is probably context specific and presents a therapeutic dilemma. Although small molecule autophagy inhibitors are in development, no autophagic specific inhibitors are available. The commonly used are lysosomotropic and anti-malaria agents which block the degradation of autophagic products by inhibiting lysosome function.
Nevertheless, the primary interest when it comes to therapeutic considerations is whether autophagy antagonizes or supports cellular demise. Inevitably, it is important to dissect whether it is indeed the effect of autophagy on the overall cellular response or whether it is only a bystander. To this point, further dissection of the interplay between autophagy and cell death in MCC cells is currently underway.
Notwithstanding these issues, the activation of autophagic cell death appears attractive for the treatment of cancer cells that are often resistant to the standard inducers of apoptosis and raise the exciting possibility that apoptosis-resistant cancers will no longer be able to escape death. Thus, our data provides a new insight in MCC pathogenesis and potential therapeutic options.
Supplementary Material
(A–B) MCC-2 and MCC-3 cells form clusters in vitro. (C–D) Hematoxylin and eosin staining of MCC-2 and MCC-3 cells illustrating large nuclei with loosely textured chromatin as well as scant cytoplasm. (E) Immunofluorecent staining demonstrating paranuclear dot staining of CK20 (green) in MCC-2 cells. (F) Expressions of MCC markers as indicated by RT-PCR analysis. (G) Activation of mTOR pathway and suppressed autophagy in MCC cells.
(A) Inhibition of mTOR pathway by mTOR kinase inhibitors. MCC-3 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. Lysates were prepared and subjected to immunoblotting analysis with indicated antibodies. (B) Induction of autophagy through inhibition of mTOR. MCC-3 cells were treated with mTOR kinase inhibitors in the presence of the lysosomal protease inhibitors E64d (10 μg/ml) and pepstatin A (10 μg/ml) for 24h, and cell lysates were analyzed by Western blot. The ratio of LC3-II/LC3-I expressions was calculated by using ImageJ software. Tubulin was used as a loading control.
(A–B) Representative cytograms showing percentages of total Annexin V positive cells. MCC-3 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. The cells were then stained with propidium iodide (PI) and Annexin-V and analyzed by flow cytometry. Each cytogram consists of data showing total Annexin V. (C–D) PP242-induced Cell death is not blocked by pan caspase inhibitor but an autophagy inhibitor bafilomycin A1. MCC-3 cells were treated with PP242 (2.5μM) for 24 hours with pan caspase inhibitor z-VAD-fmk (20μM) or 18 hours followed by an autophagy inhibitor Baf A1(10nM) for additional 6 hours before PI and Annexin-V staining and flow cytometry analysis. Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells. #P < 0.05 compared with PP242-treated cells.
Acknowledgements
The project described was supported by the Translational Research Institute (TRI), grants UL1TR000039 and KL2TR000063 through the NIH National Center for Research Resources and the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This study was also supported by funds from the Department of Dermatology and the Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement: All authors have no financial disclosure.
References
- [1].Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol. 2009;27:4021–4026. doi: 10.1200/JCO.2009.22.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baehrecke EH. Autophagic programmed cell death in Drosophila. Cell Death Differ. 2003;10:940–945. doi: 10.1038/sj.cdd.4401280. [DOI] [PubMed] [Google Scholar]
- [3].Becker JC. Merkel cell carcinoma. Ann Oncol. 2010;21(Suppl 7):vii81–vii85. doi: 10.1093/annonc/mdq366. [DOI] [PubMed] [Google Scholar]
- [4].Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM, Wong SL. Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer. 2007;110:1–12. doi: 10.1002/cncr.22765. [DOI] [PubMed] [Google Scholar]
- [5].Blobel GA, Gould VE, Moll R, Lee I, Huszar M, Geiger B, Franke WW. Coexpression of neuroendocrine markers and epithelial cytoskeletal proteins in bronchopulmonary neuroendocrine neoplasms. Lab Invest. 1985;52:39–51. [PubMed] [Google Scholar]
- [6].Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, Wang X, Larsson O, Selvaraj A, Liu Y, Kozma SC, Thomas G, Sonenberg N. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516–521. doi: 10.1038/modpathol.2009.3. [DOI] [PubMed] [Google Scholar]
- [8].Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42:23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- [9].Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359:497–498. doi: 10.1016/S0140-6736(02)07668-7. [DOI] [PubMed] [Google Scholar]
- [10].Fazi B, Bursch W, Fimia GM, Nardacci R, Piacentini M, Di Sano F, Piredda L. Fenretinide induces autophagic cell death in caspase-defective breast cancer cells. Autophagy. 2008;4:435–441. doi: 10.4161/auto.5669. [DOI] [PubMed] [Google Scholar]
- [11].Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grander D, Kharaziha P, Laane E, Pokrovskaja K, Panaretakis T. Autophagy as the main means of cytotoxicity by glucocorticoids in hematological malignancies. Autophagy. 2009;5:1198–1200. doi: 10.4161/auto.5.8.10122. [DOI] [PubMed] [Google Scholar]
- [14].Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, Coller HA, Dipaola RS, Gelinas C, Rabinowitz JD, White E. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hafner C, Houben R, Baeurle A, Ritter C, Schrama D, Landthaler M, Becker JC. Activation of the PI3K/AKT pathway in Merkel cell carcinoma. PLoS One. 2012;7:e31255. doi: 10.1371/journal.pone.0031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S, Natsume T, Takehana K, Yamada N, Guan JL, Oshiro N, Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Houben R, Ortmann S, Schrama D, Herold MJ, Berberich I, Reichardt HM, Becker JC. Activation of the MAP kinase pathway induces apoptosis in the Merkel cell carcinoma cell line UISO. J Invest Dermatol. 2007;127:2116–2122. doi: 10.1038/sj.jid.5700857. [DOI] [PubMed] [Google Scholar]
- [18].Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010;17:249–261. doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, Kundu M, Kim DH. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- [21].Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, Zur Hausen A. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- [22].Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- [23].Laane E, Tamm KP, Buentke E, Ito K, Kharaziha P, Oscarsson J, Corcoran M, Bjorklund AC, Hultenby K, Lundin J, Heyman M, Soderhall S, Mazur J, Porwit A, Pandolfi PP, Zhivotovsky B, Panaretakis T, Grander D. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16:1018–1029. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- [24].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- [27].Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Menon S, Yecies JL, Zhang HH, Howell JJ, Nicholatos J, Harputlugil E, Bronson RT, Kwiatkowski DJ, Manning BD. Chronic activation of mTOR complex 1 is sufficient to cause hepatocellular carcinoma in mice. Sci Signal. 2012;5:ra24. doi: 10.1126/scisignal.2002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [30].Moll I, Bohnert E, Herbst C, Forster W, Moll R, Franke WW. Establishment and characterization of two Merkel cell tumor cultures. J Invest Dermatol. 1994;102:346–353. doi: 10.1111/1523-1747.ep12371794. [DOI] [PubMed] [Google Scholar]
- [31].Moll R, Franke WW. Cytoskeletal differences between human neuroendocrine tumors: a cytoskeletal protein of molecular weight 46,000 distinguishes cutaneous from pulmonary neuroendocrine neoplasms. Differentiation. 1985;30:165–175. doi: 10.1111/j.1432-0436.1985.tb00528.x. [DOI] [PubMed] [Google Scholar]
- [32].Mujumdar N, Saluja AK. Autophagy in pancreatic cancer: an emerging mechanism of cell death. Autophagy. 2010;6:997–998. doi: 10.4161/auto.6.7.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nardi V, Song Y, Santamaria-Barria JA, Cosper AK, Lam Q, Faber AC, Boland GM, Yeap BY, Bergethon K, Scialabba VL, Tsao H, Settleman J, Ryan DP, Borger DR, Bhan AK, Hoang MP, Iafrate AJ, Cusack JC, Engelman JA, Dias-Santagata D. Activation of PI3K signaling in Merkel cell carcinoma. Clin Cancer Res. 2012;18:1227–1236. doi: 10.1158/1078-0432.CCR-11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Penn I, First MR. Merkel's cell carcinoma in organ recipients: report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- [35].Quaglino D, Di Leonardo G, Lalli G, Pasqualoni E, Di Simone S, Vecchio L, Ventura T. Association between chronic lymphocytic leukaemia and secondary tumours: unusual occurrence of a neuroendocrine (Merkell cell) carcinoma. Eur Rev Med Pharmacol Sci. 1997;1:11–16. [PubMed] [Google Scholar]
- [36].Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- [37].Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, Tsujimoto Y. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- [38].Shuda M, Kwun HJ, Feng H, Chang Y, Moore PS. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shvets E, Fass E, Elazar Z. Utilizing flow cytometry to monitor autophagy in living mammalian cells. Autophagy. 2008;4:621–628. doi: 10.4161/auto.5939. [DOI] [PubMed] [Google Scholar]
- [40].Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- [41].Tang F, Watkins JW, Bermudez M, Gray R, Gaban A, Portie K, Grace S, Kleve M, Craciun G. A life-span extending form of autophagy employs the vacuole-vacuole fusion machinery. Autophagy. 2008;4:874–886. doi: 10.4161/auto.6556. [DOI] [PubMed] [Google Scholar]
- [42].Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- [43].Tresse E, Kosta A, Luciani MF, Golstein P. From autophagic to necrotic cell death in Dictyostelium. Semin Cancer Biol. 2007;17:94–100. doi: 10.1016/j.semcancer.2006.10.010. [DOI] [PubMed] [Google Scholar]
- [44].Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ye S, Fowler TW, Pavlos NJ, Ng PY, Liang K, Feng Y, Zheng M, Kurten R, Manolagas SC, Zhao H. LIS1 regulates osteoclast formation and function through its interactions with dynein/dynactin and Plekhm1. PLoS One. 2011;6:e27285. doi: 10.1371/journal.pone.0027285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, Kim J, Verheijen J, Curran K, Malwitz DJ, Cole DC, Ellingboe J, Ayral-Kaloustian S, Mansour TS, Gibbons JJ, Abraham RT, Nowak P, Zask A. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- [50].Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- [51].Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan J, Lu Z, Bast RC, Jr., Feng Y, Yu Y. Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer. 2011;11:22. doi: 10.1186/1471-2407-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A–B) MCC-2 and MCC-3 cells form clusters in vitro. (C–D) Hematoxylin and eosin staining of MCC-2 and MCC-3 cells illustrating large nuclei with loosely textured chromatin as well as scant cytoplasm. (E) Immunofluorecent staining demonstrating paranuclear dot staining of CK20 (green) in MCC-2 cells. (F) Expressions of MCC markers as indicated by RT-PCR analysis. (G) Activation of mTOR pathway and suppressed autophagy in MCC cells.
(A) Inhibition of mTOR pathway by mTOR kinase inhibitors. MCC-3 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. Lysates were prepared and subjected to immunoblotting analysis with indicated antibodies. (B) Induction of autophagy through inhibition of mTOR. MCC-3 cells were treated with mTOR kinase inhibitors in the presence of the lysosomal protease inhibitors E64d (10 μg/ml) and pepstatin A (10 μg/ml) for 24h, and cell lysates were analyzed by Western blot. The ratio of LC3-II/LC3-I expressions was calculated by using ImageJ software. Tubulin was used as a loading control.
(A–B) Representative cytograms showing percentages of total Annexin V positive cells. MCC-3 cells were treated with WYE354 (3μM), PP242 (2.5μM), and Ku-0063794 (5μM) for 24h, respectively. The cells were then stained with propidium iodide (PI) and Annexin-V and analyzed by flow cytometry. Each cytogram consists of data showing total Annexin V. (C–D) PP242-induced Cell death is not blocked by pan caspase inhibitor but an autophagy inhibitor bafilomycin A1. MCC-3 cells were treated with PP242 (2.5μM) for 24 hours with pan caspase inhibitor z-VAD-fmk (20μM) or 18 hours followed by an autophagy inhibitor Baf A1(10nM) for additional 6 hours before PI and Annexin-V staining and flow cytometry analysis. Results shown are the means ± SEM of at least three independent experiments. *P < 0.05 compared with DMSO-treated cells. #P < 0.05 compared with PP242-treated cells.