Abstract
Objective
In the Mexican-American population, the prevalence of Type 2 diabetes mellitus (T2DM) is as high as 50% of the population. This randomized controlled clinical trial was designed to elucidate how treatment of periodontal disease affects HbA1c values in this population.
Materials and Methods
154 T2DM patients with periodontal disease were enrolled in the study. The test group was treated with scaling and root planing (SRP); the control group received oral hygiene instructions. At baseline and 4–6 weeks after therapy, a complete periodontal examination was performed. Blood was collected at baseline and 4 months later for HbA1c levels.
Results
126 individuals completed the study. Baseline mean ± SD HbA1c for the test and control groups were 9.0 ± 2.3% and 8.4 ± 2.0%, respectively. Non-significant difference in HbA1c reductions (0.6±2.1% and 0.3±1.7%) was found between test and control groups at 4 months. Comparisons of the periodontal clinical parameters between the test and control groups found significant differences with improved results in the test subjects.
Conclusions
No statistically significant differences were found in the changes of HbA1c levels between test and control groups. Non-surgical periodontal therapy improved the magnitude of change in periodontal parameters as compared to the control subjects.
ClinicalTrials.gov Identifier: NCT01128374
Keywords: type 2 diabetes mellitus, periodontitis, scaling and root planning, HbA1c, Hispanic population
Type 2 diabetes mellitus (T2DM) is a growing health concern, with incidence increasing in parallel with obesity (CDC, 2011). Diabetes has been linked to nephropathy, retinopathy, neuropathy, cardiovascular disease, and periodontitis (Soskolne and Klinger, 2001). Its total direct and indirect medical costs surpass $147 billion annually in the US alone (Center for Disease Control and Prevention, 2012b). Periodontitis is a known risk factor for the deterioration of glycemic control over time (Mealey and Oates, 2006). In a 2 year longitudinal trial, patients with diabetes and severe periodontitis had a six-fold increased risk of deteriorating glycemic control compared to patients with diabetes and no periodontitis (Taylor et al., 1996). Periodontitis may also be associated with an increased risk of other diabetic complications. For example, 82% of diabetic patients with severe periodontitis experienced the onset of one or more major cardiovascular, cerebrovascular, or peripheral vascular events compared to 21% of diabetic subjects without periodontal disease (Thorstensson et al., 1996). A longitudinal trial examined the effect of periodontal disease on overall mortality and cardiovascular disease-related mortality in more than 600 individuals with T2DM. The overall mortality rate from cardio-renal disease in patients with diabetes was 3.5 times higher in subjects with severe periodontitis (Saremi et al., 2005). The death rate from ischemic heart disease and diabetic nephropathy was 2.3 and 8.5 times, respectively, higher in severe periodontitis than in healthy or mild periodontal disease subjects. These results suggest that the presence of the chronic inflammatory status associated with periodontal disease in patients with diabetes may be associated with systemic deterioration.
Intervention trials have assessed the potential effects of periodontal therapy on glycemic control in diabetics. A meta-analysis (Engebretson and Kocher, 2013) showed an overall decrease in glycohemoglobin (HbA1c) of 0.36 (95% CI 0.19, 0.54) in diabetic subjects treated with periodontal therapy as compared to non-treated control. Another meta-analysis by (Sgolastra et al., 2013) showed a HbA1c reduction of 0.65% (95% CI 0.43, 0.88) after scaling and root planing (SRP). Whereas, a multicenter trial by Engebretson et. al., (2013) contradicted such effect, showing a non-statistically significant increase on HbA1c after SRP therapy. Therefore, conflicting evidence on the effect of SRP on glycemic control remains. Some racial/ethnic groups with a large proportion of immigrants have a high prevalence of cardiovascular risk factors including hypertension, hypercholesterolemia, and diabetes (Taylor and Borgnakke, 2008, Borrell et al., 2007). A study (Haffner et al., 1990) demonstrated that Mexican-Americans have an increased prevalence of T2DM compared to the general United States population. The Hispanic population, comprising 14.8% (44.3 million) of the US population (Center for Disease Control and Prevention, 2012a), is an underserved group facing several challenges such as socioeconomic status, language barriers and limited exposure to medical treatments which increases the risk of diabetic complications (Novak et al., 2008, Trevino et al., 2008).
Therefore, the aim of this randomized controlled trial was to evaluate the effect of non-surgical periodontal therapy, compared to no instrumentation, on glycemic control in a Hispanic population with diabetes and moderate to severe chronic periodontitis. Changes in periodontal clinical parameters were evaluated as secondary outcome measures.
Material and Methods
Study design
This randomized controlled trial allocated patients via computer randomization into the treatment group consisting on scaling and root planing and oral hygiene instructions or the control group which only received oral hygiene instructions.
After initial screening, subjects were invited to participate in a baseline diagnostic appointment when a medical history was obtained as well as a complete periodontal examination including probing depths (PD), clinical attachment levels (CAL), distance from the free gingival margin to the cementoenamel junction (FGM-CEJ), and bleeding on probing (BOP). Blood was drawn for initial HbA1c values. At this appointment patient education regarding periodontal disease and its association with diabetes was given along with oral hygiene instructions including the modified Bass technique, use of interproximal toothbrushes and dental floss. Experimental subjects were scheduled to return for SRP. Scaling and root planing was performed with an ultrasonic scaler (Dentsply, NY,) and Gracey curettes (Hu-Friedy, IL). Two quadrants were instrumented at each appointment under local anesthesia, using 2% lidocaine with 1:100,000 epinephrine (Dentsply, NY), with an endpoint of achieving smooth root surfaces (Greenstein, 1997). The procedure was performed by two calibrated periodontists.
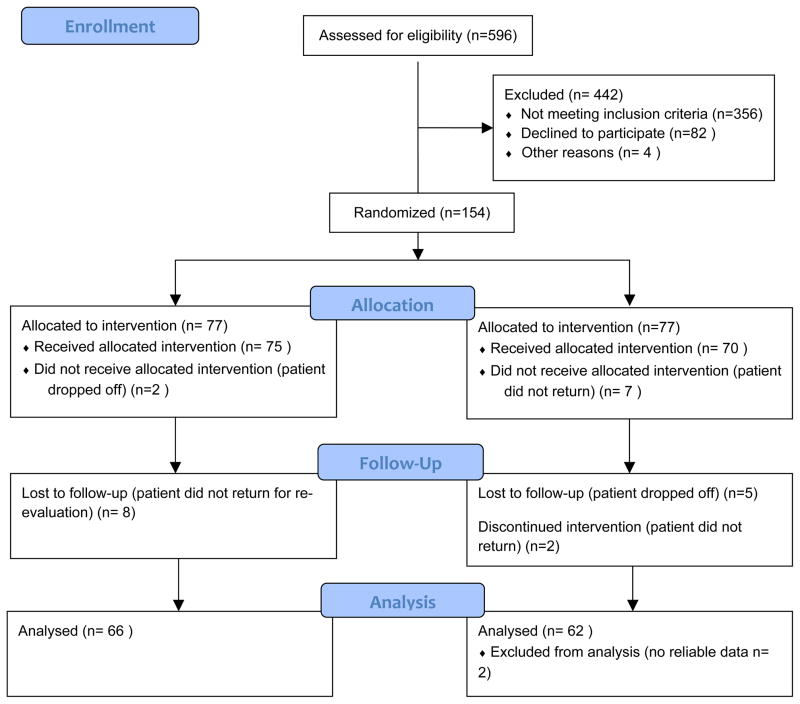
Test subjects were appointed 4–6 weeks after scaling and root planing, while control subjects were seen 4–6 weeks after baseline examination for periodontal re-evaluation (Morrison et al., 1980, Greenstein, 1997). At this appointment, all periodontal measurements were repeated, oral hygiene instructions reinforced, and any changes in medical history were recorded according to a standardized questionnaire. The medication change was determined to be related to diabetes management or not. If the medication was prescribed to decrease HbA1c values, the patient was labeled as “increased”. On the other hand, if the patient discontinued a medication for any reason we considered a “decrease”. Four months after the baseline appointment, all subjects returned for the 5th and final appointment where blood was drawn for final HbA1c values (Figure 1).
Figure 1.
Participant Flow Diagram
Subject population
This study was performed at the University of Texas Health Science Center at Houston. Following approval of the study by the Committee for the Protection of Human Subjects, 596 subjects were screened. 154 subjects met the inclusion criteria: nonsmokers, at least 18 years old, diagnosed with T2DM, Hispanic origin, dental treatment no less than 1 year ago, no systemic antibiotic therapy within 6 months of recruitment, and presence of localized or generalized severe chronic periodontitis according to the American Academy of Periodontology (AAP) criteria (Armitage, 1999). HbA1c levels for inclusion in the study equaled ≥ 6.5%. Subjects with initial HbA1C values between 5.7–6.5 % were included if they were taking hypoglycemic medications (n=16).
Permuted blocks randomization with varying block sizes using Stata 11 was performed by a statistician (DT) to generate allocation sequences. These sequences were used by the research coordinator (AC) to recruit and blindly randomize 154 participants either to a control (n=77) or experimental group (n=77) with a 1:1 allocation ratio (Figure 1). The sample size was calculated based on the primary outcome HbA1c. A previous study (Koromantzos et al., 2011) reported a difference of 0.5% (SD, 0.85%) in HbA1c change between experimental and control groups at 3 months. To detect this difference, a sample of 77 patients in each group (including 20% attrition) was required to achieve 90% power with two-sided alpha=0.05 (PS-Power and sample size calculation version 3.0) (Dupont and Plummer, 1990).
HbA1c assessment
Change in HbA1c levels at 4 months was the primary outcome. Upon patient recruitment, approximately 6ml of blood was collected from a peripheral vein and transported at 20°C to Memorial Herman Hospital (Houston, TX) for HbA1c reading. The personnel who conducted the readings were blinded as to which group the patient belong. The analyzer used was the Afinion HbA1c AS100 Analyzer (Axis Shield, Oslo, NW) which measure all reportable ranges of HbA1c values from 4.0–15.0%; (0.1% intervals). High value samples were run in duplicate and control cartridges were run twice, once on every new batch of cartridges that was used as well as on a monthly basis. Also, in compliance with the College of American Pathologists and Clinical Laboratory Improvement Amendments, several samples were run in duplicates. HbA1c was measured by an automated boronate affinity assay for the determination of the percentage of hemoglobin A1c in human whole blood. The standardization of the machine is traceable to the International Federation of Clinical Chemistry and Laboratory Medicine Reference Method for measurement of HbA1c. HbA1c values are reported according to the National Glycohemoglobin Standardization Program recommendations at Diabetes Control and Complications Trial level. Based on the experience obtained from internal and external documentation of Afinion HbA1c, a precision of <3%, expressed by the coefficient of variation, is expected in a controlled laboratory setting.
Periodontal Disease Assessment
Changes in clinical periodontal parameters at 4–6 weeks were the secondary outcomes. Periodontal status was evaluated for extent (frequency of affected sites) and severity of clinical parameters under the classification given by the American Academy of Periodontology (Armitage, 1999). This study evaluated the following periodontal parameters: Probing depth (PD): measured from the free gingival margin to the bottom of the sulcus; Clinical attachment level (CAL): measured from the cemento-enamel junction to the bottom of the sulcus; Recession (REC): measured as the distance from the free gingival margin to the exposed cement-enamel junction; Bleeding on probing (BOP): measured as the percentage of sites with presence of blood upon probing.
All measurements were recorded at six sites per tooth (mesio-buccal, disto-buccal, mid-facial, mesio-lingual, disto-lingual and mid-lingual) using a calibrated North Carolina probe (Hu-Friedy, Chicago IL). Two examiners (IG, JC) who have been treating patients for 3 years at a university setting showed an inter-examiner calibration agreement average of Kappa= 0.86.
In consideration of the periodontal status of the entire dentition, and following the guidelines provided by the American Academy of Periodontology (Armitage, 1999) and peer reviewed literature (Morrison et al. 1980, Hill et al. 1981), PDs were stratified into categories: mild (1–3mm), moderate (4–6 mm) and severe (≥ 7mm), whereas CALs were stratified into categories of: mild (1–2mm), moderate (3–4mm) and severe (≥ 5mm). PD and CAL data are presented in millimeters as well as the percentage of sites within each stated category. Probing data was entered and CAL calculated electronically by an electronic dental patient record system (Axium, Coquitlam, BC).
Statistical Analyses
Mean ± SD and percentages were used to summarize baseline continuous and categorical variables, respectively. Individual means of PD, CAL and REC were averaged across subjects to compute means of PD, CAL and REC within each treatment group. Between-group comparisons for baseline characteristics were performed using independent samples t-tests or Wilcoxon-Mann-Whitney tests for continuous variables and Chi-squared tests for categorical variables. Wilcoxon signed ranks tests were used to compare changes of HbA1c from baseline to 4 months and clinical periodontal parameters from baseline to 4 weeks. Independent samples t-tests or Wilcoxon-Mann-Whitney tests were used to compare changes of HbA1c and clinical periodontal parameters between the experimental and control groups. Multiple linear regression analysis was performed to examine the effect of periodontal therapy and all other independent variables on the change in HbA1c level at 4 months. Additional post hoc subgroup analyses were also conducted. P values less than 0.05 were adjusted for multiple comparisons using Bonferroni correction. Statistical analysis was performed using the Stata 12 software program (StataCorp LP).
Results
A total of 126 participants completed this study. Table 1 presents the demographics, clinical periodontal parameters and HbA1c values at baseline. There were no statistically significant differences between any baseline parameters of experimental and control subjects regarding age, gender, teeth numbers, diabetes control medications, initial periodontal status, and HbA1c levels. Percentages of test and control subjects taking insulin were 21% and 12%, respectively. 18 test and 13 control subjects had changes in their medications during the 4 month trial.
Table 1.
Demographic characteristics of the participants
| Experimental group (n = 66) | Control group (n =60) | |
|---|---|---|
| Age (years) | ||
| Mean ± S.D | 51.5 ± 9.0 | 54.0 ± 10.2 |
| Gender (%) | ||
| Male | 45.5 | 41.7 |
| Female | 54.6 | 58.3 |
| Number of teeth (n) | ||
| Mean ± S.D | 23.6 ± 5.7 | 24.7 ± 5.6 |
| Diabetic treatment (%) | ||
| No | 21.2 | 20.0 |
| Medicine | 78.8 | 80.0 |
| Medical change (%) | ||
| No | 72.7 | 78.3 |
| Increase | 13.6 | 16.7 |
| Decrease | 13.7 | 5.0 |
| % of sites with BOP | ||
| Mean ± S.D | 51.2 ± 29.4 | 51.8 ± 30.0 |
| % of sites with PD | ||
| 1–3 mm | 54.3 ± 21.8 | 54.4 ± 21.9 |
| 4–6 mm | 40.4 ± 17.4 | 38.6 ± 16.0 |
| ≥ 7 mm | 5.2 ± 7.3 | 7.0 ± 10.4 |
| PD (sites with initial PD 1–3 mm) | ||
| Mean ± S.D (mm) | 2.6 ± 0.2 | 2.6 ± 0.2 |
| PD (sites with initial PD 4–6 mm) | ||
| Mean ± S.D (mm) | 4.8 ± 0.3 | 4.7± 0.3 |
| PD (sites with initial PD ≥ 7 mm)* | ||
| Mean ± S.D (mm) | 7.4 ± 0.5 | 7.4 ± 0.6 |
| % of sites with CAL | ||
| 1–2 mm | 23.0 ± 16.2 | 22.4 ± 18.6 |
| 3–4 mm | 39.3 ± 14.0 | 39.2 ± 16.0 |
| ≥ 5mm | 37.5 ± 25.4 | 38.3 ± 28.3 |
| CAL (sites with initial PD 1–3 mm) | ||
| Mean ± S.D (mm) | 3.1 ± 1.0 | 3.2 ± 1.0 |
| CAL (sites with initial PD 4–6 mm) | ||
| Mean ± S.D (mm) | 5.1 ± 1.0 | 5.1 ± 1.2 |
| CAL (sites with initial PD ≥ 7mm)* | ||
| Mean ± S.D (mm) | 7.7 ± 1.4 | 8.1 ± 1.7 |
| REC (sites with initial PD 1–3 mm) | ||
| Mean ± S.D (mm) | −0.5 ± 0.8 | −0.6 ± 0.9 |
| REC (sites with initial PD 4–6 mm) | ||
| Mean ± S.D (mm) | −0.3 ± 0.9 | −0.4 ± 1.1 |
| REC (sites with initial PD ≥ 7mm) * | ||
| Mean ± S.D (mm) | −0.4 ± 1.4 | −0.7 ± 1.5 |
| HbA1c (%) | ||
| Mean ± S.D (minimum–maximum) | 9.0 ± 2.3 (5.7 – 16) | 8.4 ± 2.0 (5.8–14.2) |
All comparisons between test and control groups: non-significant
Experiment group (n = 48), control group (n = 42)
Effect of periodontal treatment on HbA1c
Table 2 shows the comparison of HbA1c status at baseline and at the end of the study (4 months after baseline) in experimental and control groups. Baseline mean ± SD HbA1c percentages for the experimental group measured 9.0 ± 2.3% and controls 8.4 ± 2.0%. The percentages of HbA1c decreased for the experimental group 0.6% (8.4 ± 1.9), whereas the control group decreased 0.3% (8.1 ± 1.8). There was a non-statistically significant difference between groups (p = 0.89) (Table 2). Multiple regression analysis of all subjects showed that the association between change in HbA1c levels and the periodontal treatment was not significant (regression coefficient = 0.04, 95% CI = −0.50 to 0.59).
Table 2.
HbA1c at baseline and 4th month in experiment and control groups
| HbA1c | Baseline | 4th months | Δ | p* | p** |
|---|---|---|---|---|---|
| Experimental group (n = 66) | 9.0 ± 2.3 | 8.4 ± 1.9 | −0.6 ± 2.1 | 0.09 | 0.89 |
| Control group (n = 60) | 8.4 ± 2.0 | 8.1 ± 1.8 | −0.3 ± 1.7 | 0.10 |
Δ: difference between HbA1c at baseline and at 4th month.
p*: comparison of baseline and 4th month data
p**: comparison of the changes in HbA1c between treatment and control groups.
Effect of demographic, glycemic control medications, and periodontal parameters on the change in HbA1c levels
Effect of demographic, glycemic control medications, and clinical parameters on the change in the levels of HbA1c four months after baseline measurements was examined using multiple linear regression analysis. The change in HbA1c levels was correlated significantly with baseline HbA1c (regression coefficient = −0.46, 95% CI = −0.60 to −0.33) and increase in the glycemic control medications (regression coefficient = −1.17, 95% CI = −1.97 to −0.38). Therefore, a subgroup analysis was conducted to compare the change in the levels of HbA1c at four months between well-glycemic controlled subjects (baseline HbA1c <7%) in the treatment group versus HbA1c change in the control group as well as between poor-glycemic controlled subjects (baseline HbA1c ≥ 7%) in the treatment group versus HbA1c change in the control group. The differences in HbA1c changes were not statistically significant (p = 0.29 and 0.59 for well and poorly controlled groups, respectively). When all patients who reported medication changes were removed, the differences in HbA1c changes between test and control groups were not statistically significant either (p= 0.58).
Effect of periodontal treatment in periodontal parameters
Table 3 shows the changes in periodontal parameters between baseline and reevaluation for the experimental and control groups. Experimental groups showed statistically significant reductions on the percentages of sites with PD in the 4–6mm and ≥7mm stratifications, as well as significant increases in the percentage of sites in the 1–3mm PD category. Whereas the control group showed statistically significant reduction in PD 4–6 mm category and increase in the 1–3 mm PD number. Only the experimental group showed statistically significant changes in CAL in the percentage of sites in the 1–2mm and ≥5mm stratifications (Table 3). Comparisons of the changes in clinical parameters between the experimental and control groups are found in Table 4. Comparisons of the mean changes in PD as well as the percentage of sites in the 1–3mm and 4–6mm stratifications found statistically significant differences between experimental and control groups (Bonferroni corrected p<0.05). Comparisons of the mean changes in CAL found significant differences between experimental and control groups at sites with initial PD of 1–3mm and 4–6mm (Bonferroni corrected p<0.05). Comparisons of the percentage of sites with CAL measuring 1–2mm and ≥5mm found significant differences between the experimental and control groups. The percentage of sites exhibiting BOP significantly decreased in the experimental subjects as compared to the control subjects (Table 3). The median (and range) of BOP changes were −18.7% (−85% to 42.2%) and −8.7% (−100% to 35%) for the experiment and control groups, respectively.
Table 3.
Clinical periodontal status at baseline and 4–6th week in experimental and control groups
| Experimental group (n = 66) | Control group (n = 60) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 4th week | p | Baseline | 4th week | p | |
| % of sites with BOP | ||||||
| Mean ± S.D | 51.2 ± 29.4 | 28.2 ± 25.0 | < 0.001 | 51.8± 30.0 | 39.6 ± 27.4 | < 0.001 |
| % of sites with PD | ||||||
| 1–3 mm | 54.3 ± 21.8 | 73.3 ± 17.8 | < 0.001 | 54.4 ± 21.9 | 60.4 ± 23.3 | < 0.001 |
| 4–6 mm | 40.4 ± 17.3 | 24.5 ± 14.5 | < 0.001 | 38.6 ± 16.0 | 33.9 ± 16.4 | 0.001 |
| ≥ 7 mm | 5.2 ± 7.3 | 2.3 ± 7.8 | < 0.001 | 7.0 ± 10.4 | 5.7 ± 10.3 | 0.03** |
| PD (sites with initial PD 1–3 mm) | ||||||
| Mean ± S.D (mm) | 2.6 ± 0.2 | 2.5 ± 0.4 | 0.04** | 2.6 ± 0.2 | 2.8 ± 0.5 | < 0.001 |
| PD (sites with initial PD 4–6 mm) | ||||||
| Mean ± S.D (mm) | 4.8 ± 0.3 | 3.6 ± 0.6 | < 0.001 | 4.7 ± 0.3 | 4.2 ± 0.7 | < 0.001 |
| PD (sites with initial PD ≥ 7 mm)* | ||||||
| Mean ± S.D (mm) | 7.4 ± 0.5 | 5.3 ± 1.2 | < 0.001 | 7.4 ± 0.6 | 5.8 ± 1.5 | < 0.001 |
| % of sites with CAL | ||||||
| 1–2 mm | 23.0 ± 16.2 | 31.6 ± 19.1 | < 0.001 | 22.4 ± 18.6 | 23.0 ± 18.3 | 0.30 |
| 3–4 mm | 39.3 ± 14.0 | 41.6 ± 14.0 | 0.09 | 39.2 ± 16.0 | 40.0 ± 17.0 | 0.65 |
| ≥ 5mm | 37.5 ± 25.4 | 26.7 ± 23.8 | < 0.001 | 38.3 ± 28.3 | 36.8 ± 29.4 | 0.36 |
| CAL (sites with initial PD 1–3 mm) | ||||||
| Mean ± S.D (mm) | 3.1 ± 1.0 | 3.1 ± 1.1 | 0.85 | 3.2 ± 1.0 | 3.3 ± 1.1 | < 0.001 |
| CAL (sites with initial PD 4–6 mm) | ||||||
| Mean ± S.D (mm) | 5.1 ± 1.0 | 4.6 ± 1.0 | < 0.001 | 5.1 ± 1.2 | 4.9 ± 1.3 | < 0.001 |
| CAL (sites with initial PD ≥ 7mm)* | ||||||
| Mean ± S.D (mm) | 7.7 ± 1.4 | 6.9 ± 1.4 | < 0.001 | 8.1 ± 1.7 | 7.3 ± 1.6 | < 0.001 |
| REC (sites with initial PD 1–3 mm) | ||||||
| Mean ± S.D (mm) | −0.5 ± 0.8 | −0.6 ± 0.9 | 0.01** | −0.6 ± 0.9 | −0.6 ± 1.0 | 0.11 |
| REC (sites with initial PD 4–6 mm) | ||||||
| Mean ± S.D (mm) | −0.3 ± 0.9 | − 0.5 ± 0.9 | < 0.001 | −0.4 ± 1.1 | −0.5 ± 1.1 | 0.003** |
| REC (sites with initial PD ≥ 7mm) * | ||||||
| Mean ± S.D (mm) | −0.4 ± 1.4 | −0.8 ± 1.3 | < 0.001 | −0.7 ± 1.5 | −0.8 ± 1.4 | 0.06 |
p: comparison of baseline and 4th week data.
experimental group (n=48), control group (n=42)
p values were > 0.05 after adjustment for multiple comparisons using Bonferroni’s correction
Table 4.
Comparison of changes of clinical periodontal status between experimental and control groups
| Changes in experiment group (N = 66) | Changes in control group (N = 60) | P* | |
|---|---|---|---|
| % of sites with BOP | |||
| Mean ± S.D | −23.0 ± 25.3 | −12.3 ± 21.5 | 0.004# |
| % of sites with PD | |||
| 1–3 mm | 18.9 ± 12.9 | 6.0 ± 10.9 | < 0.001** |
| 4–6 mm | −16.0 ± 10.6 | −4.7 ± 10.0 | < 0.001** |
| ≥ 7 mm | −3.0 ± 6.7 | −1.3 ± 4.6 | 0.008# |
| PD (sites with initial PD 1–3 mm) | |||
| Mean ± S.D (mm) | −0.05 ± 0.4 | 0.2 ± 0.4 | < 0.001 |
| PD (sites with initial PD 4–6 mm) | |||
| Mean ± S.D (mm) | −1.1 ± 0.5 | −0.5 ± 0.5 | < 0.001 |
| PD (sites with initial PD ≥ 7 mm)*** | |||
| Mean ± S.D (mm) | −2.2 ± 1.2 | −1.7 ± 1.4 | 0.009# |
| % of sites with CAL | |||
| 1–2 mm | 8.6 ± 9.4 | 0.6 ± 10.3 | < 0.001 |
| 3–4 mm | 2.3 ± 10.6 | 0.8 ± 11.1 | 0.44** |
| ≥5mm | −10.9 ± 10.4 | −1.5 ± 9.7 | < 0.001 |
| CAL (sites with initial PD 1–3 mm) | |||
| Mean ± S.D (mm) | 0.02 ± 0.2 | 0.1 ± 0.2 | < 0.001 |
| CAL (sites with initial PD 4–6 mm) | |||
| Mean ± S.D (mm) | −0.5 ± 0.2 | −0.2 ± 0.3 | < 0.001** |
| CAL (sites with initial PD ≥ 7mm)*** | |||
| Mean ± S.D (mm) | −0.9 ± 0.6 | −0.8 ± 0.9 | 0.14 |
| REC (sites with initial PD 1–3 mm) | |||
| Mean ± S.D (mm) | −0.1 ± 0.3 | −0.04 ± 0.2 | 0.60 |
| REC (sites with initial PD 4–6 mm) | |||
| Mean ± S.D (mm) | −0.2 ± 0.3 | −0.1 ± 0.3 | 0.16** |
| REC (sites with initial PD ≥ 7mm) *** | |||
| Mean ± S.D (mm) | −0.4 ± 0.7 | −0.2 ± 0.8 | 0.24 |
Change of clinical periodontal status = re-evaluation – initial
Wilcoxon-Mann-Whitney test
Student t-test
experimental group (n=48), control group (n=42)
p values were > 0.05 after adjustment for multiple comparisons using Bonferroni’s correction
Discussion
The relationship between diabetes and periodontal disease has been related to systemic immunoinflammatory responses and subsequent wound healing (Mealey and Oates, 2006). Up-regulation of inflammatory cytokines has been reported in individuals with diabetes and chronic periodontitis (Salvi et al., 1997, Loos et al., 2000). In a two way relationship, therapeutic measures to control hyperglycemia and or chronic periodontitis may improve the systemic health of individuals afflicted with these disease processes. This study evaluated the effects of periodontal therapy on glycemic control in a Hispanic population with T2DM and generalized moderate to severe chronic periodontitis. Non-surgical periodontal therapy reduced HbA1c levels by 0.6%, twice the effect observed in the control subjects who received extensive oral hygiene instructions along with home care devices, with no professional mechanical therapy. These results are corroborated by comparison with similarly designed clinical trials, where changes in HbA1c ranged from 0.4 – 0.8% for subjects treated with SRP (Chen et al., 2012, Kiran et al., 2005, Manouchehr-Pour et al., 1981, Koromantzos et al., 2011).
The secondary outcome measures evaluated the clinical responses of the study participants at 4 weeks after initiation of the study. The data were stratified according to initial PD severity. Within each stratification, the changes in percentage of sites with slight, moderate, and severe pockets showed three fold increases at test sites compared to the control sites. Only the test sites exhibited statistically significant improvements in CAL; reflecting a shift toward more slight to moderate attachment loss. Comparison of our results to the body of literature is difficult due to differences in severity of periodontitis, study designs, and data presentation. Of close parallel was a trial evaluating the effects of non-surgical periodontal therapy on glycemic control in a Greek population with T2DM and moderate to severe chronic periodontitis (Koromantzos et al., 2011). A similar shift in the percent of sites with shallower PDs was reported. After non-surgical periodontal therapy, both studies found a 19% increase in the percent of site measuring 1–3mm, with 11% and 16% reductions in the moderate pocket range, and single digit reductions in the severe pocket range. The control groups in both studies also followed a similar trend toward reduction of deeper pockets and an increase in number of shallow pockets, albeit 3 times less in magnitude as compared to the test group results. Comparison of changes in the percentage of sites with slight, moderate, or severe attachment loss found, in both studies, an 11% reduction in the number of sites with severe attachment. In other trials evaluating subjects with T2DM and slight, or slight to moderate chronic periodontitis, the effect of SRP on periodontal parameters was approximate 1/3 the magnitude observed in subjects with T2DM and moderate to severe periodontitis (Tervonen et al., 1991, Chen et al., 2012).
While no subgingival instrumentation was performed during the study period on our control subjects, statistically significant changes in periodontal parameters were observed. For the slight to moderately deep pockets, the observed changes measured less than or equal to 0.5mm. While statistically significant, these values are not clinically significant. At sites initially ≥7mm, PD reduction and CAL gain equaled 1.7mm and 0.8mm, respectively. The importance of oral home care as a lifestyle improvement toward systemic health was stressed to all subjects. Toothbrushes and interproximal brushes were given to all participants with instructions for use twice daily. Improvements in home care in the control subjects may have reduced the subgingival putative pathogen microflora yielding a reduction in probing depth at the deep sites. (Page and Rams, 2013, Christou et al., 1998). With reduced inflammation in the tissues surrounding the periodontal pockets and the continued presence of subgingival calculus, the ability to accurately measure the location of the CEJ and the true depth of the periodontal pocket may have been impeded yielding better than expected CAL gains.
Periodontal therapy and improved home care yielded significant reductions in the percentage of sites eliciting BOP (23% and 12%, for test and control groups, respectively). Linear regression analysis found no statistically significant correlation between changes in HbA1c and changes in BOP. Thus, local changes in gingival inflammation, as measured by evaluating sulcular bleeding after mild provocation with a periodontal probe within the gingival sulci, did not correlate with systemic changes in glycemic control.
How does our 4 week evaluation of periodontal parameters compare to studies extending 3–6 months? Chen et al. (2012) published periodontal changes 1.5, 3, and 6 months after SRP in a Chinese population with T2DM and slight to moderate periodontitis. Results found after 1.5 months were similar (within 1% of change) to the 3 and 6-month results. In a healthy population with moderate to severe periodontitis, it was found that 3 months after the hygienic phase of therapy an additional 0.5–1mm of PD reduction and CAL gain was found at sites with moderate to deep pocket depths (Kaldahl et al., 1988). A second round of SRP was performed 4 weeks after the initial SRP in the Kaldahl (1988) study but not in this or the Chen study (2012), which may have influenced the reported outcomes.
Possible limitations of this study should be considered. We should note that the population included in this study was Hispanic individuals belonging to a low socioeconomically disadvantaged group and therefore these results may not represent the general population. Dietary choices and daily physical activities were not assessed which may have influenced glycemic control (Jones et al., 2003). The baseline HbA1c levels for test and control subjects ranged from 5.7–16% and 5.8–14.2%, respectively. The study participants reported limited access to medical and oral care services. At baseline, twenty percent of the test and control subjects were not taking medications to treat their medical condition. As a component of the present research, we had counseled all participants on living a healthy lifestyle and provided NIH/NIDCR brochures with information in oral health habits and therapies as well as oral hygiene kits. While participants were asked not to change their daily eating, exercise, and medications for the duration of the study, subjects with baseline HbA1c levels > 10% were referred for medical consultation to a medical care facility servicing indigent populations. Prior to completion of the study, nine test and ten control subjects were either placed on hypoglycemic medications, or had increased medications or dosages of hypoglycemics, resulting in mean HbA1c reductions of 2.7% and 1.8% for test and control subjects, respectively. Linear regression analysis found a correlation between the change in HbA1c and change in medications. Recalculation of the change in HbA1c after elimination of the responses from these nineteen subjects found reductions of 0.3±1.9% and 0.06±1.7% for the test and control groups, respectively. Comparisons of this data between and within groups found no statistically significant differences. While our test group HbA1c results did not reach statistical significance, they were similar to those reported in a recently published meta-analysis that showed a modest (.36% [CI, 0.54% to 0.19%]), but significant reduction following periodontal therapy (Engebretson and Kocher, 2013). Within the limits of this study, we can conclude that non-surgical periodontal therapy had no statistically significant impact on changes in HbA1c levels. Non-surgical periodontal therapy resulted in statistically significant improvements in periodontal status as compared to the control subjects and thus, should be considered as a component in the routine medical care of individuals with type 2 diabetes mellitus and periodontitis.
CLINICAL RELEVANCE.
Scientific Rationale
Texas Hispanic populations are a high risk population for type 2 Diabetes mellitus with an increased prevalence due to significant socioeconomic barriers. This study aimed to determine if non-surgical periodontal therapy improves glycemic control in patients with diabetes and chronic periodontitis.
Principal Findings
Non-surgical periodontal therapy had no statistically significant changes on HbA1c levels in experimental versus control groups. Non-surgical periodontal therapy resulted in statistically significant improvements in periodontal status as compared to the control subjects.
Practical Implications
In individuals afflicted with diabetes and chronic periodontitis, periodontal therapy and plaque control is recommended as a component of diabetic control.
Acknowledgments
We want to thank Dr. James Katancik at the Oregon Health Sciences Institute, Portland, OR. We deeply appreciate Dr. Jon Tyson at UTMS, Houston, TX for his unconditional support.
Supported by NIH/NIDCR Grant # 5K12 RR024149
References
- Armitage GC. Development of a classification system for periodontal diseases and conditions. Annals of Periodontology. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Kunzel C, Lamster I, Lalla E. Diabetes in the dental office: using NHANES III to estimate the probability of undiagnosed disease. Journal of Periodontal Research. 2007;42:559–565. doi: 10.1111/j.1600-0765.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Number (in millions) of persons with diagnosed diabetes, United States, 1980–2005 [Online] Atlanta: CDC; 2012a. [Accessed September 18 2013]. Diabetes data & trends. Available: http://www.cdc.gov/diabetes/pubs/factsheet11.htm. [Google Scholar]
- Center for Disease Control and Prevention. Overweight and obesity. US Obesity Trends 1985–2006 [Online] Atlanta: CDC; 2012b. [Accessed Semtember 18 2013]. Diabetes data & trends. Available: http://www.cdc.gov/obesity/data/adult.html. [Google Scholar]
- Chen L, Luo G, Xuan D, Wei B, Liu F, Li J, Zhang J. Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized study. Journal of Periodontology. 2012;83:435–443. doi: 10.1902/jop.2011.110327. [DOI] [PubMed] [Google Scholar]
- Christou V, Timmerman MF, Van Der Velden U, Van Der Weijden FA. Comparison of different approaches of interdental oral hygiene: interdental brushes versus dental floss. Journal of Periodontology. 1998;69:759–764. doi: 10.1902/jop.1998.69.7.759. [DOI] [PubMed] [Google Scholar]
- Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. Journal of Periodontology. 2013;84:S153–169. doi: 10.1902/jop.2013.1340017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein G. Contemporary interpretation of probing depth assessments: diagnostic and therapeutic implications. A literature review. Journal of Periodontology. 1997;68:1194–1205. doi: 10.1902/jop.1997.68.12.1194. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK. Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes. 1990;39:283–288. doi: 10.2337/diab.39.3.283. [DOI] [PubMed] [Google Scholar]
- Jones H, Edwards L, Vallis TM, Ruggiero L, Rossi SR, Rossi JS, Greene G, Prochaska JO, Zinman B. Changes in diabetes self-care behaviors make a difference in glycemic control: the Diabetes Stages of Change (DiSC) study. Diabetes Care. 2003;26:732–737. doi: 10.2337/diacare.26.3.732. [DOI] [PubMed] [Google Scholar]
- Kaldahl WB, Kalkwarf KL, Patil KD, Dyer JK, Bates RE., Jr Evaluation of four modalities of periodontal therapy. Mean probing depth, probing attachment level and recession changes. Journal of Periodontology. 1988;59:783–793. doi: 10.1902/jop.1988.59.12.783. [DOI] [PubMed] [Google Scholar]
- Kiran M, Arpak N, Unsal E, Erdogan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. Journal of Clinical Periodontology. 2005;32:266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- Koromantzos PA, Makrilakis K, Dereka X, Katsilambros N, Vrotsos IA, Madianos PN. A randomized, controlled trial on the effect of non-surgical periodontal therapy in patients with type 2 diabetes. Part I: effect on periodontal status and glycaemic control. Journal of Clinical Periodontology. 2011;38:142–147. doi: 10.1111/j.1600-051X.2010.01652.x. [DOI] [PubMed] [Google Scholar]
- Loos BG, Craandijk J, Hoek FJ, Wertheim-Van Dillen PM, Van Der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. Journal of Periodontology. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- Manouchehr-Pour M, Spagnuolo PJ, Rodman HM, Bissada NF. Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. Journal of Periodontology. 1981;52:410–415. doi: 10.1902/jop.1981.52.8.410. [DOI] [PubMed] [Google Scholar]
- Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. Journal of Periodontology. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- Morrison EC, Ramfjord SP, Hill RW. Short-term effects of initial, nonsurgical periodontal treatment (hygienic phase) Journal of Clinical Periodontology. 1980;7:199–211. doi: 10.1111/j.1600-051x.1980.tb01963.x. [DOI] [PubMed] [Google Scholar]
- Novak MJ, Potter RM, Blodgett J, Ebersole JL. Periodontal disease in Hispanic Americans with type 2 diabetes. Journal of Periodontology. 2008;79:629–636. doi: 10.1902/jop.2008.070442. [DOI] [PubMed] [Google Scholar]
- Page LR, Rams TE. Subgingival root brushing in deep human periodontal pockets. Journal of the International Academy of Periodontology. 2013;15:55–63. [PubMed] [Google Scholar]
- Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. Journal of Periodontology. 1997;68:127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, Shlossman M, Bennett PH, Genco R, Knowler WC. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. Journal of Periodontology. 2013;84:958–973. doi: 10.1902/jop.2012.120377. [DOI] [PubMed] [Google Scholar]
- Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Annals of Periodontology. 2001;6:91–98. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Diseases. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. Journal of Periodontology. 1996;67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- Tervonen T, Knuuttila M, Pohjamo L, Nurkkala H. Immediate response to nonsurgical periodontal treatment in subjects with diabetes mellitus. Journal of Clinical Periodontology. 1991;18:65–68. doi: 10.1111/j.1600-051x.1991.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Thorstensson H, Kuylenstierna J, Hugoson A. Medical status and complications in relation to periodontal disease experience in insulin-dependent diabetics. Journal of Clinical Periodontology. 1996;23:194–202. doi: 10.1111/j.1600-051x.1996.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Trevino RP, Fogt DL, Wyatt TJ, Leal-Vasquez L, Sosa E, Woods C. Diabetes risk, low fitness, and energy insufficiency levels among children from poor families. Journal of the American Dietetic Association. 2008;108:1846–1853. doi: 10.1016/j.jada.2008.08.009. [DOI] [PubMed] [Google Scholar]