Abstract
Haploidentical SCT (HaploSCT) has been most commonly performed using a myeloablative, TBI-based preparative regimen; however, the toxicity with this approach remains very high. We studied the feasibility of a reduced-intensity conditioning regimen in a phase II clinical trial using fludarabine, melphalan and thiotepa and antithymocyte globulin (ATG) for patients with advanced hematological malignancies undergoing T-cell depleted HaploSCT. Twenty-eight patients were entered in the study. Engraftment with donor-derived hematopoiesis was achieved in 78% of patients after a median of 13 days. Six patients experienced primary graft failure, three out of four tested patients had donor-specific anti-HLA antibodies (DSA) (P = 0.001). Toxicity included mostly infections. A total of 21 out of 22 patients with AML/myelodysplastic syndrome (MDS) achieved remission after transplant (16 with relapsed/refractory AML). Five out of the 12 patients (42%) with AML/MDS with <15% BM blasts survived long term as compared with none with more advanced disease (P = 0.03). HaploSCT with this fludarabine, melphalan and thiotepa and ATG RIC is an effective, well-tolerated conditioning regimen for patients with AML/MDS with low disease burden at the time of transplant and allowed a high rate of engraftment in patients without DSA. Patients with overt relapse fared poorly and require novel treatment strategies.
Keywords: haploidentical SCT, T-cell depletion, AML, myelodysplastic syndromes
Introduction
Hematopoietic SCT is an effective treatment for advanced hematological malignancies.1,2 An HLA-identical donor is preferred, however a matched sibling or unrelated donor is unavailable for many patients.3,4 Mismatched relatives represent an alternative donor source, and virtually all patients will have an available haploidentical donor.
Historically, haploidentical SCT (HaploSCT) has been limited by the high rates of graft rejection and acute GVHD (aGVHD).5 T-cell depletion decreased the rate of GVHD at the expense of a higher risk of rejection and, in some settings, a reduced GVL effect associated with an increased relapse rate and severe infections.6 Reisner et al.7 reported that ‘megadoses’ of CD34+, collected by immunomagnetic separation as a T-cell depleted graft, can overcome HLA incompatibility and promote engraftment. Aversa et al. showed the feasibility of using high doses of CD34+ cells in patients undergoing myeloablative HaploSCT.8-11 The preferred composition of the haploidentical graft should contain more than 10 × 106 CD34+ cells/kg and less than 5 × 104 CD3+ cells/kg body weight.12 A greater degree of T-cell depletion was required when antithymocyte globulin (ATG) is not included in the regimen.13 In children, higher numbers of CD34+ cells infused resulted in more rapid immunological reconstitution.14
Most studies have used a myeloablative, total body radiation-based preparative regimen for haploidentical transplantation.8-11,15-19 This has been associated with a high rate of regimen-related toxicities, particularly in adult patients. A recent review identified median non-relapse mortality (NRM) of approximately 50% in HaploSCT patients treated with myeloablative conditioning.20
Reduced-intensity preparative regimens have been developed to decrease regimen-related toxicities and allow treatment of patients with concurrent medical conditions or older.21 The experience with such regimens for haploidentical transplantation has been limited, where a more profound immune suppression is required to prevent rejection.22,23 We studied the feasibility of a non-TBI preparative regimen using fludarabine, melphalan, thiotepa and ATG in adult patients with advanced hematological malignancies.
Patients and methods
Study protocol
This was a single-arm open-label phase II clinical trial, designed to evaluate the feasibility of the reduced-intensity conditioning regimen fludarabine, melphalan, thiotepa and ATG for haploSCT in patients less than 55 years of age with advanced hematological malignancies. All patients received a CD34+ selected graft as a means of T-cell depletion. Donors received G-CSF 16–24 μg/kg subcutaneously per day for 4 days, and PBPCs were collected using the CliniMACS system24 (Miltengi Biotec Inc, Auburn, CA, USA) and cryopreserved. Grafts were required to contain >5 × 106 CD34+ cells/kg stem cells.
The study was reviewed and approved by the University of Texas MD Anderson Cancer Center Institutional Review Board. All patients provided written informed consent. Primary end points of the study were safety, engraftment and development of grade III–IV aGVHD. Secondary end points included the development of chronic GVHD, OS and disease-free survival.
HLA typing, matching, KIR-ligand mismatching
Patients and donors were typed for alleles at HLA-A, B, C, DRB1, DRB3/4/5 and DQB1 by PCR amplification and oligonucleotide hybridization by molecular methods using commercial kits from Invitrogen (Carlsbad, CA, USA), ELPHA and/or One Lambda (Canoga Park, CA, USA), which achieve intermediate resolution. The patients were also typed for these loci by high-resolution methods using PCR amplification and nucleotide sequencing (Abbott, Abbott Park, IL, USA). HLA haplotypes were assigned by the analysis of the segregation of HLA alleles as genetically transmitted units. The parental origin of haplotypes was then ascertained. HLA matching was evaluated from the intermediate- and high-resolution results of the patient and the donors; the transplant pairs were classified in two categories: those that had two mismatches and those that had three or more mismatches in HLA-A, B, C, and DRB1 and DQB1 loci.
HLA-killer Ig-like receptor (KIR) ligands were identified by analyzing the intermediate-resolution and high-resolution results of HLA-B and C loci in the patient and donors. The polymorphism at residue 80 of HLA-B and C loci was analyzed to classify for the presence of the KIR ligands. The assignment into KIR-ligand groups was made from the interpretation of the high-resolution results or from the reactivity of oligonucleotide probes detecting sequence polymorphisms in codon 80. For the GvH and GVL vectors, the KIR-ligand mismatch was evaluated from the ‘missing self’ hypothesis of HLA-KIR ligands between the donor and the patient. The host-vs-graft vector for KIR-ligand mismatch correlated with the development of graft failure, whereas the GVL vector correlated with disease relapse disease, leukemia-free survival and OS.
Anti-HLA Ab testing
The presence of donor-specific anti-HLA antibodies (DSA) was determined in all patients starting after September 2005 by testing the patients’ sera against a panel of fluorescent beads coated with single-HLA Ab preparations (LABScreen Single-Antigen, One Lambda) detected by a Luminex platform (Luminex, Austin, TX, USA). Ab titers were interpreted as normalized fluorescence intensity as defined by the kit’s manufacturer against DSA mismatch. Fluorescence intensity less than 500 was considered negative, whereas positive fluorescence intensity ranging 500–1500, 1500–3000, 3000–7000 and more than 7000 were classified as weak, intermediate, strong and very strong, respectively.
Transplant conditioning regimen and supportive care
The conditioning regimen consisted of melphalan 140 mg/m2 on day −8, thiotepa 10 mg/m2 on day 7, fludarabine 160 mg/m2 in divided doses given on days −6, −5, −4 and −3 and 1.5 mg/kg/day of rabbit ATG on days −6, −5, −4 and −3. No other GVHD prophylaxis except T-cell depletion and no growth factors were routinely administered. All patients received anti-bacterial, anti-viral and anti-fungal prophylaxis with pentamidine or atovaquone, foscarnet, followed by gancyclovir or valacyclovir, and voriconazole or caspofungin, respectively. Pneumocystis carinii and CMV prophylaxis with pentamidine and foscarnet, started to be administered on day −1 after earlier experience revealed a high rate of Pneumocystis carinii and CMV infections in previously treated haploSCT patients.
Definitions
Engraftment was defined as achieving an ANC greater than 5 × 109/l for greater than 3 consecutive days before day 30, with donor-derived cells detected by DNA microsatellite analysis.25 Plt recovery was defined as the first day on which the plt count was greater than 20 × 109/l unsupported by plt transfusions for 7 days. Primary graft failure was defined as failure to achieve an ANC>5 × 109/l by day before day + 30, and secondary graft failure as sustained graft loss (fall of ANC to <5 × 109/l) for >5 days after the initial engraftment. Acute GVHD and chronic GVHD were defined and graded according to previously described criteria.26,27 Other toxicity was scored using the National Cancer Institute criteria.28
Statistical considerations
Time to event was assessed starting on the day of transplantation. Actuarial OS and PFS were estimated by the method of Kaplan–Meier considering death from any cause, and disease progression or death in the absence of disease progression as the outcomes of interest, respectively.29 The incidence of disease progression, NRM and GVHD was estimated using the cumulative incidence method to account for competing events.30 Death in the absence of disease progression, disease progression and death without GVHD were considered as competing events for the respective outcomes. Comparison of outcomes was performed on univariate analysis using Cox’s proportional hazards model.31 Statistical significance was defined as P-value of ≤0.05. Analysis was performed using STATA software (StataCorp. 2001. Stata Statistical Software: Release 7.0. College Station, TX, USA: Stata Corporation).
Results
Patients
Twenty-eight patients with advanced hematological malignancies, all adults except one, were enrolled in this study. All received a full-haplotype mismatched 1 transplant (≥2 Ag mismatch) between October 2001 and April 2008. The patients’ characteristics are summarized in Table 1. The median age was 36 years (range 6–56). Twenty-two patients had AML or myelodysplastic syndrome (MDS) (79%), two had CML in blastic phase, and one had relapsed peripheral T-cell lymphoma (Table 1). Overall, 78% of patients with AML/MDS were not in remission at the time of transplant. Twelve patients (48%) had poor-risk cytogenetics, most frequently involving chromosomes 5, 7 or 8 (six patients). All patients received filgrastim-mobilized PBSCs, in the majority of cases from a sibling donor (47%) (Table 1). The median follow-up interval for surviving patients was 40 months (range 7–76 months).
Table 1.
Characteristics of 28 patients with hematological malignancies treated with FMT preparative regimen
| FMT (n = 28) | |
|---|---|
| Median age (years) (range) | 36 (6–54) |
| Gender (%) | |
| M | 46 |
| F | 54 |
| Donor (%) | |
| Child | 21 |
| Parent | 32 |
| Sibling | 47 |
| Cell type (%) | |
| PB | 100 |
| BM | 0 |
| Disease (number of patients) | |
| AML/MDS | 22 |
| ALL | 3 |
| CML blast phase | 2 |
| Peripheral T-cell lymphoma | 1 |
|
Cytogenetic risk (%)
(for patients with acute leukemia/MDS) (n = 25) |
|
| Poor | 48 |
| Intermediate | 52 |
| Median % of BM blasts at transplant (for patients AML/MDS; n = 22) (range) |
13 (0–92) |
| Median number of Ag mismatches | 3 |
| Median number of CD34 cells infused ( × 106) (range) |
10.2 (6–20.85) |
| Median number of CD3 cells infused (× 106) | 0.01 |
Abbreviations: F = female; FMT = fludrabine, melphalan and thiotepa and antithymocyte globulin; M = male; MDS = myelodyspastic syndrome; PB = peripheral blood.
Engraftment
Twenty-two out of the 28 (79%) patients achieved primary engraftment verified by peripheral blood PCR. One patient died on day 27 post transplant with hematological recovery because of respiratory failure. All patients who engrafted had 100% donor T cells and myeloid cells except one whose initial T-cell chimerism was 67%. Six patients failed to achieve primary engraftment presumably because of rejection. Five patients who rejected were treated with second transplants involving T-cell replete grafts; four were from the same donor and one from a different donor. The preparative regimen for the second transplants consisted of alemtuzumab (Campath, Genzyme, Cambridge, MA, USA) in three patients, and fludarabine/ATG in two patients. The remaining patient with graft failure received infusion of cryopreserved autologous cells and recovered without detectable donor cells. Four out of five patients treated with second haploidentical transplants engrafted and recovered hematopoiesis after the second transplant, three with full donor and two with mixed chimerism. The final engraftment was of 96% (Table 2).
Table 2.
Results for 28 patients with hematological malignancies treated with FMT preparative regimen
| FMT (n = 28) | |
|---|---|
| Engraftment (%o) | |
| Primary | 78 |
| Final | 96 |
| Days to ANC 500 (median, range) | 13 (9–26) (n = 21) |
| Days to plt 20 000 (median, range) | 12 (7–25) (n = 19) |
| Non-relapse mortality (n = 11) (%) | |
| Median | Not reached |
| 100 days | 18 |
| 1 year | 40 |
| Overall | 40 |
| Disease progression (n = 12) (%) | |
| Median | Not reached |
| 100 days | 21 |
| 1 year | 40 |
| Overall | 44 |
| GVHD (%) | |
| Acute GVHD all grades (n = 27) | 19 |
| Acute GVHD III–IV | 0 |
| Chronic GVHD (n = 21) | 20 |
| DFS | (n=5) |
| Median (months) | 4.1 |
| 100 days (%) | 64 |
| 1 year (%) | 20 |
| Overall (%) | 16 |
| OS | (n=6) |
| Median (months) | 5 |
| 100 days (%) | 82 |
| 1 year (%) | 22 |
| Overall (%) | 18 |
| Median follow-up survivors (months) | 40 (n = 6) |
Abbreviations: DFS = disease-free survival; FMT = fludarabine, melpha-lan, thiotepa and antithymocyte globulin.
Evaluation of causes of graft failure
To investigate the causes of primary graft failure, we started testing for the presence of DSA after September 2005 using a method with fluorescent beads coated with single antigens and detected by a Luminex platform as described above. A total of 16 consecutive patients treated on this trial were tested between September 2005 and August 2008. Four patients enrolled had graft failure during this time and all were tested for the presence of DSA. Donor-specific anti-HLA antibodies were identified in three of these patients, all females with moderate-to-high DSA titers. No apparent cause of graft failure was identified in the fourth patient (Table 3a and b). None of the other patients who showed engraftment had detectable antibodies against donor-specific anti-HLA antigens of the donor (P = 0.001, two-sided Fisher’s exact test). No other significant differences regarding other factors that could negatively influence engraftment between the graft failure group (n = 4) and the engraftment group (n = 12) were identified, except more ABO mismatches in the graft failure group (P = 0.04) (data not shown).
Table 3a.
Correlation between donor-specific anti-HLA antibodies (DSA) and engraftment in four patients who experienced graft failure after T-cell depleted haploidentical transplantation with FMT conditioning regimen.
| Patient no. | HLA AB type a | Initial titer/before first SCT b | Engrafted Y/N c | After first SCT/before second SCT d | Engrafted Y/N e |
|---|---|---|---|---|---|
| 1 | A 3201 | + + + + | N | + + + + | Y |
| 2 | A 0211 | + + + | N | + + | N |
| B391301 | + + + | + + + | |||
| Cw70201 | + + + | NT | |||
| DRB1 0404 | + | + | |||
| 3 | DRB1*0701 | + + | N | − | Y |
| DRB4*0101 | + + + | − | |||
| DQB1 0202 | + + + | − | |||
| 4 | None | − | N | − | Y |
Abbreviations: −= < 500; + = 500–1 500; + + = 1 500–3 000; + + + = 3 000–7 000; + + + + = >7000 fluorescence intensity; N = no; Y = yes.
HLA alleles mismatched in the donor for which the patient had DSA at the initial work-up.
Intensity of DSA at the initial work-up/before the first transplant.
Engraftment after the first transplant.
Strength of DSA after the first transplant and/or before the second transplant.
Engraftment after the second transplant.
Table 3b.
Characteristics of four patients tested for DSA who experienced graft failure
| Patient nos. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Donor-specific anti-HLA antibodies | Y | Y | Y | N |
| Age | 43 | 37 | 39 | 29 |
| Gender | F | F | F | M |
| Diagnosis | AML | AML | AML | AML |
| % BM blasts at transplant | 0 | 13 | 23 | 2 |
| Donor | Sib | Sib | Son | Parent |
| Sex mismatch (Y/N) | Y | N | Y | N |
| No. of allele mismatched* | 3 | 4 | 5 | 5 |
| Alloreactive KIR-LM in HVG direction | 0 | 0 | 0 | 0 |
| CMV mismatch (R/D) | R+/D− | R+/D+ | R+/D− | R+/D + |
| ABO mismatch | Major | Minor | Minor | Minor |
| Cell type | PB | PB | PB | PB |
| No. of CD34+ cells infused (× 106/kg) | 14.8 | 6.6 | 17.5 | 11.1 |
| No. of pregnancies | 0 | 3 | 2 | NA |
| No. of PRBCs transfused before transplant | 37 | 41 | 65 | 13 |
Abbreviations: ABO = blood group; D = donor; DSA = donor-specific anti-HLA antibodies; F = female; HVG = host-vs-graft; KIR-LM = killer Ig-like receptor-ligand mismatch; M = male; NA = not applicable; PB = peripheral blood; PRBCs = packed RBCs; R = recipient; Sib = sibling. Patients with antibodies were all females, two out of three multiparous, and all heavily transfused before transplant. *Number of allele mismatches were appreciated on the basis of HLA A, B, C, DRB1, DQB1 typing.
GVHD
After the first transplant, five patients developed aGVHD (19%) with only two patients having grade II aGVHD (7%). None had grade III–IV aGVHD. Four out of 21 patients had chronic GVHD, two with extensive manifestations (both ocular involvement). These results confirmed the low rate of GVHD previously seen in patients receiving a T-cell depleted haploSCT8-11,14,22,23 (Table 2). One out of the five patients developed both acute (grade III) and extensive chronic GVHD after the second transplant.
Toxicity of the conditioning regimen, survival, relapse and causes of death
The preparative regimen was relatively well tolerated with limited regimen-related toxicity. Grade III gastrointestinal toxicity (nausea, vomiting, diarrhea, mucositis) was rare, occurring only in three patients (10%). Reversible renal toxicity occurred in three patients (grade III in two patients; grade IV in one patient associated with progressive BK virus infection). One patient developed transplant-related microangioapathy (grade III toxicity) and one hemolytic anemia, which both resolved. Hemorrhagic cystitis (grade III) occurred in three patients. Reversible liver toxicity (grade III) occurred in two patients without the development of veno-occlusive disease.
The primary cause of death was divided between disease relapse in 11 patients and NRM in 11 patients. Four patients died before day 100. Seven patients succumbed to infections (mostly lung infections/pneumonia, usually with multiple organisms), two because of multiorgan failure caused by disseminated adenovirus infection and sepsis, and two secondary to graft failure.
Infections
Infectious complications represented the major cause of morbidity and NRM in these patients, likely related to prolonged immune deficiency because of the T-cell depletion process. A total of 21 patients (75%) developed at least grade III infections. Most common type of infections was pneumonia in 19 and bacteriemia in 14 patients. Most common organisms most likely responsible for the lung infections were fungal (eight patients), viral (seven patients), Gram-positive bacteria (five patients), Gram-negative bacteria (four patients), nocardia (one patient), toxoplasma (one patient) and unknown organisms (four patients). Bacteriemia was because of Gram-positive organisms in seven and Gram-negative organisms in six patients.
Disease response and relapse
Twelve patients (44%) relapsed after a median of 72 days post transplant (Table 2). All relapses occurred in patients with active disease at the time of transplant, and all AML/MDS patients with relapsed disease achieved remission after transplant with the fludarabine, melphalan and thiotepa and ATG regimen; however, this was obviously not enough to prevent relapse in these patients.
Outcomes of patients with AML/MDS
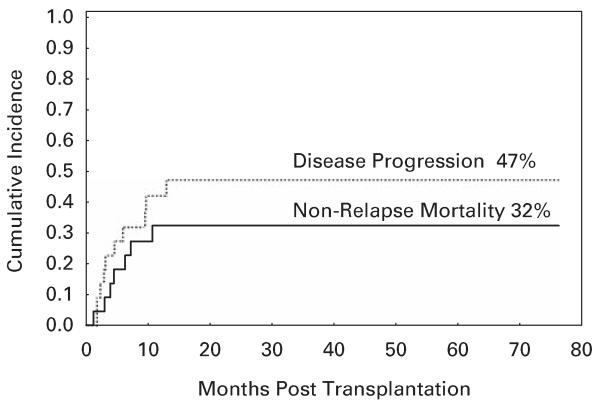
A total of 22 patients had AML/MDS, comprising the majority of patients in this study. Sixteen patients had relapsed/refractory disease at transplant (14 patients had unresponsive disease to the last chemotherapy received and 2 had untreated disease). CR was achieved in all evaluable patients after transplant. Only one patient died within 100 days of transplant. Overall, NRM for this group was 32% (Table 4, Figure 1), which appears lower than in the published studies with myeloablative conditioning.20
Table 4.
Results in 22 patients with AML/MDS treated with FMT preparative regimen
| AML FMT (n = 22) | |
|---|---|
| Engraftment (%) | |
| Primary | 77 |
| Final | 91 |
| Days to ANC 500 (median, range) | 13 (9–26) |
| n = 17 | |
| Days to plt 20 000 (median, range) | 13.5 (7–25) |
| n = 16 | |
| Non-relapse mortality (n = 7) (%) | |
| Median | Not reached |
| 100 days | 9 |
| 1 year | 32 |
| Overall | 32 |
| Disease progression (n = 10) (%) | |
| Median | Not reached |
| 100 days | 18 |
| 1 year | 42 |
| Overall | 47 |
| GVHD (%) | 18 |
| Acute GVHD III–IV | 0 |
| Chronic GVHD | 18 |
| OS | n=6 |
| Median (months) | 7 |
| 100 days (%) | 91 |
| 1 year (%) | 29 |
| Overall (%) | 23 |
| Median follow-up survivors (months) | 40 (n = 6) |
Abbreviations: FMT = fludrabine, melphalan and thiotepa and antithy-mocyte globulin; MDS = myelodysplastic syndrome.
Overall, 78% of these patients had relapsed or refractory disease at the time of transplant.
Figure 1.
Cumulative incidence of non-relapse mortality and disease progression for patients with AML undergoing haploidentical SCT with fludarabine, melphalan and thiotepa and ATG preparative regimen.
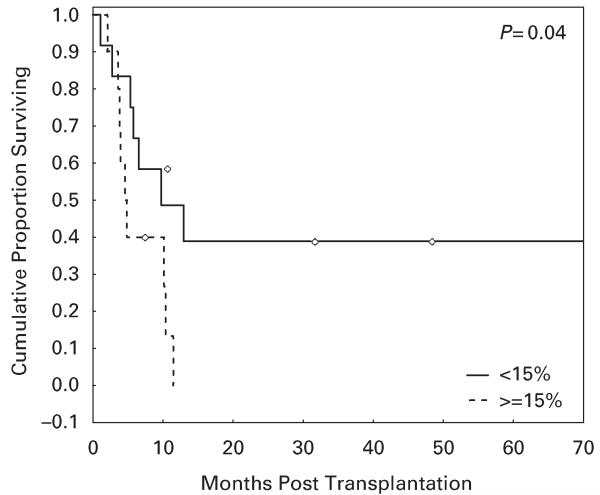
Five out of 12 patients (42%) with AML/MDS in remission or with low disease burden at transplant (≤15% BM blasts) survived long term as compared with none of those with high disease burden (>15% blasts) (P = 0.03, hazard ratio 3.3; Figure 2).
Figure 2.
OS in patients with AML/myelodysplastic syndrome (MDS) treated with fludarabine, melphalan and thiotepa and antithymocyte globulin regimen based on disease status at transplant. Overt relapse was associated with very poor outcomes.
Natural killer alloreactivity has been reported to have an important function in engraftment, disease relapse and transplant outcomes.32,33 The source of the mismatched haplotype could confer a survival advantage due to the natural tolerance to maternal antigens.34 Although this was a small study and no definite conclusions can be drawn, the patients who survived long term in this study had both KIR-ligand mismatch in the GVL direction and a maternal source of the mismatched haplotype, effects that could not be separated because of small number of patients.
Discussion
HaploSCT using T-cell depletion and ‘megadoses’ of stem cells represents an important treatment option for patients who lack an HLA-matched related or unrelated donor. HaploSCT has been most commonly performed using a total body radiation-based myeloablative conditioning regimen. The use of reduced-intensity preparative regimens has lowered the toxicity and treatment-related mortality in patients receiving histocomplatible transplants, and this approach holds the promise for haploSCTs as well.
We have evaluated a non-TBI-based reduced-intensity regimen using fludarabine, melphalan, thiotepa and ATG in patients with advanced hematological malignancies. This regimen was initially suggested by Aversa and Martelli who treated five patients with unfavorable results (Aversa, personal communication, 2000).
A modified version of this regimen was previously reported in a small number of patients by Lacerda et al.35 who performed haploSCT with T-cell depletion followed by preemptive donor lymphocyte infusion to prevent disease relapse. These patients also received CYA for GVHD prophylaxis. The fludarabine melphalan thiotepa preparative regimen was well tolerated. A total of 72% of surviving patients developed aGVHD with two cases of moderate-to-severe bronchiolitis obliterans.35 The use of preemptive donor lymphocyte infusion could have been the reason for the high rate of aGVHD.
The Tuebingen group also used a variant of this regimen in combination with selective T-cell depletion for CD3/CD19 cells to maintain the natural killer cells, monocytes and APCs in the graft.36 Bethge et al.36 used conventional doses of CD3/CD19 depleted stem cells and a preparative regimen which included fludarabine (150–200 mg/m2), melphalan (120 mg/m2), thiotepa (10 mg/m2) and OKT-3 (5 mg/day on days −5 to + 14) in 10 patients with hematological malignancies. No post transplant immunosupression was administered. All patients engrafted, day 100 TRM was 30%, and 60% of patients developed GVHD. After a median follow-up of 14 months relapsefree survival was 40%.36 A similar approach was used by Handgretinger et al.37 who achieved higher doses of CD34+ cells and used an anti-CD3 MoAb added to the same preparative regimen. This group of patients, mostly children, included both malignant and non-malignant diseases. A total of 17% of 38 patients had primary graft rejection, and engrafted after the second transplant.37 Low rates of GVHD were noted by applying post transplant immunosupression with mycofenolate mofetil.37 In both studies, there was a high incidence of viral infections, predominantly CMV reactivation and adenovirus infections, and represented the major cause of NRM at least in the first study.36,37
With the present trial, we evaluated the feasibility of the fludarabine, melphalan, thiotepa and ATG reduced-intensity conditioning regimen in 28 patients with hematological malignancies, predominantly AML/MDS, all adults except one, undergoing HaploSCT, and report long-term followup results obtained with this regimen. Engraftment occurred promptly in 96% of the patients, in which antidonor HLA antibodies were not identified. A high rate of graft failure was identified in the presence of DSA, three out of four patients with DSA rejected; a different donor should be sought or alternative treatment strategies may be needed in these patients. Regimen-related toxicity was limited, and the major cause of nonrelapse mortality was because of infectious complications, most likely related to the post transplant immune deficiency intrinsic to T-cell depleted allo-SCT.37
The great majority of patients in our study had AML/MDS, most with advanced disease at transplant. This reduced-intensity preparative regimen produced a relatively low rate of regimen-related toxicity and early nonrelapse mortality. Overall, 20% of patients treated with this regimen achieved long-term remission with this approach. Disease burden was the most important factor in determining treatment outcomes; 42% of patients with low disease burden at transplant achieved durable CR, whereas patients with overt relapse fared poorly, similar to myeloablative conditioning, as recently reported in a large retrospective study.38 Alternative treatment strategies are required for these patients.
We conclude that haploSCT using reduced-intensity conditioning with fludarabine, melphalan, thiotepa and ATG is relatively well tolerated and successful to achieve engraftment after haploidentical T-cell depleted transplantation in patients without donor-specific anti-HLA antibodies. This approach merits further study and ultimately a prospective comparison with more intense conditioning regimens. The presence of DSA appears to be associated with a high rate of graft rejection and routine DSA screening should be pursued in this setting.
Acknowledgements
We thank all our pharmacists, transplant coordinators and nursing staff for their dedication to patient care; research nurses, technicians and laboratory personnel who contributed to the collection, recording and reporting of the data.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:1511–1533. [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Beatty PG, Mori M, Milford E. Impact of racial genetic polymorphism on the probability of finding an HLA-matched donor. Transplantation. 1995;60:778–783. [PubMed] [Google Scholar]
- 4.Schipper RF, D’Amaro J, Bakker JT, Bakker J, van Rood JJ, Oudshoorn M. HLA gene haplotype frequencies in bone marrow donors worldwide registries. Hum Immunol. 1997;52:54–71. doi: 10.1016/s0198-8859(96)00257-1. [DOI] [PubMed] [Google Scholar]
- 5.Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ, et al. Mismatched family donors for bone marrow transplantation as treatment for acute leukemia. Lancet. 1983;8325:612–615. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 6.Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, Champlin RE, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 7.Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Cunningham-Rundles S, Dupont B, et al. Transplantation for severe combined immunodeficiency with HLA-A, B, D, DR incompatible parental marrow cells fractionated by soybean agglutinin and sheep red blood cells. Blood. 1983;61:341–348. [PubMed] [Google Scholar]
- 8.Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical ‘three-loci’ incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994;84:3948–3955. [PubMed] [Google Scholar]
- 9.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA Haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 10.Aversa F, Terenzi A, Felicini R, Carotti A, Falcinelli F, Tabilio A, et al. Haploidentical stem cell transplantation for acute leukemia. Int J Hematol. 2002;76:165–168. doi: 10.1007/BF03165238. [DOI] [PubMed] [Google Scholar]
- 11.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 12.Champlin R, Hesdorffer C, Lowenberg B, Martelli MF, Martelsmann RH, Reisner Y, et al. Haploidentical ‘megadose’ stem cell transplantation in acute leukemia: recommendations for a protocol agreed upon at the Perugia and Chicago meetings. Leukemia. 2002;16:427–428. doi: 10.1038/sj.leu.2402386. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly RJ. T-cell depletion and allogeneic bone marrow transplantation. Semin Hematol. 1992;29:20–26. [PubMed] [Google Scholar]
- 14.Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27:777–783. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 15.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplant for leukemia using donors other then HLA-identical sibling. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Yabe H, Yasui M, Kawa K, Yoshida T, Watanabe A, et al. Allogeneic hematopoietic transplantation of CD34+ selected cells from an HLA haploidentical related donor. Along-term follow-up of 135 patients and a comparison of stem cell source between the bone marrow and the peripheral blood. Bone Marrow Transplant. 2000;26:1281–1290. doi: 10.1038/sj.bmt.1702707. [DOI] [PubMed] [Google Scholar]
- 17.Dobyski WR, Klein J, Flomenberg N, Pietryga D, Vesole DH, Margolis DA, et al. Superior survival associated with transplantation of matched unrelated versus on-antigen mismatched unrelated or highly human-leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood. 2002;99:806–814. doi: 10.1182/blood.v99.3.806. [DOI] [PubMed] [Google Scholar]
- 18.Kanda Y, Chiba S, Hirai H, Sakamaki H, Iseki T, Kodera Y, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991-2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 19.Mehta J, Singhal S, Gee AP, Chiang KY, Godder K, Rhee Fv F, et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant. 2004;33:389–396. doi: 10.1038/sj.bmt.1704391. [DOI] [PubMed] [Google Scholar]
- 20.Koh LP, Rizzieri DA, Chao NJ. Allogeneic hematopoietic stem cell transplant using mismatched/haploidentical donors. Biol Blood Marrow Transplant. 2007;13:1249–1267. doi: 10.1016/j.bbmt.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, et al. Engraftment of allogneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 22.Spitzer TR, McAfee SL, Dey BR, Colby C, Hope J, Grossberg H, et al. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1448–1751. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]
- 23.Rizzieri DA, Koh LP, Long GD, Gasparetto C, Sullivan KM, Horwitz M, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 24.Miltenyi S. Isolation of CD34+ Hematopoietic Progenitor Cells By High-Gradient Magnetic Cell Sorting (MACS) In: Wunder E, editor. Hematopoietic Stem Cells: The Mullhouse Manual. AlphaMed Press; Dayton, OH: 1994. pp. 201–213. [Google Scholar]
- 25.Thiede C, Florek M, Bornhauser M, Ritter M, Mohr B, Brendel C, et al. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–1060. doi: 10.1038/sj.bmt.1701779. [DOI] [PubMed] [Google Scholar]
- 26.Przepiorka D, Martin P, Klingmann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 27.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 28.Common Toxicity Criteria Version 3 0. Available at http://ctep.info.nih.gov/reporting/ctc.html.
- 29.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 31.Cox DR. Regression models and life tables [with discussion] J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 32.Ruggeri L, Capanni M, Tosti A, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 33.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KiR haplotypes improve relapse-free survival after unrelated hematopoietic stem cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–731. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rood JJ, Loberiza FR, Jr, Zhang MJ, Oudshoorn M, Claas F, Cairo MS, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or and HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 35.Lacerda JF, Martins C, Carmo JA, Lourenço F, Juncal C, Rodrigues A, et al. Haploidentical stem cell transplantation with purified CD34+ cells after a chemotherapy-alone conditioning regimen. Biol Blood Marrow Transplant. 2003;9:633–642. doi: 10.1016/s1083-8791(03)00263-5. [DOI] [PubMed] [Google Scholar]
- 36.Bethge WA, Haegele M, Faul C, Lang P, Schumm M, Bornhauser M, et al. Haploidentical allogeneic stem cell transplantation in adults with reduced-intensity conditioning and CD3/CD19 depletion: fast engraftment and low toxicity. Exp Hematol. 2006;34:1746–1752. doi: 10.1016/j.exphem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA, et al. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci. 2007;1106:279–289. doi: 10.1196/annals.1392.022. [DOI] [PubMed] [Google Scholar]
- 38.Ciceri F, Lobopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008;112:3574–3581. doi: 10.1182/blood-2008-02-140095. [DOI] [PubMed] [Google Scholar]