Abstract
Ipilimumab is a fully human monoclonal antibody against Cytotoxic TLymphocyte Antigen 4 (CTLA-4). CTLA-4 negatively regulates immune cell activation. In patients with metastatic melanoma, ipilimumab increases survival time and induces complete remission in some patients. However, immune related adverse events including endocrinopathies have been reported. Bevacizumab, an angiogenesis inhibitor, has been used in combination with ipilimumab in patients with advanced melanoma. Here, we report three patients who received ipilimumab alone or with bevacizumab therapy and developed thyroiditis and the first report of euthyroid Graves’ ophthalmopathy.
Case 1 is a 51 year old female who presented with severe eye pain, proptosis and periorbital edema. Laboratory results revealed normal TSH, elevated thyroid antibodies but low titer of anti-thyrotropin receptor antibody. Imaging was consistent with Graves’ ophthalmopathy. Cases 2 and 3 were referred for hyperthyroidism and work up revealed thyroiditis. These 3 cases suggest that patients with advanced melanoma treated with ipilimumab +/- bevacizumab may be susceptible to a variety of thyroid disorders.
Anti-CTLA4 therapy has shown promising results in treating advanced malignancy such as melanoma and renal carcinoma. A number of endocrinopathies, including thyroid disorders, may develop during ipilimumab therapy. The association of bevacizumab with endocrinopathies is not clear although a few reports suggest a link to hypothyroidism. All patients on ipilimumab and/or bevacizumab therapy should be monitored for signs or symptoms of thyroiditis.
Introduction
CTLA-4 is a checkpoint molecule present on the cell surface of activated T lymphocytes. It counterbalances the T–cell activation mediated by CD28, a positive immune response regulator. As a result, the proliferation of T lymphocytes and secretion of interleukin 2 are inhibited (1). Ipilimumab is a fully human monoclonal antibody against CTLA-4. Clinical studies have revealed a variety of immune related adverse events (IRAEs) associated with ipilimumab therapy, including endocrinopathies. The most common endocrinopathy has been hypophysitis. In a large series of 163 patients with advanced melanoma or renal cell cancer, eight patients developed autoimmune hypophysitis while receiving ipilimumab (2) . The incidence of autoimmune hypophysitis in anti-CTLA-4 clinical trials ranges from 0-17% (3). Thyroiditis has been listed as an adverse event but details are scant (3). Bevacizumab is a humanized monoclonal antibody that acts as an anti-angiogenic agent by directly inhibiting VEGF and is widely used in advanced malignancies(4). Here, we report three patients with advanced melanoma who received ipilimumab with or without bevacizumab, and developed autoimmune thyroiditis or ophthalmopathy.
Case Report
Case 1: A 51 year old female with a 4 year history of stage IV melanoma was hospitalized for acute onset of severe eye pain, conjunctivitis, proptosis, and periorbital edema. Ipilimumab (10 mg/kg) therapy was started two months prior to hospitalization. She had no history of thyroid disease and was euthyroid at baseline with thyrotropin (TSH) 3.7 (normal range: 0.5-5 mIU/L) and free T4 1.1 (normal range 0.93-1.7 ng/dL) (Table 1). After receiving four doses of ipilimumab at 10 mg/kg, she developed the eye symptoms noted above. Physical examination revealed bilateral proptosis, conjunctival redness and periorbital edema. Hertel exophthalmometry showed OD 23 mm, and OS 23 mm (Normal range 12-22 mm) indicating mild proptosis. Intraocular pressures were slightly increased (OD 24 mmHg and OS 20 mmHg, normal range: 10-20 mmHg). Thyroid examination was negative for goiter, nodules or tenderness. Her laboratory studies revealed high anti-TPO antibody (662 IU/ml, n< 20) and thyroglobulin antibody (148.5 IU/ml, n< 3.9) though her thyroid function tests remained normal with TSH 1.01 and free T4 1.1 (normal range: 0.8-1.8 ng/dl) (Table 1) . Computed tomography of the brain and orbital magnetic resonance imaging showed bilateral thickening of extraocular muscles compatible with Graves’ ophthalmopathy. Ipilimumab was discontinued. She received intravenous Solu-Medrol 1 gm daily for 3 days, and subsequently a course of oral prednisone. Her symptoms and ophthalmopathy resolved initially following treatment with glucocorticoids, but relapsed 2 months later as prednisone was tapered. High dose intravenous Solu-Medrol was again initiated. She received intravenous Solu-Medrol 100 mg daily for the first day followed by intravenous Solu-Medrol 250 mg every 6 hours for a total of 12 doses. Prednisone 100 mg po twice daily with slow taper was initiated after finishing the course of intravenous Solu-Medrol. Five months later, her ocular symptoms persisted. The levels of anti-TPO and thyroglobulin antibodies remained elevated though decreased significantly over one year. Thyroid stimulating immunoglobulin was not initially checked but was 1.4 (normal range <1.3) seventeen months after initial presentation. She has subsequently been able to stop glucocorticoids with almost complete resolution of ocular symptoms and signs.
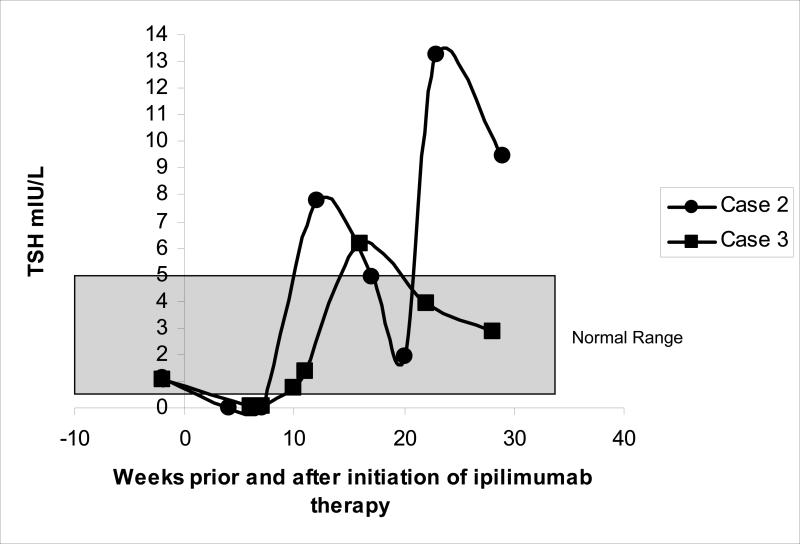
Case 2: A 48 year old male with advanced melanoma was enrolled in a clinical trial with ipilimumab (10 mg/kg) and bevacizumab (7.5 mg/kg) combined therapy. He had no history of thyroid disease (Table 2). His baseline TSH was 1.13 mIU/L with negative anti-thyroglobulin antibodies (Table 1). Following two infusions of ipilimumab and bevacizumab his TSH declined to 0.01 mIU/L. He denied symptoms of hyper- or hypothyroidism though physical exam revealed hand tremor. Thyroid examination was unremarkable. Laboratory studies revealed elevated thyroxine and strongly positive anti- TPO and anti-thyroglobulin antibodies (Table 1). A radioiodine-123 thyroid uptake showed low uptake of 0.9% at 6 hours (normal range 7-15%) consistent with a thyroiditis. He had not received any iodinated contrast in the previous two months. We observed him without treatment and three weeks later, his thyroxine normalized. Three months after presentation, his TSH increased to 7.78 mIU/L and remains high with the most recent TSH 9.46, consistent with an autoimmune thyroiditis (Figure 1).
Figure 1.
Change in TSH in cases 2 and 3 prior to and after ipilimumab therapy
Case 3: A 28 year old female received surgery, radiation therapy and systematic chemotherapy with interferon-alpha and autologous tumor cell vaccine for advanced melanoma. She enrolled in a clinical trial with ipilimumab (10 mg/kg)/bevacizumab (7.5 mg/kg) after her disease progressed. She received the interferon-alpha therapy for 6 months. She was clinically euthyroid during the therapy. Her levels of TSH were normal before and after interferon-alpha therapy. The interferon-alpha treatment was completed 2 years prior to ipilimumab therapy. TSH was 1 mIU/ml prior to the trial and declined to 0.06 after three doses of combined therapy (Table 1). She had not received iodinated contrast in the previous two months. Her free T4 was high normal and anti-TPO antibody was elevated at 60 IU/ml. Physical exam revealed tachycardia without goiter or neck tenderness. PET scan revealed persistently increased FDG positive uptake in both thyroid lobes, consistent with a thyroiditis (5-7). One month later, her TSH normalized, three months later it increased to 6.19, and 5 months after presentation it normalized to 3.9, consistent with a resolving painless thyroiditis (Figure 1).
Discussion
These three patients had no history of thyroid disorders and were euthyroid prior to initiation of ipilimumab alone or combined with bevacizumab therapy (Table 2). Each developed a different thyroid associated disorder. Although case 2 and 3 both developed thyroiditis, case 2 progressed to a Hashimoto-type permanent hypothyroidism while case 3 had findings more typical of a transient, painless thyroiditis. Case 1 has remained euthyroid but developed physical and radiologic evidence of bilateral Graves’ ophthalmopathy. All cases have no family history of autoimmune endocrinopathies. The onset of abnormalities in all patients occurred relatively quickly, after 2 to 4 cycles or 6 to 12 weeks of ipilimumab +/- bevacizumab therapy. The tests for other autoimmune endocrinopathies especially hypophysitis were negative in all patients.
Our clinical observations extend the findings of previous studies with ipilimumab. Most recently, Hodi et al described a phase III study of ipilimumab in patients with metastatic melanoma(8). In addition to an improvement in overall survival, treatment with ipilimumab was associated with thyroid disorders or abnormal thyroid function tests in approximately 2% of the study population. Interestingly, several studies have reported a positive correlation between clinical response to anti-CTLA-4 therapy and IRAEs. (9-12).
Case 1 had striking ocular symptoms and signs. The imaging studies were consistent with Graves’ ophthalmopathy. She had a slightly elevated thyroid stimulating antibody over 1 year after presentation but low titer of TSH-BII (thyrotropin binding inhibitory immunoglobulins) of 9% (ref 0-16%) and normal thyroid function tests (Table 1) suggesting euthyroid Graves’ ophthalmopathy. Up to 5% of patients with Graves’ ophthalmopathy are euthyroid or hypothyroid and have low titers of anti–thyrotropin-receptor antibodies at the time of presentation (13). To our knowledge, this is the first case of euthyroid Graves’ ophthalmopathy associated with ipilimumab therapy.
CTLA4 gene polymorphisms have been associated with autoimmune thyroid diseases (AITD) including Graves’ disease and Hashimoto's thyroiditis (14, 15). A recent meta-analysis supported these associations (16). Sanderson et al. (11) performed polymorphism analysis in a small anti-CTLA-4 clinical trial and noted that the GG allele in JO33 that encodes three alleles correlating with the level of CTLA4 expression on T cells is related to higher risk of developing autoimmunity with CTLA-4 blockade while the GG allele is associated with low CTLA-4 expression. There are controversies about the correlation of CTLA-4 polymophisms with Graves’ ophthalmopathy. Early studies from a UK team suggested the association of CTLA-4 gene polymorphism with increased incidence of Graves’ ophthalmopathy(17, 18). Studies in different populations were inconsistent (19). A meta-analysis failed to find a significant association of CTLA-4 SNP with Graves’ ophthalmopathy (20) .
Management of ipilimumab-related endocrinopathies can be challenging since glucocorticoids are usually used as anti-inflammatory agents. As the goal of anti-CTAL4 therapy is to stimulate an immune response against cancer cells, treating the patients with glucocorticoids as in Case 1, theoretically reverses the anti-cancer benefit of CTLA4 blockade. Interestingly, the accumulated evidence suggests that systemic steroid administration does not significantly alter the anti-tumor activity of CTLA4 blockade (2, 12, 21). Blansfield et al reported that a glucocorticoid therapy protocol with intravenous dexamethasone 4 mg every 6 hours for 7 days and then rapidly tapered to replacement doses of hydrocortisone appeared to have no adverse impact on tumor response (2). In this report, case 1 had been on chronic high dose glucocorticoid treatment for her ophthalmopathy, yet her melanoma remains in remission.
Two of our patients received combination therapy with bevacizumab and ipilimumab. A recent retrospective study revealed a relation of bevacizumab therapy and hypothyroidism in children with primary CNS tumor (22). However, there are no details describing the possible etiology for hypothyroidism in these patients. Based on animal studies, the mechanism of anti-VEGF related hypothyroidism is associated with capillary regression in the thyroid gland rather than autoimmune thyroiditis (23). Since all of our reported patients had significantly high TPO antibodies and received ipilimumab, it is possible that the thyroiditis was related to the independent effects of this medication. Alternatively , an independent and/or synergistic effect of bevacizumab on thyroid function cannot be excluded.
In conclusion, we have described 3 patients with advanced melanoma who developed autoimmune thyroiditis or Graves ophthalmopathy early in the course of ipilimumab +/- bevacizumab therapy. Further research on the relation between polymorphisms of CTLA-4 and autoimmunity may be useful in identifying populations at highest risk for AITD and other endocrinopathies while on anti-CTLA-4 therapy. Identifying the possible association of CTLA-4 polymorphism with ipilimumab related autoimmune endocrinopathies may help to unveil the mechanism and pathogenesis of these disorders. Based on the recent findings supporting the efficacy of ipilimumab in treating patients with advanced melanoma(8), we expect that more patients with advanced malignancies will receive this therapy. This may result in increased autoimmune related endocrinopathies. For now, we recommend that all patients on ipilimumab alone or combined with bevacizumab therapy have baseline thyroid function tests and careful monitoring for new onset of thyroid disease, particularly during the first 3 months of treatment.
Acknowledgement
We thank Dr. Frank S. Hodi and Dr. Philip A. Friedlander for referring the above patients to our endocrine clinic.
Funding: This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Footnotes
Disclosure Statement: No competing financial interests exist.
Contributor Information
Le Min, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, 02115 Phone: 617 732 5666 Fax: 617 264-5220 lmin1@partners.org
Anand Vaidya, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, 02115 Phone: 617 732 5666 Fax: 617 264-5220 Avaidya1@partners.org
Carolyn Becker, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, 221 Longwood Avenue, Boston, 02115 Phone: 617 732 5666 Fax: 617 264-5220 cbbecker@partners.org.
References
- 1.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 2.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 4.Boehm S, Rothermundt C, Hess D, Joerger M. Antiangiogenic drugs in oncology: a focus on drug safety and the elderly - a mini-review. Gerontology. 2010;56:303–309. doi: 10.1159/000262450. [DOI] [PubMed] [Google Scholar]
- 5.Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, Peller PJ, Bahn RS, Lowe VJ. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896–901. doi: 10.2967/jnumed.106.039024. [DOI] [PubMed] [Google Scholar]
- 6.Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, Horiuchi M. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207:775–778. doi: 10.1148/radiology.207.3.9609903. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y. Clinical significance of thyroid uptake on F18-fluorodeoxyglucose positron emission tomography. Ann Nucl Med. 2009;23:17–23. doi: 10.1007/s12149-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M, Weber J. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 12.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O'Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresecTable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 13.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 15.Chistiakov DA, Turakulov RI. CTLA-4 and its role in autoimmune thyroid disease. J Mol Endocrinol. 2003;31:21–36. doi: 10.1677/jme.0.0310021. [DOI] [PubMed] [Google Scholar]
- 16.Kavvoura FK, Akamizu T, Awata T, Ban Y, Chistiakov DA, Frydecka I, Ghaderi A, Gough SC, Hiromatsu Y, Ploski R, Wang PW, Bednarczuk T, Chistiakova EI, Chojm M, Heward JM, Hiratani H, Juo SH, Karabon L, Katayama S, Kurihara S, Liu RT, Miyake I, Omrani GH, Pawlak E, Taniyama M, Tozaki T, Ioannidis JP. Cytotoxic T-lymphocyte associated antigen 4 gene polymorphisms and autoimmune thyroid disease: a meta-analysis. J Clin Endocrinol Metab. 2007;92:3162–3170. doi: 10.1210/jc.2007-0147. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya B, Imrie H, Perros P, Dickinson J, McCarthy MI, Kendall-Taylor P, Pearce SH. Cytotoxic T lymphocyte antigen-4 (CTLA-4) gene polymorphism confers susceptibility to thyroid associated orbitopathy. Lancet. 1999;354:743–744. doi: 10.1016/S0140-6736(99)01465-8. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya B, Oakes EJ, Imrie H, Dickinson AJ, Perros P, Kendall-Taylor P, Pearce SH. CTLA4 gene and Graves’ disease: association of Graves’ disease with the CTLA4 exon 1 and intron 1 polymorphisms, but not with the promoter polymorphism. Clin Endocrinol (Oxf) 2003;58:732–735. doi: 10.1046/j.1365-2265.2003.01778.x. [DOI] [PubMed] [Google Scholar]
- 19.Bednarczuk T, Gopinath B, Ploski R, Wall JR. Susceptibility genes in Graves’ ophthalmopathy: searching for a needle in a haystack? Clin Endocrinol (Oxf) 2007;67:3–19. doi: 10.1111/j.1365-2265.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- 20.Han S, Zhang S, Zhang W, Li R, Li Y, Wang Z, Xie Y, Mao Y. CTLA4 polymorphisms and ophthalmopathy in Graves’ disease patients: association study and meta-analysis. Hum Immunol. 2006;67:618–626. doi: 10.1016/j.humimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reismuller B, Azizi AA, Peyrl A, Heinrich M, Gruber-Olipitz M, Luckner D, Rothschild KV. Slavc I Feasibility and tolerability of bevacizumab in children with primary CNS tumors. Pediatr Blood Cancer. 54:681–686. doi: 10.1002/pbc.22409. [DOI] [PubMed] [Google Scholar]
- 23.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]