Abstract
Spermatogenesis takes place in the seminiferous tubules in adult testes such as rats, in which developing germ cells must traverse the seminiferous epithelium while spermatogonia (2n, diploid) undergo mitotic and meiotic divisions, and differentiate into elongated spermatids (1n, haploid). It is conceivable that this event involves extensive junction restructuring particularly at the blood-testis barrier (BTB, a structure that segregates the seminiferous epithelium into the basal and the adluminal compartments) that occurs at stages VII–VIII of the seminiferous epithelial cycle. As such, cross-talk between tight (TJ) and anchoring junctions [e.g., basal ectoplasmic specialization (basal ES), adherens junction (AJ), desmosome-like junction (DJ)] at the BTB must occur to coordinate the transient opening of the BTB to facilitate preleptotene spermatocyte migration. Interestingly, while there are extensively restructuring at the BTB during the epithelial cycle, the immunological barrier function of the BTB must be maintained without disruption even transiently. Recent studies using the androgen suppression and Adjudin models have shown that anchoring junction restructuring that leads to germ cell loss from the seminiferous epithelium also promotes the production of AJ (e.g., basal ES) proteins (such as N-cadherins, catenins) at the BTB site. We postulate the testis is using a similar mechanism during spermatogenesis at stage VIII of the epithelial cycle that these induced basal ES proteins, likely form a “patch” surrounding the BTB, transiently maintain the BTB integrity while TJ is “opened”, such as induced by TGF-b3 or TNFa, to facilitate preleptotene spermatocyte migration. However, in other stages of the epithelial cycle other than VII and VIII when the BTB remains “closed” (for ~10 days), anchoring junctions (e.g., AJ, DJ, and apical ES) restructuring continues to facilitate germ cell movement. Interestingly, the mechanism(s) that governs this communication between TJ and anchoring junction (e.g., basal ES and AJ) in the testis has remained obscure until recently. Herein, we provide a critical review based on the recently available data regarding the cross-talk between TJ and anchoring junction to allow simultaneous maintenance of the BTB and germ cell movement across the seminiferous epithelium.
Introduction
In adult rats, spermatogenesis takes place in the seminiferous tubules in testes, in which spermatozoa (1n, haploid) differentiate from spermatogonia (type A, 2n, diploid) in the seminiferous epithelium in ~58 days via six mitotic and two meiotic divisions consecutively.1,2 During this time, germ cells also undergo different phases of cellular differentiation with changes in morphology particularly at the head region associated with chromatin condensation, as well as tail elongation (see Fig. 1). Earlier studies using PAS (periodic acid-Schiff reaction) staining has divided these cellular changes in the seminiferous epithelium in adult rats into 14 stages, which, in turn, are comprised of one seminiferous epithelial cycle, from stages I to XIV. Each stage displays a unique pattern of association of germ cells at different stages of their development with Sertoli cells in the seminiferous epithelium.1–6 If an investigator is visualizing a specific section of the tubule at stage I in the epithelium under a transillumination stereomicroscope, this stage progresses to II through XIV and returns to stage I in ~12.9 days in rats. As such, a type A spermatogonium requires to complete the epithelial cycle 4.5 times in ~58 days utilizing much of these time for mitosis before it divides and matures into 256 spermatozoa.1,4 This process is precisely regulated under FSH, LH, and testosterone via coordination between the hypothalamus, pituitary gland, and the testis, which is known as the hypothalamic-pituitary-testicular axis (see Fig. 1).
Figure 1.
The physiological relationship along the hypothalamic-pituitary-testicular axis that regulates testicular function, and the intimate structural relationship between Sertoli and germ cells in the seminiferous epithelium during spermatogenesis. A) This is a schematic drawing that shows the hormonal regulation of spermatogenesis in the testis via the hypothalamic-pituitary-testicular axis. LHRH released from the hypothalamus stimulates the production of LH and FSH by the pituitary gland, which in turn regulates Leydig and Sertoli cell functions, residing in the interstitium and the seminiferous epithelium, respectively. Testosterone (T) and inhibin (and others, e.g., follistatin) released by Leydig and Sertoli cells, respectively, provide the necessary feed-back loops to maintain the endogenous levels of LH and FSH to regulate spermatogenesis. Any changes in the homeostasis of these hormones can perturb spermatogenesis. For instance, a reduction of intra-testicular T level can suppress spermatid (step 8 and beyond) adhesion, leading to spermatid depletion from the epithelium without disrupting the blood-testis barrier (BTB) integrity.60–62 The right panel represents a stage II–VI seminiferous tubule dissected from the testis by using a transilluminating stereomicroscope as described.1 B) A semi-thin cross-section from an adult rat testis showing a seminiferous tubule at stage V of the epithelial cycle, illustrating the intimate relationship between Sertoli (SC) and different germ cells (e.g., sg, spermatogonium; SP, pachytene spermatocyte; RS, round spermatid; es, elongating/elongate spermatid) that constitute the seminiferous epithelium, resting on the basement membrane (see asterisks). C) A schematic drawing of the seminiferous epithelium that illustrates germ cells at different stages of their development are associated with Sertoli cells via specialized cell junctions, such as AJ, apical ectoplasmic specialization (apical ES) and desmosome-like junctions (DJ), and apical tubulobulbar complex (apical TBC). The BTB, which is composed of tight junction (TJ), basal ES, basal TBC, DJ, and AJ, anatomically divides the seminiferous epithelium into the basal and adluminal compartments. However, the BTB must “open” to accommodate the movement of preleptotene spermatocytes across the barrier at stage VIII of the epithelial cycle.7 This figure was prepared based on recent findings and reviews in the field.6,17,45,110 Bar in (B), 10 μm.
Besides these well defined hormonal events that occur along the hypothalamic-pituitary-testicular axis, spermatogenesis cannot proceed unless preleptotene spermatocytes residing in the basal compartment can traverse the blood-testis barrier (BTB) at late stage VII through early stage VIII (these two cycles combined last for ~3.5 days in rats), entering into the adluminal compartment for further development7 (Fig. 1). The BTB is a functional term initially coined by Chiquoine8 and others9 to illustrate the presence of a physiological barrier in the testis. The tightness of the BTB is comparable to the blood-brain barrier. It prevented staining of the seminiferous tubules when dyes (e.g., acriflavine used by Kormano9) were injected into animals (for a review, see ref. 10). Subsequent studies by Setchell and colleagues have illustrated many unique features of the BTB versus other barriers in mammals (e.g., the blood-brain barrier or the blood-retina barrier).10 For instance, besides Sertoli cells, peritubular myoid cells in rodents contribute to the BTB function by limiting the diffusion of lanthanum and colloidal carbon,11,12 however, in other blood-tissue barriers, the barrier function is contributed almost exclusively by the epithelial or endothelial cells alone.
Several hypotheses regarding the movement of germ cells across the BTB have been suggested. For instance, the “zipper theory” proposes that during germ cell migration across the BTB, new occluding fibrils form below the preleoptotene/leptotene spermatocytes, to be followed by the break down of occluding fibrils above these spermatocytes.3,13 The “intermediate cellular compartment theory” of Russell7,14 suggests the presence of a unique compartment inhabited by the preleptotene/leptotene spermatocytes in transit where these spermatocytes are trapped in between two occluding zonules as confirmed by electron microscopy. The “repetitive removal of membrane segments theory” of Pelletier15 suggests that the upward movement of the migrating germ cells create stresses on the Sertoli cell junctional complexes, which, in turn, induces the formation of intercellular pockets. Each developing germ cell (except elongating spermatid) is trapped in one of these intercellular pockets, sealed at both ends by tight junction (TJ), and anchoring junctions [e.g., adherens junction (AJ) and desmosome-like junctions (DJ). Note: while DJ is an intermediate filament-based anchoring junction that localizes to the BTB, virtually no functional study is found in the literature regarding this junction type. Also, the molecular composition is virtually unknown, and as such, DJ is not included in our remaining discussion herein] and moves progressively across the seminiferous epithelium until it is internalized in autophagic vesicles. However, neither the “zipper”, the “intermediate cellular compartment”, nor the “repetitive removal of membrane segment” theories can perfectly describe the processes of preleptotene spermatocyte migration across the BTB during stage VIII of the epithelial cycle, taking into account the interactions between integral membrane proteins at the BTB, their peripheral adaptors, kinases, phosphatases, cytokines, proteases and protease inhibitors.3,5,6,14–17 This is not entirely unexpected since these postulates by and large were based on morphological studies without any biochemical and/or molecular data available at the time. A recent hypothesis known as the “junction restructuring theory” has provided a more solid basis in delineating the complicated biochemical and molecular cascade of events behind this phenomenon (for a review, see refs. 6,17). The essence of this hypothesis suggests that germ cell movement is composed of waves of junction disassembly and reassembly that occur under the influence of cytokines (e.g., TGF-β3 and TNF-α) that are released by Sertoli and/or germ cells into the microenvironment to facilitate cell movement. For instance, these cytokines regulate the steady-state levels of proteins at the BTB (e.g., occludin, ZO-1, cadherins and catenins) and the homeostasis of proteases and protease inhibitors in the epithelium.17 The net result of these interactions determines the “opening” or the “closing” state of the BTB to facilitate germ cell movement. This biochemical theory, if confirmed and adequately characterized, should provide new clues for developing novel male contraceptives, such as by “shutting-down” the BTB to prohibit the migration of preleptotene spermatocytes from the basal to the adluminal compartment. Recent studies have also illustrated that this hypothesis fits quite well with the “zipper theory” (see below for further discussion).
The Concept of Endocytosis in BTB Dynamics
In other epithelia, endocytosis has been shown to play a pivotal role in junction restructuring to facilitate cell migration.18–20 This mechanism also provides the efficient means for restructuring junctional complexes at the TJ-barrier by perturbing its permeability without requiring de novo protein synthesis.18 For instance, it is suggested that intercellular movement is achieved by coendocytosis of apposing TJ-integral membrane proteins into the adjacent cells in a study using mouse Eph4 cells in vitro.19 Besides, there is growing evidence that the “leaky” intestinal epithelia in chronic disorders is mediated by abnormal internalization of TJ-integral membrane proteins via endocytosis.21,22 Interferon-γ (IFNγ), a proinflammatory cytokine, was shown to increase the intestinal permeability through its effects on TJ proteins such as occludin, junctional adhesion molecule-A (JAM-A) and claudin-1 in T84 cells.22,23 For instance, TJ proteins were internalized into large vacuoles via the RhoA/ROCK signaling pathway.22
In the testis, endocytosis is also employed by Sertoli cells for house-keeping activities, such as phagocytosis of germ cells undergoing apoptosis or anoikis. This process is also used to eliminate residual bodies from elongating spermatids prior to spermiation when fully mature spermatids (spermatozoa) are emptied into the tubular lumen.17 At present, it is not clear if testes employ similar mechanism as other epithelia to regulate the rapid turnovers of TJ- and AJ-integral membrane proteins at the BTB to facilitate germ cell migration. Obviously, this can certainly be possible. Cytokines, such as TGF-β and TNF-α, have been shown to increase BTB permeability via different signaling pathways.24,25 However, the mechanism(s) that reduces the TJ protein levels following cytokine treatment is not known. Does this simply the result of a decline in de novo protein synthesis or involve internalization of TJ-integral membrane proteins? Recent findings have suggested that endocytosis does occur in the testis at the apical ES to facilitate spermiation. For instance, the formation of tubulobulbar complexes (TBC, another testis-specific AJ type) at the Sertoli cell-elongated spermatid interface at late stage VIII of the epithelial cycle prior to spermiation by replacing the apical ES has been speculated to assist the release of mature spermatids via internalization of TBC-junction molecules.16 This postulate has been confirmed in a recent study26 which showed that the TBC indeed appeared at the concave surface of the head of spermatids that was previously occupied by apical ES. Furthermore, the adhesion domains of nectin-2 and 3 were found to be internalized as membrane vesicles near the TBC at spermiation.26 The fate of these internalized adhesion molecules at TBC remains to be determined; they can either be degraded or recycled back to the cell surface. In other epithelia, most of the endocytosed molecules (e.g., E-cadherin and occludin) enter the recycling pathway so that they can be rapidly recycled back to the cell surface to maintain junction integrity, especially in unstable cell-cell contacts. For instance, a significant increase in E-cadherin recycling was detected in MDCK cells during [Ca2+]-depletion-induced loss of cell adhesion.27,28
It is conceivable that internalization of TJ and/or AJ integral membrane proteins takes place at the BTB between adjacent Sertoli cells. However, this would be a more complicated event since TJ, AJ (e.g., basal ES and basal TBC), and desmosome-like junctions are coexisting at the BTB (see Figs. 1, 2). In other epithelia, it is believed that endocytic recycling of TJ proteins (e.g., occludin) is mediated by Rab GTPases,28 and Rab8B was shown to structurally associate with E-cadherin in the rat testis at both the BTB and the apical ES29 (see Fig. 3). Moreover, other than intracellular movement, endocytosis can also mediate the disassembly of cell-matrix focal adhesion to facilitate cell movement along the extracellular matrix.30,31 Interestingly, the basal ES is a hybrid cell-cell and cell-matrix anchoring junction type because it is composed of proteins that are usually restricted to focal adhesion complex at the cell-matrix interface, such as FAK and vinculin.32 The disassembly of focal adhesion at the fibroblast-matrix interface is independent of Rho GTPases, instead, another GTPase called dynamin is involved. Dynamin is known to play a pivotal role in endocytic process. Among the three isoforms of dynamin, dynamin-2 and 3 are highly expressed in the testis, particularly in Sertoli and germ cells33,34 while dynamin-1 is neuron-specific. It is likely that dynamin may be involved in the internalization of junction molecules at the BTB. Ubiquitination is another physiological process that has recently been shown to play a crucial role in junction protein turnover using Sertoli cells cultured in vitro.35 For instance, ubiquitination is used to facilitate the rapid turnover of occludin at the inter-Sertoli TJ-barrier by targeting the ubiquitin-conjugating enzymes, such as Itch and UBC4, to occludin to induce its degradation by proteasomes or lysosomes.35 Obviously, much research is needed in this area to investigate the role(s) of integral membrane protein recycling and ubiquitination at the BTB to facilitate germ cell movement. These studies will also identify new targets for male contraceptive research, such as by disrupting the events of protein recycling and/or internalization at the BTB to perturb spermatogenesis.
Figure 2.
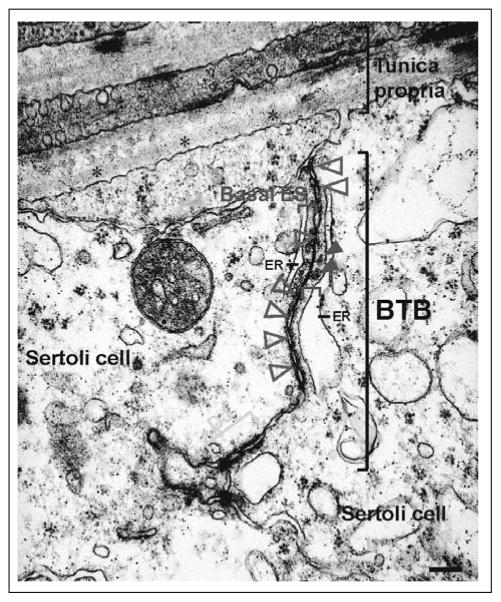
Electron micrograph of normal rat testes illustrating the typical BTB ultrastructural features. The features shown herein for BTB from an adult rat are applicable to mice and most other mammals. The basement membrane (asterisk), which is part of the tunica propria, appears as a homogeneous substance. This is a modified form of extracellular matrix (ECM)42 and is clearly visible underneath the collagen (type 1) fibrils. The BTB is composed of coexisting tight junction (TJ, blue arrowheads), basal ES (bracketed in red), desmosome-like junction (DJ, bracketed in green, see electron dense substances on both sides of the apposing Sertoli cell plasma membranes typified of desmosome), and adherens junction (AJ, bracketed in yellow). Basal ES refers to the ultrastructure between two adjacent Sertoli cells typified by the presence of actin bundles (red arrowheads) sandwiched between the endoplasmic reticulum (ER) and the plasma membrane that are found on both sides of the two adjacent Sertoli cells (SC). Bar, 0.2 μm.
Figure 3.
A schematic drawing depicting the currently known protein complexes at the apical ectoplasmic specialization (ES) and the BTB. Apical ES (top panel) is limited to the interface between Sertoli cells and spermatids (step 8 and beyond) in the apical compartment of the seminiferous epithelium in adult rat testes. As discussed in the text, the apical ES is a testis-specific hybrid cell-cell actin-based adherens junction (AJ), tight junction (TJ), and cell-matrix focal contact anchoring junction type since it contains protein complexes that are found in AJ (e.g., cadherins/catenins, nectins/afadins), TJ (e.g., JAM/CAR/ZO-1), and focal adhesion complex (FAC) (e.g., α6β1 integrin/laminin-333) of other epithelia. Some of the peripheral adaptors (e.g., α- and β-catenins, ZO-1), kinases (c-Src) and signaling molecules (e.g., p120ctn) that are known to associate with these complexes in adult rat testes are also shown. The BTB (bottom panel) is found between two adjacent Sertoli cells near the basement membrane that segregates the seminiferous epithelium into the adluminal and the basal compartments (see Figs. 1C, 2). In adult rat testes, the BTB is constituted by coexisting tight and anchoring junctions (see Figs. 1C, Fig. 2). The BTB is composed of protein complexes that are found in TJ (e.g., JAMs/ZO-1, claudins/ZO-1, occludin/ZO-1), AJ/basal ES/basal TBC (e.g., N-cadherin/catenin), desmosome-like junction [e.g., connexins, desmogleins (Dsg), desmocollins (Dsc)] of other epithelia. This drawing was prepared based on several recent papers and reviews.17,74,89,111–113 Because of space limitation, only the integral membrane proteins and some of the better studied adaptors, kinases and signaling molecules are shown.
Some Unique Physiological Phenomena at the BTB: Unidirectional and Bidirectional Cross-Talk between TJ and Anchoring Junction (e.g., Basal ES and AJ)
In multi-cellular organisms, cell-cell adhesion in epithelia is mediated through junction complexes which are constituted by TJ, AJ, desmosomes and gap junctions (for a review, see ref. 36). Each of these junction types has a specific physiological role. For instance, the TJ functions as a barrier to limit the passage of ions, water and other molecules between cells, and maintains cell polarity. The AJ links neighboring cells together by forming a continuous adhesion belt, desmosomes serve as an anchoring site and unite cells together while the gap junction is for communication between cells. Interestingly, BTB is unique in its morphological layout versus other blood-tissue barriers found in other epithelia/endothelia (for reviews, see refs. 2,3,5,6). In brief, in other epithelia/endothelia excluding the seminiferous epithelium, junctions are organized typically from apical to basal, in which TJ is present at the most apical region, to be followed by AJ and then desmosomes (for reviews, see refs. 37,38–40). As such, these junctions, in particular TJ, are furthest away from ECM. However, the TJ in the testis is located at the basal compartment of the seminiferous epithelium, closest to the basement membrane, a modified form of ECM41,42 and coexisting with AJ, basal ES, basal TBC (both basal ES and basal TBC are testis-specific AJ types restricted to the Sertoli-Sertoli cells interface at the BTB), gap junctions, and desmosome-like junctions (see Figs. 1, 2).3,16,17,43–45
Apart from the unique morphological features of BTB, the testis apparently is using a specialized junction restructuring mechanism in assisting preleptotene spermatocytes to migrate across the BTB, such that the post-meiotic germ cell antigens can be sequestered from the immune system while permitting continuous restructuring of the TJ-barrier and/or anchoring junction (e.g., basal ES and AJ) at the BTB. This apparently is in contrast to other blood-tissue barriers (e.g., blood-brain and blood-retina barriers), even though they are suggested to be dynamic in nature.18 Unlike the BTB, these blood-tissue barriers do not undergo such drastic restructuring to facilitate the migration of preleptotene spermatocytes which are 10–15 μm in diameter. Besides, except for stage VIII of the epithelial cycle, the BTB remains closed to limit the entry of preleptotene spermatocytes into the adluminal compartment. This implicates that there are unique molecules and mechanisms in place to coordinate these events.
In the past decade, different in vivo models have been used to study BTB dynamics and regulation.17 There are a number of interesting observations stemming from this body of work. For instance, studies using CdCl2,46–48 an environmental toxicant, and glycerol49–51 have shown that these toxicants can irreversibly perturb TJ which, in turn, lead to anchoring junction damage, dislodging germ cells of all classes from the epithelium. These studies thus illustrate the presence of cross-talk between tight and anchoring junctions that a disruption in TJ can spread to anchoring junction such as apical ES, which is located at the opposite end of the TJ in the seminiferous epithelium (see Fig. 1C). These findings are consistent with the cellular physiology in other epithelia, such as the skin, in which a disruption of TJ leads to underlying AJ damage and vice versa,52–54 illustrating bi-directional cross-talk between TJ and anchoring junction. However, in studies using antispermatogenic drugs such as Adjudin55 and lonidamine,56 it was shown that anchoring junction (e.g., apical ES) disruption could be limited to the interface between Sertoli cells and elongating spermatids/elongate spermatids/round spermatids/spermatocytes (but not spermatogonia) without compromising anchoring (e.g., basal ES) and tight junctions between Sertoli cells at the BTB,17,57,58 illustrating unidirectional cross-talk between TJ and anchoring junction in the testis. These findings were also supported in a recent study using the model of McLachlan and O’Donnell by suppressing the endogenous testosterone level in the testis using testosterone/estradiol (TE) implants.59,60 For instance, suppression of intratesticular androgen level by using TE implants in adult rats can lead to disruption of apical ES at the Sertoli-germ cell interface (step 8 spermatids and beyond) without perturbing the BTB nor anchoring junctions between Sertoli cells and round spermatids/spermatocytes/spermatogonia.60,61 Indeed, using the androgen suppression model, we have confirmed that a disruption of apical ES can be limited to the Sertoli cell-spermatid interface without compromising the BTB integrity nor the anchoring junction between Sertoli cells and round spermatids/spermatocytes in the seminiferous epithelium.62 In short, these findings are drastically different from other blood-tissue barriers (e.g., in the skin) since AJ disruption in these barriers always leads to TJ damage,53,54 and the assembly and integrity of TJ depend on AJ.63,64 For instance, when the reassembly of junctional complexes in intestinal cells is initiated by an incubation of high-calcium medium, AJ is reassembled within ~30 min prior to TJ assembly.65 As such, these results illustrated that this unidirectional cross-talk mechanism is operating at the BTB in which a disruption of Sertoli-germ cell adhesion can be restricted to anchoring junction (e.g., apical ES) without interfering with TJ nor anchoring junction (e.g., basal ES) at the BTB.
Changes in Protein-Protein Interactions between Integral Membrane Proteins and Their Adaptors, and Cross-Talk between Junctions at the BTB in the Regulation of BTB Dynamics
To address the observations regarding the cross-talk between TJ and AJ (e.g., basal ES) at the BTB that regulate its timely opening to facilitate preleptotene spermatocyte migration at stage VIII of the cycle, recent studies have shifted the focus on the role of protein-protein interactions in BTB dynamics.
Studies based on two different in vivo models, namely the Adjudin32,55,57 and the androgen suppression model have yielded some interesting observations that leads to a hypothesis to be tested in future studies. First, in the testosterone suppression model of O’Donnell60,61 in which subdermal testosterone/estradiol implants were placed in adult rats to suppress the intratesticular T level, it was shown that a loss of testosterone in the seminiferous epithelium selectively perturbed apical ES junction (from step 8 spermatids and beyond), leading to spermatid depletion from the seminiferous epithelium. While the BTB integrity and functionality were not compromised,62 there were significant changes at the BTB as well, reminiscence of the seminiferous epithelium during spermatogenesis, such as stage VIII of the epithelial cycle. For instance, at the time of apical ES restructuring that led to spermatid depletion from the epithelium, there were enhanced production of several basal ES proteins (e.g., cadherins, integrins, JAM-A).57,66,74 Second, these observations were consistent with other findings using the Adjudin model in which the Adjudin-induced anchoring junction restructuring in the seminiferous epithelium also led to progressive depletion of germ cells from the seminiferous epithelium, beginning with elongating/elongate spermatids, to be followed by round spermatids and spermatocytes.6,75 However, the BTB apparently remained “intact”.17 Likewise, an induction of an array of proteins at the BTB was also detected, such as cadherins, cateneins, integrins,32,74–76 illustrating restructuring indeed occurred at the BTB, but unlike the apical ES at the Sertoli cell-spermatid interface that led to spermatid loss from the epithelium, such BTB restructuring did not lead to functional damage which compromise the immunological barrier, reminiscent of the seminiferous epithelium at stage VIII of the epithelial cycle. We thus postulate that these transiently induced basal ES proteins that form a “patch” are being used to temporarily “reinforce” the TJ-barrier function at the BTB during androgen suppression- or Adjudin-induced junction restructuring in the seminiferous epithelium so that the BTB integrity can be maintained as observed in these models. However, this transient induction in basal ES proteins can also “supersede” the TJ-barrier function temporarily when TJ must open to accommodate preleptotene spermatocyte migration at stage VIII of the epithelial cycle. This speculation was also supported by a study using fluorescent microscopy. For instance, in studies using the androgen suppression model, it was shown that during the androgen depletion-induced spermatid (step 8 and beyond) loss, the increased basal ES proteins (e.g., N-cadherin, β-catenin) at the BTB had surrounded the entire basal compartment, forming a transient “patch”, and extended into the seminiferous epithelium well beyond the BTB site.57,62,66 As such, these basal ES proteins could “patch” the TJ-barrier, reinforcing the BTB integrity, which is also the mechanism being used to maintain the transiently disrupted TJ-barrier at the Sertoli-Sertoli interface at stage VIII of the cycle, facilitating preleptotene spermatocyte migration while maintaining the immunological barrier at the BTB. It is obvious that this possibility must be carefully evaluated and investigated in future studies. While functional data are not yet available to support this “patch” hypothesis, recent findings in the field seem to favor this postulate. For instance, several TJ proteins, such as JAM-C and coxsackievirus and adenovirus receptor (CAR) are putative integral membrane proteins at the TJ of multiple epithelia,67–70 yet recent studies have shown that they are also putative Sertoli and/or germ cell products that are restricted to the apical ES.71,72 A recent report has also identified the presence of JAM-A in germ cells,73 which is also detected at the BTB colocalizing with ZO-1 by fluorescent microscopy,62 suggesting that it is likely a component of the basal ES at the BTB. These findings are significant because they illustrate that ectoplasmic specialization (e.g., apical and basal ES), a testis-specific AJ type, is indeed a hybrid of AJ and TJ as it consists components of TJ-integral membrane proteins. Thus, these TJ component proteins at the basal ES can confer the necessary tight junction functionality, however, when the TJ fibrils are disrupted, the transiently induced basal ES proteins serve as a transient “patch”. Nonetheless, this concept must be vigorously investigated in future studies.
One may argue that since there was a surge in anchoring junction proteins in the seminiferous epithelium, and these proteins were being used to “reinforce” the TJ-barrier function at the BTB, why would such an increase in anchoring junction protein levels (e.g., cadherins and catenins) in the seminiferous epithelium fail to retain germ cells outside the BTB. Based on limited available data, it seems that this loss of cell adhesion is the result of a loss of protein-protein association between cadherins and catenins at the Sertoli-germ cell interface.66 This is likely mediated by a surge in tyrosine phosphorlyation of β-catenin, resulting in a reduction in its adhesive activity.66 Other studies have shown that tyrosine phosphorylation of AJ proteins (e.g., cadherins) facilitates junction disassembly in various epithelia,77,78 as well as in pathological conditions, such as tumor metastasis79 whereas Ser/Thr phosphorylation, on the contrary, promotes adhesion. In addition, other molecules are operating side-by-side to regulate BTB. For instance, the integrity of endothelial TJ-barrier was found to be partly regulated by reactive oxygen species, such as hydrogen peroxide. These reactive oxygen species up-regulate tyrosine phosphorylation of FAK and paxillin (note: these are cell-matrix focal adhesion proteins but have now been found at the apical and basal ES, such as the BTB), as well as β-catenin and VE-cadherin in the vascular endothelium.80 Likewise, recent studies have shown that NO, a reactive oxygen species produced by NOS, regulates Sertoli cell TJ-barrier and Sertoli-germ cell anchoring junction function via the cGMP/PKG signaling pathway81,82 which is also associated with a significant decline in cadherin-NOS and catenin-NOS interaction.74,76 It is likely that these changes can lead to an increase in phosphorylation of the cadherin/catenin complex via the action of PKG.76,82 These observations thus support the notion that as preleptotene spermatocytes traverse the BTB, there must be a coordination in the disintegration of TJ and anchoring junction. We speculate that when TJ is “opened” to accommodate cell migration, the transiently induced basal ES proteins form a “patch” to supersede the temporarily loss TJ-barrier function (see text above). Thereafter, cell adhesion complexes in the “patch” also transiently “open” to facilitate cell movement by altering their phosphorylation status so that preleptotene spermatocytes can continue their migration. However, one may also argue if the basal ES and perhaps AJ transiently loss their adhesion function at the BTB to facilitate preleptotene spermatocyte migration, how can BTB maintains its cell adhesion and TJ-barrier function at the time. Interestingly, in studies using the Adjudin and the androgen suppression model, it was shown that there was a transient induction in TJ-proteins (e.g., occludin, ZO-1, JAMs) at the BTB when spermatids were depleting from the epithelium.57,62,66,73 We speculate that these induced TJ-proteins are being used to supersede the temporal loss of AJ function at the BTB to confer adhesion between migration preleptotene spermatocytes and Sertoli cells. For instance, it is known that the first extracellular domain of occludin can confer cell adhesion function.83 In short, it is likely that TJ and basal AJ at the BTB are working in concert to facilitate preleptotene spermatocyte migration while maintaining the TJ-barrier function and cell adhesion in this microenvironment.
In the testis, apical ES is considered to be a hybrid cell-cell actin-based AJ and cell-matrix focal contact anchoring junction type restricted to the interface between Sertoli cells and developing spermatids in adult rat testes.32,84,85 This conclusion is reached since several focal contact proteins usually restricted to the cell-martix interface to facilitate cell movement (e.g., macrophages and fibroblasts) in other epithelia are also found at the apical ES. For instance, FAK is localized at the basal ES near the basement membrane while its phosphorylated and activated form, p-FAK-Tyr397, is detected almost exclusively at the apical ES and is structurally associated with a nonreceptor protein tyrosine kinase, c-Src.32 Furthermore, their protein levels are significantly induced when germ cells are depleting from the seminiferous epithelium, as illustrated in the Adjudin and testosterone-suppression models.32,86 Other protein kinases, including Csk, CK2 and Fer kinases have also been found to associate with N-cadherin and catenin at the basal and apical ES.74,75,87 Integrin-linked kinase (ILK) was also reported to be associated with β1 integrin at the apical ES.85 Collectively, these findings coupled with recent reports that TJ proteins are also integral components of the apical and basal ES (see discussion above) thus illustrate that ES is indeed a unique hybrid anchoring junction type of AJ, focal contact, and TJ, that can confer AJ functionality (i.e., cell adhesion) while facilitate cell movement (e.g., focal contact function) and transiently confer TJ functionality (e.g., during BTB restructuring in which the induced basal ES proteins form a “patch” at the BTB). Thus, it is not entirely unlikely that basal ES can supersede the function of TJ and vice versa as discussed herein.
Cross-Talk between TJ and Anchoring Junctions at the BTB That Regulates BTB Dynamics
The coexistence of TJ and AJ at the BTB has been illustrated by studies using electron and freeze-fracture microscopy since the 1970s.12,16,41,88 Recent studies using fluorescent microscopy have also colocalized TJ and AJ to the same site at the BTB. Even though occludin and cadherin at the BTB have no direct protein-protein interaction as demonstrated by coimmunoprecipitation, they are linked via their peripheral adaptors, such as ZO-1 and catenins.89 This finding is not entirely unexpected since at the early stage of TJ assembly, ZO-1 was shown to associate with catenins in MDCK cells.90 Besides, ZO-1 also serves as a cross-linker between the cadherin/catenin protein complex and the actin-based cytoskeleton in nonepithelial cells.91,92 In a more current study, ZO-1 was found to use the same domain for its interaction with occludin and α-catenin.93 As such, ZO-1 is not only a TJ adaptor; in fact, it shuffles between TJ and AJ and links these two junction types to cytoskeleton.
Using an in vivo model to study junction dynamics involving Adjudin, rats were treated with a single dose of this compound at 50 mg/kg b.w. by gavage to induce germ cell depletion from the seminiferous epithelium.17 The drug is targeted at the apical ES with minimal effect to TJs and basal ES.57 Interestingly, at the time of germ cell loss, the association between the protein complexes at TJ (e.g., occludin/ZO-1) and AJ (e.g., cadherin/catenin) via their corresponding adaptors was temporarily abolished, namely ZO-1 and α- and γ-catenins. In essence, ZO-1 was no longer associated with catenins, and as such, occludin and N-cadherin as well as their adaptors, ZO-1 and γ-catenin were diffusing away from the BTB site.89 Yet, their association was reestablished by day 7 after treatment when germ cells (e.g., elongating/elongate spermatids and some round spermatids) were depleted from the epithelium. Based on such timely dissociation between TJ and AJ protein complexes in the seminiferous epithelium, an “engagement and disengagement” theory was proposed to describe the unique mechanism employed by the testis to facilitate germ cell movement pertinent to spermatogenesis89 (Fig. 4). It is likely that under physiological conditions, such as at stages other than VII and VIII of the epithelial cycle, TJ and AJ (e.g., basal ES) are structurally “engaged” via their peripheral adaptors at the BTB to reinforce barrier integrity (Fig. 4). At the time of spermiation and when preleptotene spermatocytes must traverse the BTB,6,88 which occur concurrently at stage VIII of the epithelial cycle, a transient “disengagement” between adaptors (e.g., ZO-1 and catenins) of the corresponding TJ and AJ protein complexes takes place to facilitate germ cell movement across the barrier, avoiding the unnecessary damage to TJ during AJ (e.g., basal ES) restructuring. This novel mechanism thus preserves barrier integrity while facilitating germ cell movement (Fig. 4). After spermiation and the movement of preleptotene spermatocytes across the BTB, the adaptors, namely ZO-1 and α-/γ-catenins become “engaged” again to strengthen the barrier. This theory not only provides a solid biochemical basis regarding the mechanism of germ cell movement across the BTB, it also provides the rationale for the coexisting TJ and AJ at the BTB. However, the identity of the protein(s) that “pulls” ZO-1 away from catenins to maintain the “disengaged” state and facilitate germ cell movement remains to be determined. Recent studies have shown that dynamins (e.g., dynamin II), large GTPases that serve as “pinchase-like mechanoenzymes” to facilitate the formation of endocytic vesicles by severing nascent endocytic pits from the plasma membrane,94,95 are likely to maintain such a “disengaged” state at the BTB.73 This work must be vigorously validated and expanded in future studies.
Figure 4.
A–C) A schematic drawing that illustrates the engagement and disengagement mechanism between TJ and AJ at the BTB to facilitate preleptotene spermatocyte migration. A) The restructuring of TJs and AJs (e.g., basal ES) at the BTB are regulated by the “engagement (close)” and “disengagement (open)” mechanism to facilitate preleptotene spermatocyte migration across the BTB with minimal damaging effects on the barrier integrity. The left top panel shows the spatial arrangement of the seminiferous epithelium in which the BTB separates the epithelium into the basal and adluminal compartments. TJs (e.g., occludin/ZO-1) and basal ES (e.g., N-cadherin/γ-catenin) that coexist at the BTB are structurally associated with each other via the peripheral adaptors (e.g., ZO-1 and catenins). At stage VIII of the epithelial cycle, spermiation occurs when elongated spermatids emptying into the tubule lumen, and when preleptotene spermatocytes traverse the BTB, a transient opening of BTB must occur. B,C) In stages other than VIII, protein complexes occludin/ZO-1 and N-cadherin/β-catenin at the BTB are “engaged” via their corresponding adaptors to reinforce the BTB integrity. At stage VIII of the epithelial cycle during which preleptotene spermatocytes traverse the BTB, in order to minimize the damage to the BTB, adaptors (e.g., catenins) at the apical ES are ‘disengaged’ from adaptors (e.g., ZO-1) at the TJ. We postulate that at the time of AJ (e.g., basal ES) disassembly, which is likely mediated by TGF-β3 via the ERK signaling pathway,57,58 to facilitate preleptotene spermatocyte migration, TJ-proteins transiently maintain the barrier function alone as well as cell adhesion (e.g., the first extracellular domain of occludin is known to confer cell adhesion function) (B). The migrating germ cells and Sertoli cells also produce more cytokines, such as TGF-β3 and TNF-α, into the BTB microenvironment at stage VIII of the epithelial cycle. When these cytokines bind onto the receptors on the Sertoli cell, the cascade of signaling molecules (e.g., p-p38) are activated, which, in turn, reduces the levels of TJ proteins (e.g., occludin) at the BTB24,47,58 to facilitate preleptotene spermatocyte migration (C). The induced AJ (e.g., basal ES) proteins at the site likely supersede the role of the TJ-proteins at the BTB transiently by “patching” the temporarily disrupted TJ-barrier so as to maintain the BTB integrity (C). The “unpairing” of these TJ and AJ (e.g., basal ES) proteins are mediated either via changes in tyrosine phosphorylation, thereby causing the loss of adhesiveness of these protein complexes, facilitating germ cell movement. This model was prepared based on recently published reports as discussed in the text. Sg, spermatogonium; Sp, pachytene spermatocyte; es, elongating spermatid; SC, Sertoli cell; P, phosphate; ZO-1, zonula occludens-1; AJ, adherens junction; basal ES, basal ectoplasmic specialization; TJ, tight junction; GJ, gap junction; DJ, desmosomes.
In this context, it is of interest to note that this theory does not account for the temporal disruption of TJ at the BTB, which must occur to accommodate preleptotene spermatocyte migration. Recent studies have unequivocally demonstrated that TNFα and TGF-β3 produced and secreted by Sertoli and/or germ cells into the BTB microenvironment can induce reversible disruption of the barrier58,96 as receptors for these cytokines are mostly resided in Sertoli cells.6,17 This is illustrated in a functional BTB assay by monitoring the diffusion of a small molecular dye (e.g., fluorescein 5′-isothiocyanate, Mr 389) from the systemic circulation into the adluminal compartment behind the BTB following TNFα treatment.96 Thus, it is highly plausible that these cytokines at the BTB perturb TJs, transiently open the barrier to facilitate preleptotene spermatocyte migration at stage VIII of the epithelial cycle. Indeed, the expression levels of both TNFα and TGF-β3 are highest at stage VIII of the cycle.25,58
Other studies using Sertoli cell cultures have shown that TGF-β3 regulates TJ-barrier function via the p38 and ERK MAP kinase signaling pathways.97,98 Furthermore, studies using the in vivo CdCl2 model have unequivocally demonstrated that BTB dynamics are regulated by TGF-β324,47 via its effects, at least in part, on the steady-state protein levels of protease inhibitors (e.g., α2-macroglobulin), and proteases (e.g., cathepsin L),47,99 which, in turn, assist BTB restructuring. Other studies have shown that MMPs and TIMPs are both present at the ES and BTB, and whose actions are coordinated by TNFα.25 Collectively, these data suggest that at the time preleptotene spermatocytes traverse the BTB, it is initially mediated by a transient loss in protein-protein associations between AJ-proteins at the basal and apical ES (e.g., cadherins/catenins) via a surge in tyrosine phosphorylation of catenins (and/or cadherins). Additionally, AJ-associated proteins complexes are transiently disengaged from TJs and the surge in TJ proteins can supersede the temporal loss of cell adhesion at the BTB. During the migration of preleptotene spermatocytes across the BTB, Sertoli and germ cells secrete TGF-β3 and/or TNF-α into the microenvironment at the BTB, which reduce the steady-state levels of TJ-proteins at the BTB to “open” the barrier to facilitate germ cell migration. Ser/Thr phosphorylation and the temporal increase in AJ-proteins can substitute the TJ-barrier function by forming a “patch” in the “opened” BTB. After germ cells pass through the BTB, further TJ disassembly can be limited by the production of protease inhibitors such as α2-MG and TIMPs.99,100 Finally, TJ and AJ become structurally linked and “engaged”, which is mediated via their peripheral adaptors to reinforce the BTB.
Additionally, it is of interest to note that the disengagement between TJ and AJ at the time of Adjudin-induced germ cell depletion from the epithelium does not involve actin disruption, since the level of F to G-actin increases as AJ disengaged from TJ.89 This illustrates that this germ cell loss event is entirely a junction restructuring process. Perhaps the increase in F-actin content provides additional scaffolding function to the BTB during germ cell depletion. Indeed, an increase in F- to G-actin ratio is detected in human adenocarcinoma cells with an increase in invasiveness,101 illustrating active AJ restructuring pertinent to cellular migration is associated with an increase in F-actin, consistent with results obtained from the Adjudin model.
In short, the biochemical events that occur at the BTB during spermatogenesis as discussed above are in agreement with the “junction restructuring theory”.17 Also, these recent biochemical findings do not negate the “zipper theory”3,13 or the “intermediate cellular compartment theory”.7,14 Indeed, these three theories are not mutually exclusive. In essence, the “junction restructuring theory” is a biochemical version of the combined “zipper” and “intermediate cellular compartment” theories, taking into account the molecular players in the junction restructuring events that occur during spermatogenesis. For instance, in the “zipper theory”, the “old” TJ fibrils above the migrating preleptotene spermatocytes must be broken down after the formation of the nascent TJ fibrils below these migrating cells as illustrated in the “intermediate cellular compartment theory” in which preleptotene spermatocytes were shown to be trapped between TJ fibrils in the seminiferous epithelium.14 The “disengagement” between TJs and AJs at the BTB as discussed above (see also ref. 89) thus offers the biochemical mechanism to facilitate preleptotene spermatocyte migration across the BTB.
Cross-Talk between TJ, Anchoring Junction, and GJ in the Seminiferous Epithelium Is Crucial to Spermatogenesis
In other epithelia, cross-talk between different junctions has been the subject of active investigation in recent years. For instance, cross-talk between cadherins and integrins is likely mediated by Rap1 GTPase. It was shown that the disassembly of AJ through endocytosis activated Rap1, which, in turn, enhanced the integrin cell-matrix adhesion by redistributing intergrins to new adhesion sites. This thus avoids uncontrolled cell dissemination.102 Direct association between AJ proteins (e.g., nectin-1 and 3) and PAR-3 (a signaling molecule usually restricted to TJ and crucial to cell polarization working in concert with aPKC and PAR-6103) was also reported in neural epithelial cells in which the affinity of nectins toward afadin (an adaptor of nectin) and PAR-3 was found to be similar.104,105 Protein interacting with C-kinase-1 (PICK-1), a scaffolding protein, interacts with both AJ (e.g., nectin) and TJ proteins (e.g., JAM-A, B & C but not claudins) in CHO cells.106 It is believed that PICK-1 is an adaptor which coordinates cross-talk between TJ and AJ in epithelial cells. However, the significance (and its presence in the testis) of PICK-1 in cross-talk between TJ and anchoring junction (e.g., basal ES) at the BTB and between apical ES and BTB remains to be determined. Gap junctions (GJ) (e.g., connexin-43) are recently shown to associate with TJ (e.g., ZO-1 & 2)107,108 and AJ proteins (e.g., N-cadherin, α-catenin, β-catenin and p120ctn).109 These findings thus demonstrate the potential inter-dependent relationship between junction types in various epithelia including the BTB, which is likely mediated via peripheral adaptors. In some cases, their roles can be temporarily superseded by each other, as illustrated in the engagement and disengagement theory in the BTB.89 In others, the formation of a junction type requires the coassembly of another type. For instance, when either connexin-43 (GJ) or N-cadherin (AJ) was disrupted by RNA silencing, gap junctional communication as well as the mobility of NIH3T3 cells was reduced,109 illustrating a functional AJ and GJ linkage.
Concluding Remarks—Lesson from the Testis
In the past decade, much work has been done in dissecting the role of TJ and anchoring junction dynamics in spermatogenesis, however, the crucial information is still lacking in the field as highlighted in this review. For instance, how is the cross-talk between TJ and anchoring junctions initiated and regulated? Is the cross-talk limited between TJ and AJ (e.g., basal ES and apical ES) or is it extended to the desmosome-like junctions? What are the signaling molecules and pathways that are involved in this cross-talk? Answers to some of these questions will also unravel potential targets for male contraception and may offer an explanation for unexplained infertility. Nonetheless, recent findings regarding the regulation of different junction types at the BTB as reviewed herein have illustrated that the testis has a unique mechanism in place in which anchoring junction (e.g., basal ES) and TJ can be functionally segregated so that preleptotene spermatocytes can traverse the BTB without compromising the TJ-barrier function. Additionally, cytokines (e.g., TGF-β3 and TNFα) produced locally by Sertoli and/or germ cells and secreted into the microenvironment of BTB are also being used to transiently “open” the TJ-barrier to facilitate germ cell movement. It will be of interest and physiologically important to determine if this mechanism is used to facilitate food absorption across the epithelial TJ-barrier in small intestine or to facilitate transepithelial migration of neutrophils across the endothelial TJ-barrier in microvessels at the inflammation site.
Table 1.
Junction types and their molecular components at the BTB of adult rodent testes
| Junction Type | Component Proteins | Mr (kDa) | Interacting Partners |
|---|---|---|---|
| TJ | - Integral membrane proteins | ||
| Occludin, occludin IB | 60–65 | ZO-1, ZO-2, ZO-3, cingulin, NOS | |
| Claudin-1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12–24 | 20–25 | ZO-1, ZO-2, ZO-3 | |
| JAM-A, B | 40 | ZO-1, cingulin, AF-6/afadin, PAR3, PAR6, aPKC, PICK-1, CASK, MUPP-1 | |
| CAR | 46 | ZO-1, MUPP-1, MAGI-1 | |
| CLMP (CAR-liked membrane protein) | 44–48 | Occludin, ZO-1 | |
| BT-IgSF (Brain- and testis-specific immunoglobulin superfamily) | 43.77–52 | n.k. | |
| Tricellulin | 66–72 | n.k. | |
| - Peripheral membrane proteins | |||
| ZO-1, 2, 3 | 210–225 | Occludin, claudin, JAM, α and γ-cateinins, connexin 43, actin, afadin, 4.1 R | |
| Clingulin | 140 | JAM-A, ZO-1, ZO-2, ZO-3, myosin, | |
| Basal ES and/or AJ | - Integral membrane proteins | ||
| N- or E-Cadherin | 127–135 | α, β and γ-Catenins, p120ctn, c-Src, NOS-2,3, ponsin, α-actinin, actin, Rab8b | |
| Celsr cadherins 1, 2 | 320 | Protocadherin a, Rab7 | |
| Nectin-1, 2, 4 | 70–85 | Afadin, ponsin, α2-macroglobulin, ZO-1, PAR3, PAR6, aPKC, PICK-1 | |
| - Peripheral membrane proteins | |||
| α-Catenin | 100–104 | N-Cadherin, β and γ-catenins, ZO-1, p120ctn, α-actinin, actin, afadin | |
| β-Catenin | 92 | N-Cadherin, α and γ-catenins, p120ctn α-actinin, actin, NOS-2,3, PRKG | |
| γ-Catenin | 82 | N-Cadherin, α and β-catenins, ZO-1, p120ctn, α-actinin, actin, Rab8b | |
| p120ctn | 65–120 | N-Cadherin, β and γ-catenins, α-actinin, actin, | |
| Afadin/AF-6 | 205 | Nectin, ponsin, α2-macroglobulin, actin, P-cadherin | |
| Basal TBC | MN7 | 90 | |
| Actin | 42 | ||
| Cofilin | 20 | ||
| Desmosome-like junction | Connexins 26, 30.2, 30.3, 31, 31.1, 32, 33, 36, 37, 40, 43, 45, 46, 50, 57 | 27–70 | Occludin, ZO-1, ZO-2, N-cadherin, α and β-catenins, p120ctn |
| Desmocollin-1, -2, -3 | 107–115 | Plakophilin, desmoplakin vimentin | |
| Desmoglein-1α, -1β, -1γ, -2, -3, -4 | 110 | Plakoglobin, desmoplakin, vimentin | |
| Others | 4.1 B* | 110 | n.k. |
| 4.1 G* | 130–140 | Cadherin | |
| LYRIC** | 80 | Occludin, ZO-1 | |
| P-glycoprotein (P-gp) | n.k. | n.k. | |
| Multidrug resistance-associated protein (MRP1) | n.k. | n.k. | |
This table was prepared based on current findings in the field as discussed in the text. References given here are not exhaustive due to the limited page space. As such, only selected references are given herein and additional references can be found in the text and earlier reviews.6,17,30,39,40,46,108–110,113–124 In the column for “Component proteins”, proteins that have been positively identified in the testis are underlined. In the column for “Interacting partners”, underlined proteins that are bold and italic are those that were shown to interact with the corresponding integral membrane proteins at the BTB; proteins that are underlined only are found in testes but were shown to interact with the corresponding target proteins in other epithelia; proteins that are included in the table but not underlined are those that were found in similar blood-tissue barriers other than the testis. n.k. = not known.
These proteins are found at the Sertoli cell-spermatogonia interface near the basal compartment, and may not be a component of the BTB;
Protein that has not been identified in the BTB but it is known to interact with TJ-associated proteins.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (NIH, NICHD U01 HD045908 and U54 HD29990 Project 3), and the CONRAD Program (CICCR CIG 96-05-A/B, CIG 01-72, and CIG 01-74). W.M.L. was supported by a grant from the Hong Kong Research Grant Council (HKU 7599/06M). H.H.N.Y. was supported by a University of Hong Kong Postgraduate Research Fellowship Award.
References
- 1.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 2.Russell L, Ettlin R, Sinha Hikim A, et al. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990. [Google Scholar]
- 3.Russell L, Peterson R. Sertoli cell junctions: Morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 4.Hess RA, Schaeffer DJ, Eroschenko VP, et al. Frequency of the stages in the cycle of the seminiferous epithelium in the rat. Biol Reprod. 1990;43:517–524. doi: 10.1095/biolreprod43.3.517. [DOI] [PubMed] [Google Scholar]
- 5.Vogl AW, Pfeiffer DC, Redenbach DM, et al. Sertoli cell cytoskeleton. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- 6.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 7.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 8.Chiquoine AD. Observations on the early events of cadmium necrosis of the testis. Anat Rec. 1964;149:23–35. doi: 10.1002/ar.1091490104. [DOI] [PubMed] [Google Scholar]
- 9.Kormano M. Dye permeability and alkaline phosphatase activity of testicular capillaries in the post-natal rat. Histochemie. 1967;9:327–338. doi: 10.1007/BF00305816. [DOI] [PubMed] [Google Scholar]
- 10.Setchell BP, Waites GMH. The blood-testis barrier. Washington DC: American Physiological Society; 1975. [Google Scholar]
- 11.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 12.Fawcett DW, Leak LV, Heidger PMJ. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- 13.Dym M, Cavicchia J. Further observations on the blood-testis barrier in monkeys. Biol Reprod. 1977;17:390–403. doi: 10.1095/biolreprod17.3.390. [DOI] [PubMed] [Google Scholar]
- 14.Russell L. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- 15.Pelletier RM. Tight Junctions. New York: CRC Press; 2001. The tight junctions in the testis, epididymis, and vas deferens; pp. 599–628. [Google Scholar]
- 16.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: Structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 17.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: Mechanisms and possible roles in regulations of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, Kubo A, Furuse M, et al. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins AM, Walsh SV, Verkade P, et al. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 22.Utech M, et al. Mechanism of IFN-γ-induced endocytosis of tight junction proteins: Myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson CJ, Hoare CJ, Garrod DR, et al. Interferon-γ selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci. 2005;118:5221–5230. doi: 10.1242/jcs.02630. [DOI] [PubMed] [Google Scholar]
- 24.Lui WY, Wong CH, Mruk DD, et al. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: An in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 25.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 26.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod. 2004;71:548–559. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 27.Le TL, Yap AS, Stow JL. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 28.Morimoto S, et al. Rab 13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 29.Lau ASN, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- 30.Burridge K. Foot in mouth: Do focal adhesions disassemble by endocytosis? Nat Cell Biol. 2005;7:545–547. doi: 10.1038/ncb0505-545. [DOI] [PubMed] [Google Scholar]
- 31.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 32.Siu MKY, Mruk DD, Lee WM, et al. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 33.Cook TA, Urrutia R, McNiven MA. Identification of dynamin 2, an isoform ubiquitously expressed in rat tissues. Proc Natl Acad Sci USA. 1994;91:644–648. doi: 10.1073/pnas.91.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamitani A, et al. Distribution of dynamins in testis and their possible relation to spermatogenesis. Biochem Biophys Res Commun. 2002;294:261–267. doi: 10.1016/S0006-291X(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 35.Lui WY, Lee WM. cAMP perturbs inter-Sertoli tight junction permeability barrier in vitro via its effect on proteasome-sensitive ubiquitination of occludin. J Cell Physiol. 2005;203:564–572. doi: 10.1002/jcp.20254. [DOI] [PubMed] [Google Scholar]
- 36.Alberts B, et al. The Molecular Biology of the Cell. New York: Garland Publishing Inc; 1994. [Google Scholar]
- 37.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 38.Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 39.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 40.Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 41.Dym M. The mammalian rete testis—A morphological examination. Anat Rec. 1976;186:493–523. doi: 10.1002/ar.1091860404. [DOI] [PubMed] [Google Scholar]
- 42.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 43.Bart J, et al. An oncological view on the blood-testis barrier. Lancet Oncol. 2002;3:357–363. doi: 10.1016/s1470-2045(02)00776-3. [DOI] [PubMed] [Google Scholar]
- 44.Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cell: New vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 45.Vogl AW, Pfeiffer DC, Mulholland DJ, et al. Unique and multifunctional adhesion junctions in the testis: Ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 46.Prozialeck WC. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- 47.Wong CH, Mruk DD, Lui WY, et al. Regulation of blood-testis barrier dynamics: An in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 48.Hew KW, Heath GL, Jiwa AH, et al. Cadmium in vivo causes disruption of tight junction-associaetd microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 49.Wiebe JP, Barr KJ. The control of male fertility by 1,2,3-trihydroxypropane (THP; glycerol): Rapid arrest of spermatogenesis without altering libido, accessory organs, gonadal steroidogenesis, and serum testosterone, LH and FSH. Contraception. 1984;29:291–302. doi: 10.1016/s0010-7824(84)80009-8. [DOI] [PubMed] [Google Scholar]
- 50.Wiebe JP, Barr KJ. Sustained azoospermia in squirrel, monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception. 1989;39:447–457. doi: 10.1016/0010-7824(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 51.Wiebe JP, Kowalik A, Gallardi RL, et al. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–635. [PubMed] [Google Scholar]
- 52.Troxell ML, Chen Y, Cobb N, et al. Cadherin function in junctional complex rearrangement and post-translational control of cadherin expression. Am J Physiol Cell Physiol. 1999;276:C404–C418. doi: 10.1152/ajpcell.1999.276.2.C404. [DOI] [PubMed] [Google Scholar]
- 53.Guo X, et al. Regulation of adherens junctions and epithelial paracellular permeability: A novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 54.Man Y, Hart VJ, Ring CJA, et al. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. Am J Respir Cell Mol Biol. 2000;23:610–617. doi: 10.1165/ajrcmb.23.5.4046. [DOI] [PubMed] [Google Scholar]
- 55.Cheng CY, et al. AF-2364, [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Traina ME, et al. Lonidamine transiently affects spermatogenesis in pubertal CD1 mice. Contraception. 2005;72:262–267. doi: 10.1016/j.contraception.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 58.Xia W, Mruk DD, Lee WM, et al. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 59.McLachlan RI, Wreford NG, Meachem SJ, et al. Effects of testosterone on spermatogenic cell populations in the adult rat. Biol Reprod. 1994;51:945–955. doi: 10.1095/biolreprod51.5.945. [DOI] [PubMed] [Google Scholar]
- 60.O’Donnell L, McLachlan RI, Wreford NG, et al. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- 61.Beardsley A, O’Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 62.Xia W, Wong CH, Lee NPY, et al. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: An in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 63.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 65.Ivanov AI, Hunt D, Utech M, et al. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin complex which are possibly mediated by c-Src and myotubularin-related protein 2: An in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–1284. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Padura I, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmeri D, van Zante A, Huang CC, et al. Vascular endothelial junction-associated molecule, a novel member of the immunoglobulin superfamily, is localized to intercellular boundaries of endothelial cells. J Biol Chem. 2000;275:19139–19145. doi: 10.1074/jbc.M003189200. [DOI] [PubMed] [Google Scholar]
- 69.Raschperger E, et al. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 70.Bergelson JN, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 71.Gliki G, Ebnet K, Aurrand-Lions M, et al. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 72.Mirza M, et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 73.Lie P, et al. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood-testis barrier in adult rat testes. J Endocrinol. 2006;191:571–586. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- 74.Lee NPY, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- 75.Chen YM, Lee NPY, Mruk DD, et al. Fer kinase/Fer T and adherens junction dynamics in the testis: An in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- 76.Lee NPY, Mruk DD, Wong CH, et al. Regulation of Sertoli-germ cell adherens junction dynamics in the testis via the nitric oxide synthase (NOS/cGMP/protein kinase G (PRKG)/β-catenin (CATNB) signaling pathway: An in vitro and in vivo study. Biol Reprod. 2005;73:458–471. doi: 10.1095/biolreprod.105.040766. [DOI] [PubMed] [Google Scholar]
- 77.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Matsuyoshi N, et al. Cadherin-mediated cell-cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Usatyuk PV, Natarajan V. Regulation of reactive oxygen species-induced endothelial cell-cell and cell-matrix contacts by focal adhesion kinase and adherens junction proteins. Am J Physiol Lung Cell Mol Physiol. 2005;289:L999–L1010. doi: 10.1152/ajplung.00211.2005. [DOI] [PubMed] [Google Scholar]
- 81.Lee NPY, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′,5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: An in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 82.Sarkar O, Xia W, Mruk DD. Adjudin-mediated junction restructuring in the seminiferous epithelium leads to displacement of soluble guanylate cyclase from adherens junctions. J Cell Physiol. 2006;208:175–187. doi: 10.1002/jcp.20651. [DOI] [PubMed] [Google Scholar]
- 83.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 84.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 85.Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 86.Wong CH, et al. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- 87.Wine RN, Chapin RE. Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J Androl. 1999;20:198–213. [PubMed] [Google Scholar]
- 88.Russell L. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 89.Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajasekaran AK, Hojo M, Huima T, et al. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Itoh M, Nagafuchi A, Moroi S, et al. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to α-catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muller SL, et al. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem. 2005;280:3747–3756. doi: 10.1074/jbc.M411365200. [DOI] [PubMed] [Google Scholar]
- 94.Thompson H, McNiven M. Dynamin: Switch or pinchase? Curr Biol. 2001;11:R850. doi: 10.1016/s0960-9822(01)00513-9. [DOI] [PubMed] [Google Scholar]
- 95.Cao H, et al. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li MWM, et al. Tumor necrosis factor α reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 97.Lui WY, Lee WM, Cheng CY. Transforming growth factor β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 98.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 99.Wong CH, Mruk DD, Siu MKY, et al. Blood-testis barrier dynamics are regulated by α2–macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 100.Mruk DD, et al. Role of tissue inhibitor of metalloproteases-1 in junction dynamics in the testis. J Androl. 2003;24:510–523. doi: 10.1002/j.1939-4640.2003.tb02703.x. [DOI] [PubMed] [Google Scholar]
- 101.Nowak D, Krawczenko A, Dus D, et al. Actin in human colon adenocarcinoma cells with different metastatic potential. Acta Biochem Pol. 2002;49:823–828. [PubMed] [Google Scholar]
- 102.Balzac F, et al. E-cadherin endocytosis regulates the activity of Rap1: A traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–4783. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- 103.Ohno S. Intercellular junctions and cellular polarity: The PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 104.Takekuni K, et al. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–5500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- 105.Ozaki-Kuroda K, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- 106.Reymond N, Garrido-Urbani S, Borg J, et al. PICK-1: A scaffold protein that interacts with nectins and JAMs at cell junctions. FEBS Lett. 2005;579:2243–2249. doi: 10.1016/j.febslet.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 107.Singh D, Solan JL, Taffet SM, et al. Connexin 43 interacts with Zona Occludens-1 and -2 proteins in a cell cycle stage-specific manner. J Biol Chem. 2005;280:30416–30421. doi: 10.1074/jbc.M506799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hunter AW, Barker RJ, Zhu C, et al. ZO-1 alters connexin 43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wei C, Francis R, Xu X, et al. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 110.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin: Landes Bioscience; 2007. [Google Scholar]
- 111.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 112.Cyr DG, et al. Cellular immunolocalization of occludin during embryonic and postnatal development of the mouse testis and epididymis. Endocrinology. 1999;140:3815–3825. doi: 10.1210/endo.140.8.6903. [DOI] [PubMed] [Google Scholar]
- 113.Green KJ, Bohringer M, Gocken T, et al. Intermediate filament associated proteins. Adv Protein Chem. 2005;70:143–202. doi: 10.1016/S0065-3233(05)70006-1. [DOI] [PubMed] [Google Scholar]
- 114.Beall SA, Boekelheide K, Johnson KJ. Hybrid GPCR/cadherin (Celsr) proteins in rat testis are expressed with cell type specificity and exhibit differential Sertoli cell-germ cell adhesion activity. J Androl. 2005;26:529–538. doi: 10.2164/jandrol.05003. [DOI] [PubMed] [Google Scholar]
- 115.Inagaki M, et al. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 2005;132:1525–1537. doi: 10.1242/dev.01697. [DOI] [PubMed] [Google Scholar]
- 116.Pointis G, Segretain D. Role of connexin-based gap junction channels in testis. Trends Endocrinol Metab. 2005;16:300–306. doi: 10.1016/j.tem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 117.Terada N, et al. Immunohistochemical study of a membrane skeletal molecule, protein 4. 1G, in mouse seminiferous tubules. Histochem Cell Biol. 2005;124:303–311. doi: 10.1007/s00418-005-0031-y. [DOI] [PubMed] [Google Scholar]
- 118.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15:665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 119.Britt DE, et al. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp Cell Res. 2004;300:134–148. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 120.Bart J, et al. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 121.Terada N, et al. Immunohistochemical study of protein 4. 1B in the normal and W/Wv mouse seminiferous epithelium. J Histochem Cytochem. 2004;52:769–777. doi: 10.1369/jhc.3A6192.2004. [DOI] [PubMed] [Google Scholar]
- 122.Whittock NV, Bower C. Genetic evidence for a novel human desmosomal cadherin, desmoglein 4. J Invest Dermatol. 2002;120:523–530. doi: 10.1046/j.1523-1747.2003.12113.x. [DOI] [PubMed] [Google Scholar]
- 123.Ikenouchi J, et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]