Abstract
Objective
The objective of this study was to determine the incidence of hypoglycemia after burn injury and whether hypoglycemia is associated with increased post-burn morbidity and mortality.
Design
Cohort analysis.
Setting
Academic pediatric burn hospital.
Patients
This analysis included 760 pediatric burn patients, who were stratified according the number of hypoglycemic episodes (<60 mg/dl glucose) they experienced while in the intensive care unit. Clinical outcomes as well as metabolic and inflammatory biomarkers were analyzed during the first 60 days post admission. Patients with one or more hypoglycemic events were matched with patients not experiencing any event using propensity score matching, and outcomes and biomarker expression were compared between groups.
Measurements and main results
Eighty-four patients had one episode of hypoglycemia, 108 patients had two or more episodes of hypoglycemia, and 568 patients never experienced hypoglycemia. Patients with one or more hypoglycemic episodes had longer hospitalization as well as more frequent infections, sepsis, multiple organ failure (MOF), and death (p<0.05). The 166 propensity score-matched patients with one or more hypoglycemic events had greater inflammatory and metabolic responses, incidence of sepsis, MOF, and mortality than burn patients without hypoglycemic (p<0.05).
Conclusions
Hypoglycemic episodes correlate with injury severity and inhalation injury. When adjusted for injury severity, hypoglycemia is associated with significantly higher post-burn morbidity and mortality.
Keywords: hypoglycemia, insulin, burn size, pediatric, morbidity, inflammation, critical care
INTRODUCTION
The introduction of tight euglycemic control (1, 2) quickly changed standard protocols in intensive care unit (ICU) care. These changes were the result of unicenter trials demonstrating that insulin therapy aimed at keeping glucose at 80–110 mg/dl diminishes death, infections, sepsis and sepsis-associated multiple organ failure (MOF) after surgery. In medical patients, tight euglycemic control attenuates kidney injury and accelerated weaning from mechanical ventilation and discharge from the ICU stay. Several subsequent unicenter and multicenter studies were conducted to shed light on whether tight euglycemic control provides benefits in other settings. Some showed that euglycemic control (3–5) improved outcomes, while others did not. Yet others showed that tight euglycemic control was associated with harmful effects, including a greater prevalence of hypoglycemia (blood glucose <60 mg/dl) (6, 7). Current guidelines for the treatment of critically ill, septic, trauma, and burn patients call for less restrictive glucose ranges of 130–150 mg/dl (4, 8, 9) or 160–180 mg/dl (10). Finfer et al. (11) recently reported that hypoglycemia increases ICU morbidity and mortality.
Despite the realization that hypoglycemic episodes should be avoided, the short- and long-term consequences of hypoglycemic episodes in the critical care setting, or even in the burn care setting, are not entirely clear. More importantly, it is also unclear whether the number of episodes or the duration of the periods of hypoglycemia may increase morbidity and mortality or worsen clinical outcomes. The objective of this study was to determine the incidence of hypoglycemic episodes after burn injury as well as to investigate whether hypoglycemia may contribute to morbidity and mortality after burns.
PATIENTS AND METHODS
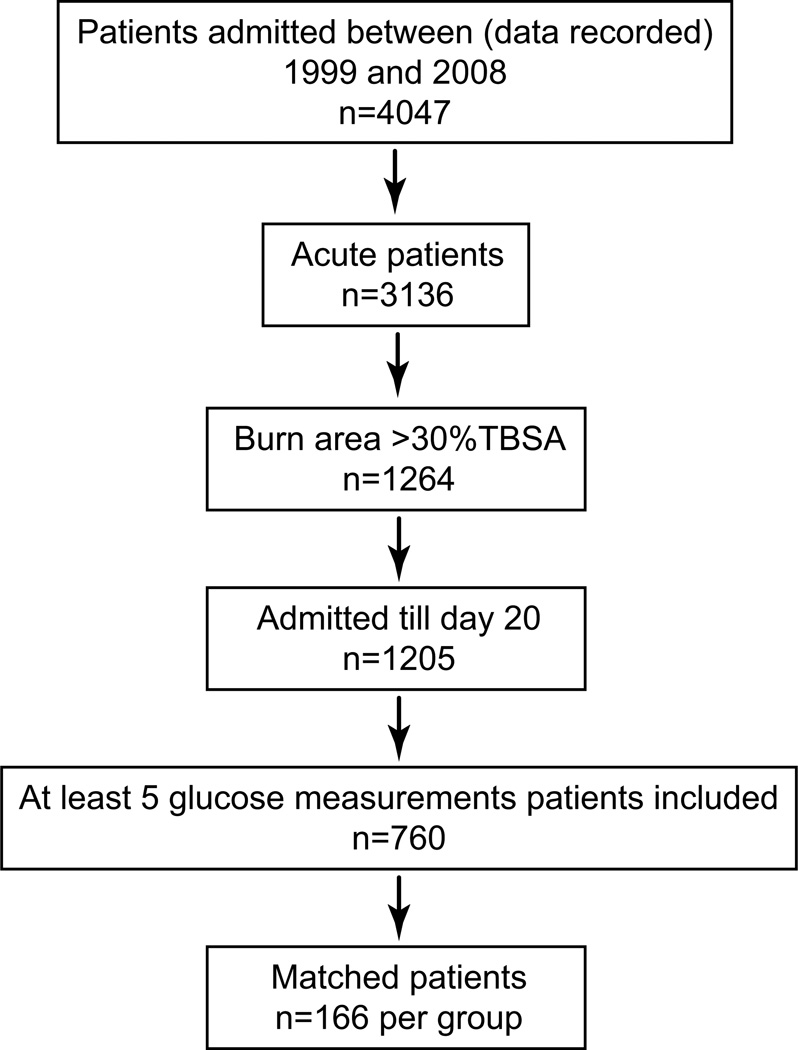
Seven-hundred sixty children who had severe burns [>30% of the total body surface area (TBSA)] and who had to undergo at least one surgical intervention were included in this study. Children were stratified according to the number of hypoglycemic episodes in the following manner: no hypoglycemic episodes (0), one hypoglycemic episode (1), or more than one episode (2+). Patients with a glucose reading < 60 mg/dl were considered hypoglycemic. Details are given in the Consort Diagram (Fig. 1).
Figure 1.
Consort diagram.
All patients were treated according to standardized clinical protocols for resuscitation (Galveston formula: 5,000 cc/m2 TBSA burned + 2,000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours) and nutrition (1,500 kcal/m2 body surface + 1,500 kcal/m2 area burn) (5, 12–15). All patients underwent complete wound excision within 2 days of admission. Wounds were covered with autograft and if necessary, remaining open areas were covered with homograft. Patients underwent grafting procedures until all open wounds were covered with autologous skin.
Demographics (age, date of burn, date of hospitalization, gender, burn size and depth), injury characteristics (severity of burn and inhalation injury), comorbidities, and clinically relevant parameters such as sepsis, morbidity, and mortality were recorded. Sepsis was assessed using SCCM criteria adjusted for burns. Criteria included a positive blood culture or pathologic tissue identifying the pathogen during hospitalization or at autopsy, with the presence of three or more of the following: leucocytosis or leucopenia (>12,000 or <4,000), hyperthermia or hypothermia (>38.5 or <36.5°C), tachycardia (exceeding 20% of the normal heart rate for age), refractory hypotension (systolic BP <90 mmHg), thrombocytopenia (platelets <50,000/mm3), hyperglycemia (serum glucose >240 mg/dl), and enteral feeding intolerance (residuals > 200 cc/hr or diarrhea > 1 L/day), as previously published (12, 13, 15). Incidence of organ failure was determined using the DENVER2 score, which includes renal, hepatic, cardiac, and pulmonary function components. Organ functions were continuously monitored during hospitalization. MOF was defined as a total score >2 for two or more organs for at least two consecutive days (16–19). The interval between surgeries served as a surrogate marker of wound healing and re-epithelization time.
Patient data were prospectively collected and recorded by staff using Emtek. Data were processed with Microsoft Access® or Excel® (Redmond, WA, USA).
Glucose Metabolism
For each patient, blood glucose concentrations were assessed on multiple occasions throughout the day. Blood glucose concentrations were quantified using the hexokinase assay (Siemens Healthcare Diagnostics, West Sacramento, CA). Daily average, minimum and maximum values were determined for each patient. Insulin doses were recorded when appropriate.
Cytokines and Biomarkers
Blood was collected in serum-separator tubes for clinical routine measurements throughout the entire duration of hospitalization. Serum was isolated by centrifugation at 1,320 rpm for 10 minutes and kept at −70°C until analysis. As already mentioned, glucose concentrations were determined using the hexokinase assay. Serum proteins were quantified using HPLC, nephelometry (BNII, Plasma Protein Analyzer; Siemens Healthcare Diagnostics, West Sacramento, CA), and ELISA. Cytokines were analyzed using Bio-Plex Suspension Arrays and the Bio-Plex Human Cytokine 17-Plex panel (Bio-Rad, Hercules, CA).
Cardiac and Pulmonary Function
Heart, lung, kidney, and liver function were assessed using DENVER2 score definitions as described by Moore et al. (20). The cardiac function score was based on the number and dosages of inotrope substances that were administered (Supplemental Table 1). Pulmonary function was defined as the ratio between partial arterial pressure of oxygen (PaO2) in mmHg and the fraction of inspired oxygen (FiO2). Matched readings were used to calculate PaO2/FiO2 (P/F).
Ethics and Statistics
This study was reviewed and approved by the IRB at the University Texas Medical Branch, Galveston, Texas. Prior to the study, the subjects, parents or child’s legal guardian agreed to participate in the study by signing an informed consent form. Children under 7 years gave assent to participate.
Continuous variables are reported as mean±SD or SEM and categorical variables as frequency and percentage. Patients comprising the full group were compared using t-test or Wilcoxon rank sum test for continuous data and Chi-square or Fisher’s exact test for the categorical data. A logistic regression model for mortality was fitted to compare patients with 1, 2, and ≥3 hypoglycemic events to those with no hypoglycemic events, adjusting for TBSA, inhalation injury, age, sex and time from burn to admission. The Hosmer-Lemshow test was used to assess the fit of the model.
The propensity score model included the following available baseline characteristics: age, inhalation injury, total percent TBSA burned, percent TBSA with third-degree burns, time from burn to admission, sex, and type of burn. Patients were matched 1:1 based on the logit of the propensity scores using a greedy matching procedure with calipers 0·2 of the standard deviation of the logit of propensity score. Standardized differences were calculated to check for balance in the matched characteristics. Clinical outcomes for the propensity-matched patients were compared using paired t-test or Wilcoxon signed rank test and McNemar’s test.
The log rank test was used to compare survival curves in unmatched patients. In matched patients, a Cox model was used by taking the clustering into consideration and using a robust sandwich estimator for the variance. We performed as sensitivity analysis Cox proportional-hazards model for time from burn to death with time-dependent covariates: time to hypoglycemia and time to cardiac, liver, kidney and lung failure for a subset of 748 patients for whom this data was available. For this analysis, patients were censored at discharge. This analysis should be interpreted with caution as the time-dependent hypoglycemia is related to subject’s health status (21). Data were analyzed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA), and p<0.05 was considered significant. Patients included in this study are part of a clinical trial registered at www.clinicaltrials.gov (#NCT00673309).
Role of the Funding Source
The funding organizations played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. Dr. Jeschke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
RESULTS
Demographics and Outcome for the Whole Patient Population
Characteristics and outcomes for the entire patient population are presented in Table 1. Five-hundred sixty-eight patients never became hypoglycemic (Control/0), while 192 patients had hypoglycemic episodes. Of these 192 patients, 84 had one (1) and 108 had more than one episode (2+) throughout acute hospitalization (up to 60 days). Patients in both groups had similar sex and ethnicity distribution. Patients had no pre-existing comorbidities. The majority of patients experiencing hypoglycemic episodes sustained flame burns (p<0.001). Patients with hypoglycemic episodes had a significantly greater burn size (Control: 51±16% TBSA burned vs. Hypoglycemia: 66±18% TBSA burned; p<0.001), third-degree component (Control: 32±24, Hypoglycemia: 55±27; p<0.001), and incidence of inhalation injury (Control: 163/29%, Hypoglycemia: 102/53%; p<0.0001). More patients from the hypoglycemic groups than the control group made up the intensive insulin therapy group of the prospective randomized trial (Jeschke et al.) [Control: 9 (2%) vs. Hypoglycemia: 32 (17%); p<0.001].
Table 1.
Demographics and outcome of hypoglycemic and control patients.
| No hypoglycemic event |
One or more hypoglycemic events |
p value | |
|---|---|---|---|
| n | 568 | 192 | |
| Gender | 0.7335 | ||
| male n (%) | 371 (65.3) | 128 (66.7) | |
| Ethnicity (n=559; n=191) | 0.2849 | ||
| African American n | 35 (6.3) | 19 (10.0) | |
| Caucasian n | 89 (15.9) | 24 (12.6) | |
| Hispanic n | 430 (76.9) | 146 (76.4) | |
| Other n | 5 (0.9) | 2 (1.0) | |
| Age at Admit (years) | 7.1 ± 5.1 | 7.9± 5.6 | 0.067 |
| Type of burn | 0.0015 | ||
| Flame n (%) | 363 (63.9) | 154 (80.2) | |
| Electrical n (%) | 46 (8.1) | 9 (4.7) | |
| Scald n (%) | 141 (24.8) | 26 (13.5) | |
| Metabolic n (%) | 12 (2.11) | 2 (1.04) | |
| Other n (%) | 6 (1.1) | 1 (0.5) | |
| Burn size | |||
| % TBSA burn (total) | 51.2 ± 15.8 | 66.1 ± 17.9 | <0.001 |
| % TBSA third degree | 32.3 ± 23.9 | 54.9 ± 26.6 | <0.001 |
| Time from burn to admit*(days) | 2 (1–5) | 2 (1–3) | 0.00034 |
| Inhalation Injury n (%) | 163 (28.7) | 102 (53.1) | <0.0001 |
| Clinical outcomes | |||
| Number OR visits (number) | 3 (1–4) | 5 (3–8) | <0.0001 |
| Time between OR (days) (n=424; n=175) | 4.7 ± 1.9 | 5.0 ± 1.8 | 0.052 |
| LOS ICU (days)* | 20 (12–30) | 32 (21.5–55.5) | <0.001 |
| LOS/TBSA | 0.5 ± 0.3 | 0.6 ± 0.4 | <0.001 |
| Died n (%) | 19 (3.3) | 49 (25.5) | <0.0001 |
| Max DENVER2 | 3.1 ± 1.3 | 4·8 ± 2.2 | <0.001 |
| MOF n (%) | 57 (10.0) | 92 (47.9) | <0.0001 |
| Sepsis n (%) | 37 (6.5) | 41 (21.4) | <0.0001 |
| Number Infections (n=257; 120)* | 0 (0–1) | 1 (0–3) | <0.001 |
Medians and interquartile ranges (IQR) reported. All the other continuous variables are expressed as mean±standard deviation.
Clinical outcomes (Table 1) differed by cohort; compared to control patients, both patient groups with hypoglycemic episodes had more operating room visits, longer stays in the ICU (even when normalized for burn size), higher mortality rates, higher incidence of MOF, greater maximum DENVER2 scores, and more septic and infectious episodes (p<0.05). Feeding intolerance was comparable between groups.
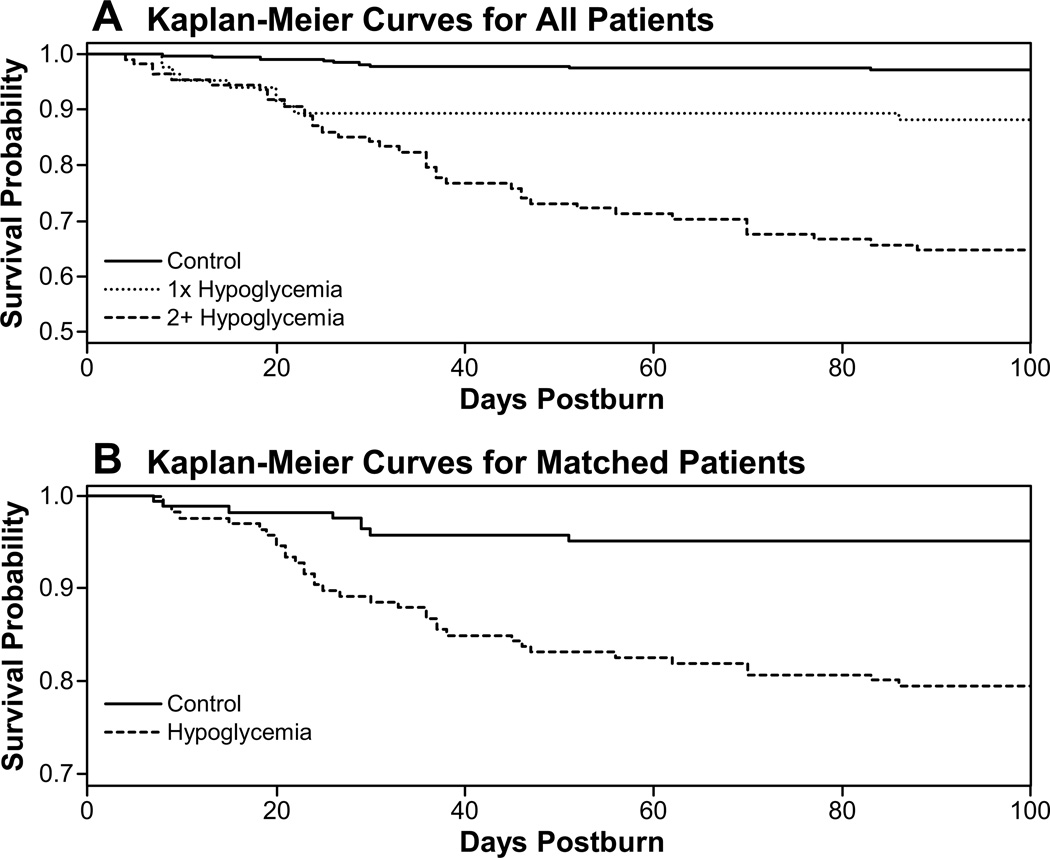
Probability of survival for the first 100 days after burn was determined by the Kaplan-Meier method (Fig. 2A). Differences were found among the three groups (log-rank test, p≤0.0001), with progressively higher mortality being found in patients with one hypoglycemic episode and then in patients with 2 or more hypoglycemic episodes. The odds of dying increased as the number of hypoglycemic events increased [Table 2; compared to no hypoglycemic event: 1 event OR=2.67 95% CI (1.15, 6.20); 2 events OR=5.58 95% CI (2.26, 13.81)]. Burn size and being female were also associated with greater odds of dying. Similar results were obtained using the survival analysis model for burn to death, with hypoglycemia as time-dependent covariate being associated with a larger hazard of dying (Table 3).
Figure 2.
Kaplan-Meier survival curves. (A) Patients without hypoglycemia and patients with either one or two or more episodes of hypoglycemia had statistically significantly different mortality compared, p<0.0001. (B) The statistically significant difference in mortality held true for propensity matched patients, p<0.0002.
Table 2.
Logistic regression for outcome mortality.
| Variable | Odds Ratio | 95%CI | p value |
|---|---|---|---|
| Number of hypoglycemic events <60 | <0.0001 | ||
| 0 | 1 | ||
| 1 | 2.67 | (1.15, 6.20) | 0.022 |
| 2 | 5.58 | (2.26, 13.81) | 0.0002 |
| 3 or more | 9.25 | (4.30, 19.88) | <0.0001 |
| Gender female | 2.60 | (1.40, 4.81) | 0.0024 |
| Age at admit (years) | 1.04 | (0.99, 1.10) | 0.11 |
| % TBSA third degree | 1.03 | (1.02, 1.05) | <0.0001 |
| Time from burn to admit (days) | 1.04 | (0.97, 1.12) | 0.28 |
| Inhalation Injury | 1.70 | (0.93, 3.10) | 0.0838 |
Table 3.
Cox proportional-hazards model with hypoglycemia, lung, cardiac, liver and kidney as time-dependent covariates.
| Variable | Hazard Ratio | 95%CI | p value |
|---|---|---|---|
| Hypoglycemia | 3.86 | (2.04, 7.28) | <0.0001 |
| Gender female | 2.79 | (1.60, 4.90) | 0.0003 |
| Age at admit (years) (1-year increase) | 0.98 | (0.93, 1.02) | 0.3865 |
| % TBSA third degree (1% increase) | 1.02 | (1.01, 1.04) | 0.0086 |
| Inhalation Injury | 1.40 | (0.80, 2.46) | 0.234 |
| Liver failure | 6.20 | (3.06, 12.57) | <0.0001 |
| Kidney failure | 6.69 | (3.00, 14.89) | <0.0001 |
| Lung failure | 3.91 | (0.51, 29.90) | 0.1888 |
| Cardiac failure | 6.49 | (2.81, 11.04) | <0.0001 |
Another logistic regression analysis included the parameters of burn size (percent TBSA burned), third-degree burn size, and intensive insulin therapy. A positive correlation existed between the number of hypoglycemic episodes throughout the hospital stay and insulin administration (Data not shown, p=0.002).
Incidence of Hypoglycemic Episodes
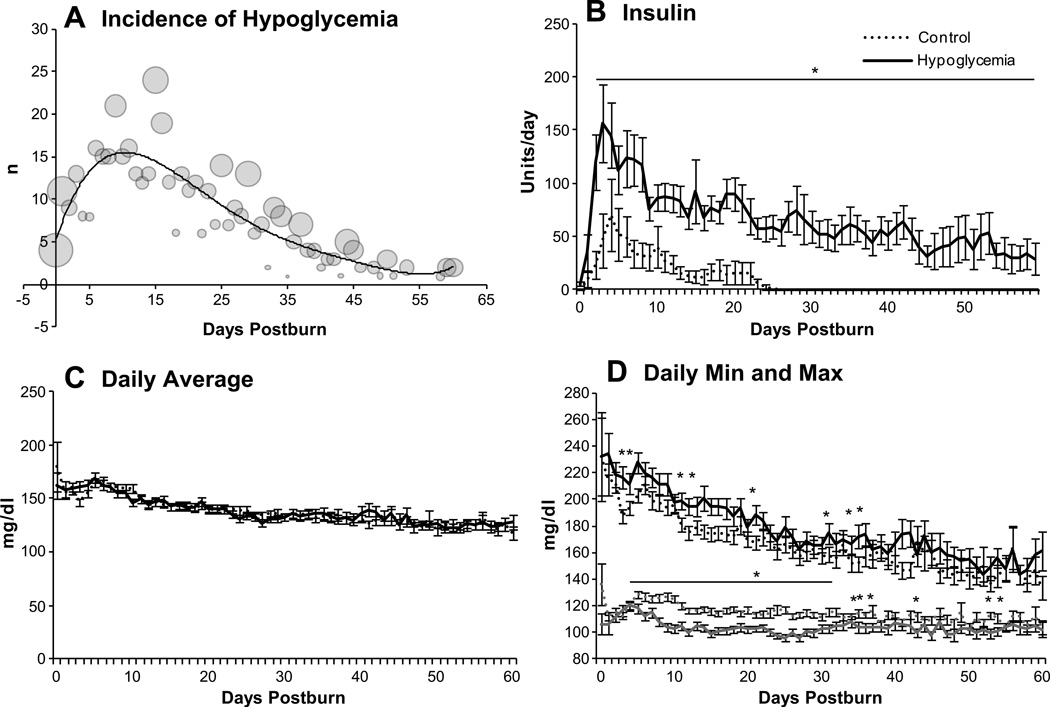
The incidence of hypoglycemic episodes in the total patient population peaked approximately 12 days after burn injury and then decreased steadily until the last studied time point, as depicted by the trend line in Figure 3A. Sizes of the circles indicate the percentage of measurements from all patients that fell below 60 mg/dl on the day in question.
Figure 3.
Glucose metabolism over time. The incidence of hypoglycemic episodes (A) peaked during the critical phase two weeks from burn. Sizes of the circles show the percentage of measurements below 60 mg/dl for the whole patient population for each day up to day 60. The following graphs show the pattern of insulin administration (B), daily average glucose levels (C), and daily minimum and maximum glucose readings (D) for propensity score matched groups. *Hypoglycemia vs. control p<0.05.
Demographics and Outcomes for Matched Patient Cohorts
Using a propensity score model, we matched 166 control to 166 hypoglycemic pediatric patients (Table 4). Patients matched for injury according to the aforementioned criteria had an average burn size of 63% TBSA with an average third-degree component of 50%. The groups had comparable incidence of inhalation injury (Control: 49% vs. Hypoglycemia: 48%), age (Control: 8.3 ± 5.4 years vs. Hypoglycemia 7.6 ± 5.6 years), and sex distribution. Significant differences in these characteristics were not detected in the matched group, though this group exhibited a standardized difference of slightly larger than 10% (non-clinically important difference) for age.
Table 4.
Demographics and outcome of propensity matched patients.
| Control | Hypoglycemia | p value | Standardized difference (%) |
|
|---|---|---|---|---|
| n | (N=166) | (N=166) | ||
| Gender male n (%) | 111 (66.9) | 110 (66.3) | 0.91 | −1.27 |
| Age at admit (years) | 8.3± 5.4 | 7.6 ± 5.6 | 0.28 | −11.8 |
| Type of burn | 0.99 | |||
| Flame n (%) | 131 (78.9) | 130 (78.3) | ||
| Electrical n (%) | 12 (7.2) | 9 (5.4) | ||
| Scald n (%) | 21 (12.7) | 24 (14.5) | ||
| Metabolic n (%) | 2 (1.2) | 2 (1.2) | ||
| Other n (%) | 0 | 1 (0.6) | ||
| Burn size | ||||
| % TBSA burn (total) | 63.5 ± 17.3 | 63.0 ± 16.9 | 0.61 | −3.0 |
| % TBSA third degree | 49.5 ± 25.2 | 50.3 ± 25.5 | 0.56 | 3.2 |
| Time from burn to admit | 2 (1–4) | 2(1–4) | 0.65 | −6.7 |
| Inhalation Injury n (%) | 81 (48.8) | 80 (48.2) | 0.90 | −1.2 |
| Clinical outcomes | ||||
| Number OR visits | 4 (2–6) | 5 (3–7) | 0.0018 | |
| Time between OR (days) | 5.3 ± 1.9 | 5.0 ± 1.7 | 0.067 | |
| LOS ICU (days) | 30 (19–45) | 32 (22–50) | 0.074 | |
| LOS/TBSA | 0.57± 0.38 | 0.62 ± 0.39 | 0.058 | |
| Mortality n (%) | 11 (6.6) | 34 (20.5) | 0.0002 | |
| Max DENVER2 | 3.6 ± 1.3 | 4.6 ± 2.1 | <0.0001 | |
| MOF n (%) | 30 (18.1) | 71 (42.8) | <0.0001 | |
| Sepsis n (%) | 16 (9.6) | 30 (18.1) | 0.020 | |
| Number Infections | 0 (0–3) | 1 (0–3) | 0.16 |
The probability of being administered intensive insulin therapy was higher in the hypoglycemia group (Control: 2% vs. Hypoglycemia: 19%; p<0.001). Clinical outcome parameters were poorer in the hypoglycemia group. Hypoglycemic patients underwent significantly more surgeries (Control: 4 [2–6] vs. Hypoglycemia: 5 [3–7], p<0.0018). The total length of the ICU stay was similar in the two groups (Control, 30 days vs. Hypoglycemia, 32 days; p=0.074). Similarly, length of stay (LOS) normalized for burn size did not differ between groups (p=0.058). Hypoglycemic patients had significantly higher overall mortality (p=0.0002) and incidence of clinical complications such as MOF (p<0.0001), combined with higher maximum DENVER2 scores (p<0.0001) and incidence of sepsis (p=0.02). The rate of infection rate did not significantly differ between the groups.
Time to death was significantly different as seen by the Kaplan-Meier curve in Figure 2B and the Cox-proportional hazards model accounting for matched patients (p=0.0002).
Glucose Metabolism and Insulin Requirements
Patients with hypoglycemic episodes received significantly more units of insulin throughout the hospital course (p<0.05, Fig. 3B). Daily average glucose values were highly similar in both groups (Fig. 3C), but the hypoglycemia group had higher daily maximum values (p<0.05) as well as lower minimum values after the first week post-burn (Fig. 3D).
Inflammatory Parameters
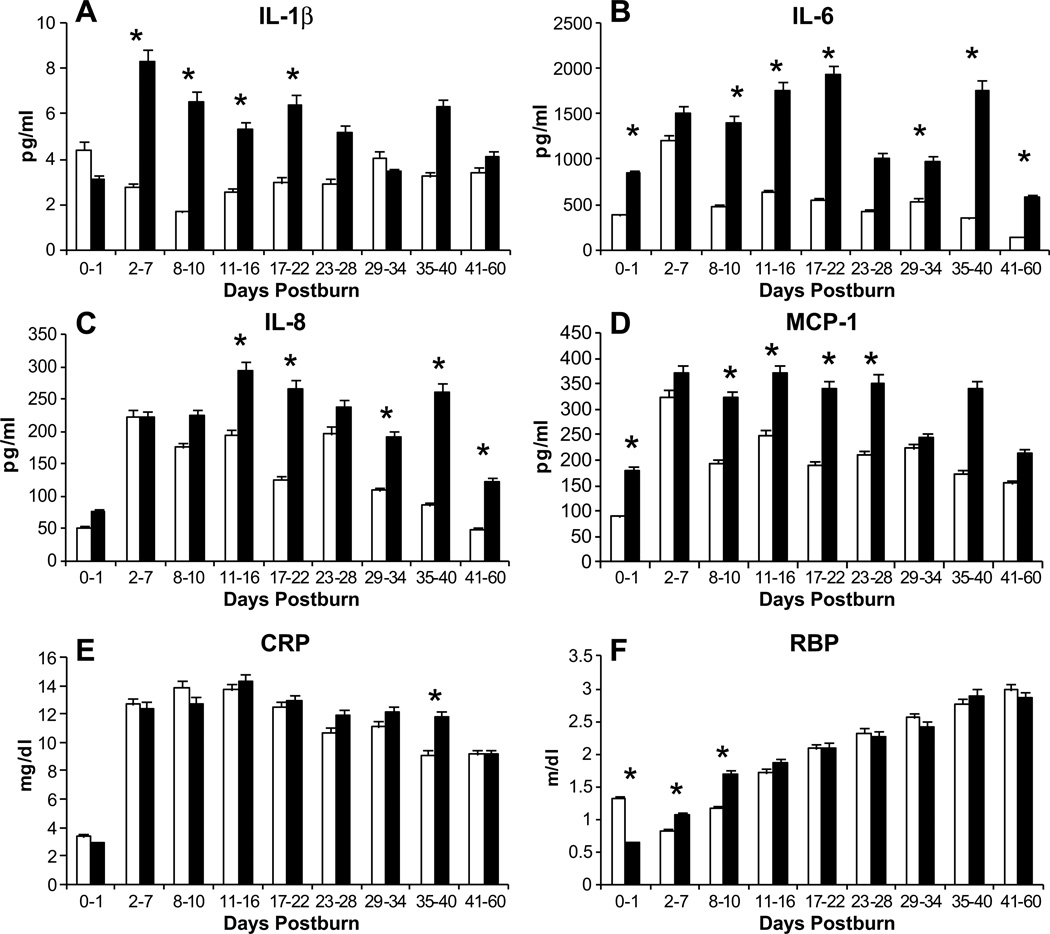
The cytokines IL-1β, IL-6, IL-8, and MCP-1 were significantly higher in the hypoglycemic group (Fig. 4A–D) (p<0.05). IL-1β, IL-6, and MCP-1 underwent immediate increases, whereas IL-8 increased around day 11. The established inflammatory marker, CRP (Fig. 4E), CRP did not show any clear pattern of differences between the groups at any time, with one exception. At the 35–40 day time point, CRP levels were significantly higher in the hypoglycemic group (p<0.05). Levels of retinol binding protein (RBP) significantly differed between the groups at the first three time points until day 10, though no uniform pattern was noted (Fig. 4F) (p<0.05). RBP levels increased at a similar rate between the groups until the end of the study period.
Figure 4.
Profiles of inflammatory markers such as Cytokines (A–D) and acute phase proteins (E) of the matched groups. Cytokines showed a significantly higher expression in the hypoglycemia group, whereas inflammatory proteins such as CRP and RBP (F) displayed only minor differences among the groups. *Hypoglycemia vs. control p<0.05.
Free Fatty Acids and Proteins
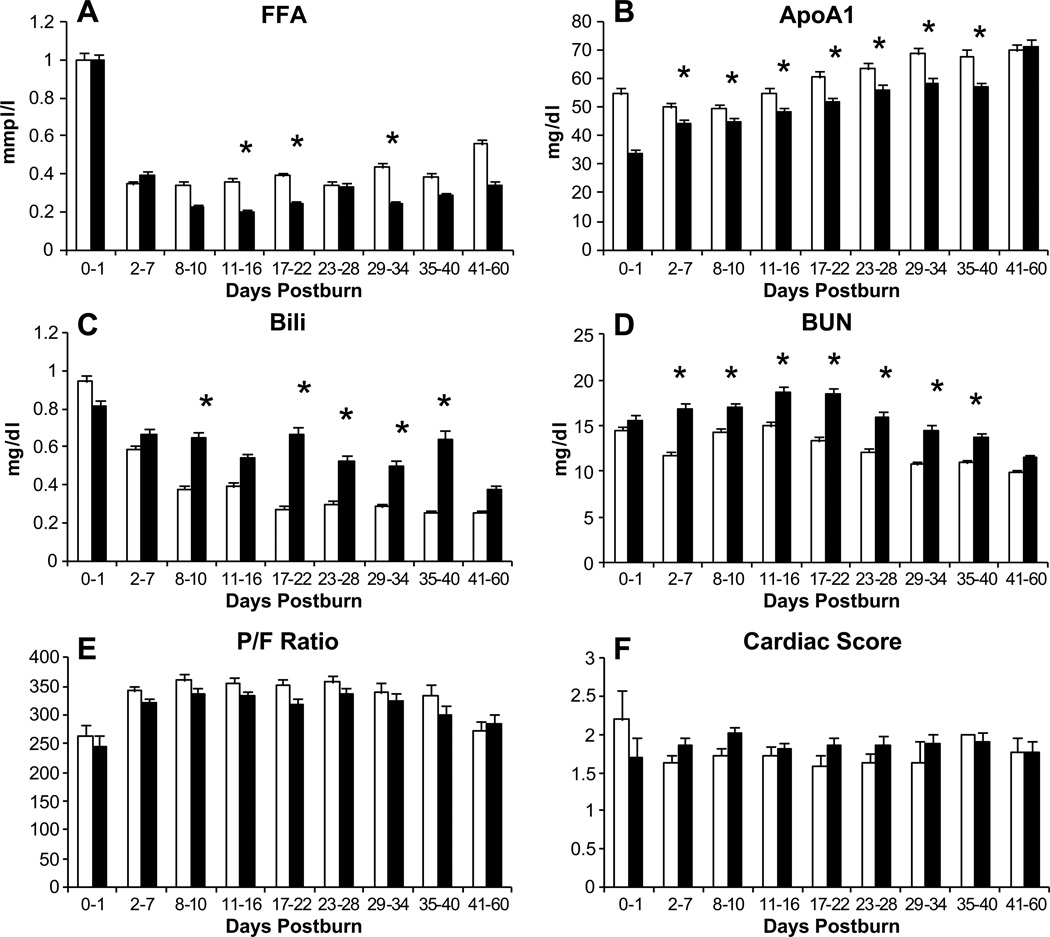
Free fatty acid (FFA) levels dropped immediately after day 1 in both groups (Fig. 5A). Control patients had significantly higher plasma levels from after day 10 until day 34 (p<0.05). Apolipoprotein A1 (ApoA1) followed a similar pattern, with significantly higher levels being seen from day 2 until day 40 (p<0.05) (Fig. 5B).
Figure 5.
Free Fatty Acids (A) and Apolipoprotein A1 (B) two major indicators for peripheral lipid breakdown displayed significantly higher values in the no-hypoglycemic group. Organ function for directly in glucose metabolism involved and sensitive organs such as liver (C) and kidney (D) were significantly impaired in the hypoglycemia group. Pulmonary (E) and cardiac (F) functions were not significantly more affected in the hypoglycemia group. *Hypoglycemia vs. control p<0.05.
Metabolic and Organ Function Markers
The hypoglycemia group had significantly impaired liver function, as assessed by bilirubin and kidney function, as assessed by blood urea nitrogen (BUN) (Fig. 5C, 5D). The hypoglycemia group exhibited elevated bilirubin levels after the first week post-admission (p<0.05) and BUN levels immediately after 1 day post-admission (p<0.05). Respiratory function (Fig. 5E) represented by the P/F ratio and cardiac function (Fig. 5F), which were assessed according to the DENVER2 definitions, did not differ between groups.
DISCUSSION
Tight euglycemic control (1, 2) has changed modern ICU practice (3, 9). However, multiple studies have recently found that tight glycemic control may contribute to morbidity and mortality, making it unclear if tight glycemic control is indeed beneficial to patients (6, 7, 11). Several investigators have hypothesized that the increased adverse events seen with intensive insulin therapy and/or tight glycemic control are related to increased hypoglycemic episodes (6, 7, 11). Although hypoglycemia is known to be a serious adverse event with the potential for severe detrimental outcomes (22), essentially nothing is known regarding the effects of hypoglycemia on outcomes in critical care or burn patients. The NICE SUGAR investigators, therefore, reexamined their data and found that hypoglycemia adversely affects morbidity and mortality in critical care patients (11). Because the effect of hypoglycemia in burns remains unknown, we investigated whether hypoglycemia (i.e., blood glucose <60 mg/dl) is associated with adverse effects in pediatric burn patients. We found that, in pediatric burn patients, hypoglycemia is associated with a significant increase in death and complications. It was interesting that the incidence of hypoglycemia was significantly increased in patients who were administered intensive insulin as part of a randomized controlled trial (5) confirming the risk of tight euglycemic control that has since been shown in other trials of glycemic control (5–7, 11, 22–24).
Hypoglycemia usually occurs in patients with diabetes, with endocrine tumors, or taking drugs that induce; the incidence of hypoglycemia has been reported to be 0.2–0.4% (25). Episodes of hypoglycemia in the critical care setting have recently gained attention because of the initiation of tight glycemic control (6, 7). Insulin administration targeting glucose at 80–110 mg/dl results in better outcomes for seriously ill adults and children (1, 2, 24) but leads to an increased incidence of mild and severe hypoglycemia. A recent prospective randomized trial by our group showed that intensive insulin therapy in pediatric burn patients significantly reduces the occurrence of infections and sepsis, an effect that is accompanied by diminished burn-induced acute-phase and inflammatory responses (5). In addition, we found that intensive insulin improves kidney and liver function. In burn patients, insulin administration provides improvements in muscle protein anabolism, lean mass, hypermetabolism, wound healing time (26–29), and the inflammatory and acute-phase responses (30–33). Others have shown that insulin administration post-burn decreases infection and sepsis and increases survival (34, 35). Therefore, there is an important role for insulin administration during the acute phase post-burn, though strict glycemic control using insulin is a double-edged sword as seen by the current study. That is, the profound and significant benefits of tight glycemic control come with a danger of hypoglycemia and associated adverse events. The recent guidelines for glucose control established by the American College of Physicians have set 140 mg/dl as the absolute minimum target for intensive insulin therapy to retain the positive effects of tight glycemic control and lessen the chance of hypoglycemia in SICU and MICU patients.
Van den Berghe et al (1, 2) have reported an approximate 5% incidence of hypoglycemia in critically ill patients, while other trials have report an incidence in range of 10–25% (6, 7, 24). Here, we found that hypoglycemia was more frequent in burn patients (26%) than in other patient populations. We believe that multiple reasons exist for increased hypoglycemia. It could be due to the nature of the injury, difficulty in maintaining tight glycemic ranges because of feeding interruption, or variability in feeds. It could be due to interruptions in enteral nutrition for surgery and daily dressing changes, which negatively affect gastrointestinal motility and makes adjustments difficult (8, 15, 31). It may also be that the caloric delivery was inadequate to meet the demand, exacerbating hypoglycemia. Finally, Wolf et al. (36, 37) have shown that blood glucose measurements in burn patients can be read incorrectly because of existing anemia. Almost all burn patients are anemic, making blood glucose readings in this population potentially inaccurate (36, 37).
This report demonstrates that hypoglycemia is associated with greater death and complications after burn injury. One can only speculate why hypoglycemia is detrimental for burn patients. The CNS uses glucose as its primary source of fuel for energy production (38). However, endogenous glucose reserves in the CNS last for only minutes, causing cellular energy production to rely heavily on adequate glucose delivery via the blood because neurons cannot generate glucose or use any other energy source (e.g., lipids or proteins). Maintaining an adequate glucose range between 60–140 mg/dl is one of the central tasks of the body’s metabolism. If blood glucose drops below 60 mg/dl, the body responds by releasing counteracting hormones (e.g., glucagon and epinephrine) as well as by inhibiting insulin release in an attempt to increase blood glucose. However, if hypoglycemia persists, cell damage occurs. One characteristic of burn injury is the large hypermetabolic response, which requires glucose as an energy source. If glucose is not accessible, proteins and lipids are used to fulfill the required metabolic demand, but these stores are depleted in the first 2–3 weeks post-burn (39–42). Hence, there are no real energy stores for burn patients, and it seems likely that hypoglycemia is more pronounced and more dangerous in burn patients simply because of the heightened need for glucose. The hypothesized increased inflammatory (15) and hypermetabolic responses were confirmed through analysis of cytokines and acute-phase proteins. We found that patients who have at least one episode of hypoglycemia have a greater inflammatory response and express significantly higher acute-phase proteins that patients who never experienced hypoglycemia. This could be an interesting link between low glucose and hyper-inflammation, because enhanced inflammation drives higher metabolic need, increasing the turnover and hence increasing the risk for hypoglycemia. Increased hypermetabolism and turnover subsequently leads to impaired organ function, suppression of the immune system, and ultimately death.
In infants, hypoglycemia may develop because of limited glycogen reserves. For this reason, fluid administration in children often involves 5% dextrose with Ringer’s Lactate. Thus, hypoglycemia in pediatric burn patients acutely may be caused by the method of fluid infusion. The practice in our hospital is to avoid giving intravenous glucose. Rather, the patient is fed as soon as possible after admission with a regular diet, via NJ or NG feeding tube, using Vivonex T.E.N., which is very high glucose nutrition and, we therefore, suggest that hypoglycemia is not due to failure of glucose delivery.
A central focus of this study was whether hypoglycemia increases post-burn morbidity and mortality or if hypoglycemia is reflective of sicker patients who are predisposed to morbidity and mortality because their more severe illness. Our data show that hypoglycemia is the factor leading to detrimental outcomes and not an indicator of sicker patients. Specifically, both the propensity-matched analysis and the logistic regression analysis showed worse outcomes for patients suffering at least one episode hypoglycemia compared to controls with similar burn sizes and injuries. We also noted that hypoglycemia occurred before MOF or infection. As shown in Figure 2, the majority of hypoglycemia occurred during the early stages after injury, while MOF, infection, sepsis and death occurred at later times. This indicates a causal association and that hypoglycemia leads to increased post-burn morbidity and mortality. This finding is of importance because it implies that burn physicians should avoid driving patients into hypoglycemia early after burn. Strategies to avoid hypoglycemia should include safe protocols, constant feeding, and the use of less restricted glucose target ranges. VISEP and Finney propose targeting blood glucose levels to 140 mg/dl and below (3, 6); the ACP recommends a minimum of 140 mg/dL. Despite the controversy regarding the safest lower limit, it is clear that further examination of the issue is necessary. Based on their review of recent glucose modulation studies, Preiser et al. (4) recommended an intermediate glucose level of 140 mg/dl considering the risk of hypoglycemia associated with intensive insulin administration and uncertainty about the ideal glucose level. In the new surviving sepsis campaign guidelines (10), the authors acknowledge that an ideal range to be targeted is unclear and the negative consequences of hypoglycemic episodes, and they advocate use of 180 mg/dl. Investigators from the NICE trial propose targeting levels to 140–150 mg/dl to avoid hyperglycemia and hypoglycemia. Our group previously conducted a trial aimed at identifying morning glucose levels associated with a better survival and fewer complications in the severely burned. An analysis of 300,000 glucose values revealed that patients with levels of 130 mg/dl for the greater part (75%) of their acute hospital stay have better outcomes than patients with glucose levels >140 mg/dl (8). In this study, the ideal glucose range was around 130–140 mg/dl. The glucose curve was U-shaped, indicating that very low and very high glucose levels are equally harmful. A glucose range of 130–150 mg/dl does not seem to induce protein glycosylation and increase risk of severe hypoglycemia. This recommended range is consistent with three studies focusing on pediatric patients and showing similar glucose cut-off values as presented here (43–45).
One limitation of this study is that this trial was never set up to prospectively determine the incidence of hypoglycemia on post-burn outcomes, and therefore, all results are based on statistical models. These results are associations, which are difficult to causally link. The propensity score analysis allowed for matching the patients to balance the measured confounders, but it does not take into account any unmeasured confounders. Because of the design of our analysis and what we had available, we may have not included all confounders. Nevertheless, based on our results, we suggest that hypoglycemia adversely affects post-burn morbidity and mortality and should be avoided.
Conclusions
One or more episodes of hypoglycemia are associated with adverse clinical outcomes in severely burned children. However, because glycemic control does improve outcomes in this population, use of management strategies that minimize iatrogenic hypoglycemia is imperative. Consistent, validated approaches should be considered for this purpose.
Supplementary Material
Acknowledgments
Source of Funding: This study was supported by the Shriners Hospitals for Children (8490, 71008, 8640, 8760, 79135, 71001, and 84080), National Institutes of Health (R01-GM56687, R01-GM087285-01, T32-GM008256, P50-GM60338, KL2RR029875, and UL1RR029876), National Institute of Disability and Rehabilitation Research (H133A020102, H133A70019, and H133A070026). CFI Leader’s Opportunity Fund: Project #25407, Canadian Institutes of Health Research (CIHR) grant #123336, and Physicians’ Services Incorporated Foundation - Health Research Grant Program.
Copyright Form Disclosures:
Drs. Jeschke, Pinto, Herndon, Finnerty, and Kraft received support for article research from Shriners Hospitals for Children (8490, 71008, 8640, 8760, 79135, 71001, and 84080), National Institutes of Health (R01-GM56687, R01-GM087285-01, T32-GM008256, P50-GM60338, KL2RR029875, and UL1RR029876), National Institute of Disability and Rehabilitation Research (H133A020102, H133A70019, and H133A070026), CFI Leader’s Opportunity Fund (Project #25407), Canadian Institutes of Health Research (CIHR - grant #123336), and Physicians’ Services Incorporated Foundation (Health Research Grant Program). Their institutions received grant support from NIH.
Footnotes
Conflicts of Interest
Authors have no conflicts of interest to declare.
REFERENCES
- 1.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 3.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA. 2003;290:2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 4.Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med. 2007;35:S503–S507. doi: 10.1097/01.CCM.0000278046.24345.C7. [DOI] [PubMed] [Google Scholar]
- 5.Jeschke MG, Kulp GA, Kraft R, et al. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 7.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 8.Jeschke MG, Kraft R, Emdad F, et al. Glucose control in severely thermally injured pediatric patients: what glucose range should be the target? Ann Surg. 2010;252:521–528. doi: 10.1097/SLA.0b013e3181f2774c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, Liu B, et al. NICE-SUGAR Study Investigators. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118. doi: 10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 12.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 14.Mlcak RP, Jeschke MG, Barrow RE, et al. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft R, Herndon DN, Finnerty CC, et al. Occurrence of multiorgan dysfunction in pediatric burn patients: incidence and clinical outcome. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828c4d04. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeschke MG, Gauglitz GG, Finnerty CC, et al. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31828dfbf1. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeschke MG, Finnerty CC, Emdad F, et al. Mild obesity is protective after severe burn injury. Ann Surg. 2013 doi: 10.1097/SLA.0b013e3182984d19. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeschke MG. Clinical review: Glucose control in severely burned patients - current best practice. Crit Care. 2013;17:232. doi: 10.1186/cc12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–512. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Langouche L, Vanhorebeek I, Van den Berghe G. Therapy insight: the effect of tight glycemic control in acute illness. Nat Clin Pract Endocrinol Metab. 2007;3:270–278. doi: 10.1038/ncpendmet0426. [DOI] [PubMed] [Google Scholar]
- 23.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31:359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 24.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 25.Hypoglycemia in the Diabetes Control and Complications Trial. The Diabetes Control and Complications Trial Research Group. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 26.Ferrando AA, Chinkes DL, Wolf SE, et al. A submaximal dose of insulin promotes net skeletal muscle protein synthesis in patients with severe burns. Ann Surg. 1999;229:11–18. doi: 10.1097/00000658-199901000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XJ, Chinkes DL, Wolf SE, et al. Insulin but not growth hormone stimulates protein anabolism in skin would and muscle. Am J Physiol. 1999;276:E712–E720. doi: 10.1152/ajpendo.1999.276.4.E712. [DOI] [PubMed] [Google Scholar]
- 30.Jeschke MG, Klein D, Bolder U, et al. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145:4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- 31.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Ann Surg. 2004;239:553–560. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeschke MG, Rensing H, Klein D, et al. Insulin prevents liver damage and preserves liver function in lipopolysaccharide-induced endotoxemic rats. J Hepatol. 2005;42:870–879. doi: 10.1016/j.jhep.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 33.Klein D, Schubert T, Horch RE, et al. Insulin treatment improves hepatic morphology and function through modulation of hepatic signals after severe trauma. Ann Surg. 2004;240:340–349. doi: 10.1097/01.sla.0000133353.57674.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmila MR, Taddonio MA, Arbabi S, et al. Intensive insulin therapy is associated with reduced infectious complications in burn patients. Surgery. 2008;144:629–637. doi: 10.1016/j.surg.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham TN, Warren AJ, Phan HH, et al. Impact of tight glycemic control in severely burned children. J Trauma. 2005;59:1148–1154. doi: 10.1097/01.ta.0000188933.16637.68. [DOI] [PubMed] [Google Scholar]
- 36.Mann EA, Mora AG, Pidcoke HF, et al. Glycemic control in the burn intensive care unit: focus on the role of anemia in glucose measurement. J Diabetes Sci Technol. 2009;3:1319–1329. doi: 10.1177/193229680900300612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pidcoke HF, Wanek SM, Rohleder LS, et al. Glucose variability is associated with high mortality after severe burn. J Trauma. 2009;67:990–995. doi: 10.1097/TA.0b013e3181baef4b. [DOI] [PubMed] [Google Scholar]
- 38.Van den Berghe G. Hypoglycemia. In: Fink M, Abraham E, Vincent J, Kochanek, editors. Textbook of Critical Care. Philadelphia: Elsevier/Saunders; 2005. pp. 82–86. [Google Scholar]
- 39.Wolfe RR, Durkot MJ, Allsop JR, et al. Glucose metabolism in severely burned patients. Metabolism. 1979;28:1031–1039. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 41.Wolfe RR, Herndon DN, Peters EJ, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–221. doi: 10.1097/00000658-198708000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe RR, Jahoor F, Hartl WH. Protein and amino acid metabolism after injury. Diabetes Metab Rev. 1989;5:149–164. doi: 10.1002/dmr.5610050205. [DOI] [PubMed] [Google Scholar]
- 43.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 44.Preissig CM, Rigby MR. Pediatric critical illness hyperglycemia: risk factors associated with development and severity of hyperglycemia in critically ill children. J Pediatr. 2009;155:734–739. doi: 10.1016/j.jpeds.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan V. Hyperglycemia in the pediatric intensive care unit: a few steps closer to sweetening the pot. Pediatr Crit Care Med. 2008;9:231–233. doi: 10.1097/PCC.0b013e318166d04b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.