Abstract
Background:
Chemotherapy combined with radiotherapy can improve outcome in locally advanced esophageal cancer.
Aim:
This study aimed to compare efficacy and toxicity between concurrent chemoradiotherapy (CCRT) and sequential chemoradiotherapy (SCRT) in unresectable, locally advanced, esophageal squamous cell carcinoma (ESSC).
Materials and Methods:
Forty-one patients with unresectable, locally advanced ESCC were randomized into two arms. In the CCRT arm (Arm A), 17 patients received 50.4 Gy at 1.8 Gy per fraction over 5.6 weeks along with concurrent cisplatin (75 mg m-2 intravenously on day 1 and 5-fluorouracil (1000 mg m-2 continuous intravenous infusion on days 1-4 starting on the first day of irradiation and given after 28 days. In the SCRT arm (Arm B), 20 patients received two cycles of chemotherapy, using the same schedule, followed by radiotherapy fractionated in a similar manner. The endpoints were tumor response, acute and late toxicities, and disease-free survival.
Results:
With a median follow up of 12.5 months, the complete response rate was 82.4% in Arm A and 35% in Arm B (P = 0.003). Statistically significant differences in frequencies of acute skin toxicity (P = 0.016), gastrointestinal toxicity (P = 0.005) and late radiation pneumonitis (P = 0.002) were found, with greater in the CCRT arm. A modest but non-significant difference was observed in median time to recurrence among complete responders in the two arms (Arm A 13 months and Arm B 15.5 months, P = 0.167) and there was also no significant difference between the Kaplan Meier survival plots (P = 0.641) of disease-free survival.
Conclusions:
Compared to sequential chemoradiotherapy, concurrent chemoradiotherapy can significantly improve local control rate but with greater risk of adverse reactions.
Keywords: Concurrent chemoradiation, esophageal cancer, sequential chemoradiotherapy
INTRODUCTION
Esophageal carcinoma is a malignancy with a poor prognosis. It is the sixth leading cause of cancer-related death worldwide.[1] Squamous cell carcinoma is still the dominant histology in Asian countries.[2] Only 30% are resectable and 5-year survival is 10% in European studies. Traditionally, carcinoma of the esophagus has been treated by surgery or radiation therapy, but overall 5 year survival rates have been only 5-10%.[3] Most patients with locally advanced esophageal cancer are either not candidates for surgical treatment or prefer not to undergo surgery. Such patients are often treated with chemotherapy and radiation therapy.[4]
Primary radiotherapy is usually reserved for patients with extensive locoregional disease that is unresectable or for patients who are not fit to undergo surgery. The combination of chemotherapy involving intravenous (IV) cisplatin and 5-fluorouracil (5-FU) with radiation has further improved outcome for patients with locally advanced disease. Several studies suggested that concurrent chemoradiotherapy (CCRT) would be more beneficial than radiotherapy alone in terms of locoregional control and survival rates for patients with locally advanced disease.[5,6,7,8,9] However, in addition to tumor outcome, toxicity must also be considered in the framework of overall therapeutic gain. Combining chemotherapy concurrently with radiotherapy runs the risk of intensifying overlapping toxicities. We therefore sought to ascertain whether sequential chemoradiotherapy (SCRT), that is radiotherapy administered sequentially to chemotherapy, would provide efficacy and toxicity outcome comparable to CCRT in the management of locally advanced esophageal squamous cell carcinoma (ESCC).
MATERIALS AND METHODS
Previously untreated patients with histologically confirmed primary ESCC were recruited from the oncology out-patient department of a tertiary care teaching hospital. The study protocol was approved by the Institutional Ethics Committee and written informed consent was obtained from all the study participants. The eligibility criteria included age 18-70 years, Eastern Cooperative Oncology Group (ECOG) status ≤2, histopathologically proven T1 to T4 ESCC with N1 status (AJCC 2010, Stage IIB and III), hematological and biochemical parameters suitable for radiotherapy or chemotherapy, no tracheoesophageal fistula, no prior chest radiotherapy or chemotherapy or definitive surgery, no other primary cancer and no other diseases that needed hospitalization. The pretreatment assessment included clinical history in detail (including grading of dysphagia) and thorough clinical examination. Acceptable baseline hematological and biochemical parameters included hemoglobin >10 g/dL, leukocyte count >4.0 X 109/mL, platelet count >150 X 109/mL, urea <40 mg/dL, creatinine <1.5 mg/dL, total bilirubin <1 mg/dL and alanine aminotransferase and aspartate aminotransferase levels <40 IU/mL. Upper gastrointestinal (GI) endoscopy, chest X-ray, ultrasonography (USG) of whole abdomen and computerized tomography (CT) scans of thorax and abdomen were done in each patient before the treatment.
Subjects fulfilling the above criteria were randomly assigned to two arms. In the CCRT arm (Arm A) patients received 50.4 Gy at 1.8 Gy per fraction over 5.6 weeks along with concurrent cisplatin (75 mg m–2) IV on day 1 and 5-FU (1000 mg m–2) continuous IV infusion on days 1-4; starting on the first day of irradiation and repeated after 28 days. In the SCRT arm (Arm B) patients received two cycles of chemotherapy, using the same schedule, followed by radiotherapy in the same dose, fractionated in a similar manner.
Radiotherapy was administered as external beam radiotherapy by a megavoltage beam utilizing tele-Cobalt-60. Conventional fractionated radiotherapy was performed throughout the entire treatment process in both arms, and a total dose of 50.4 Gy was delivered at the rate of 1.8 Gy per fraction (single fraction per day and five fractions per week). Prescription dose was calculated without tissue heterogeneity correction. The bounds of gross target volume (GTV) were delineated by barium swallow, endoscopy and CT scan. The upper and lower bounds of clinical target volume (CTV) were defined as an extension of 5 cm outside the upper and lower bounds of GTV, respectively, in phase I plan and 2 cm outside the upper and lower bounds of GTV, respectively, in phase II plan. The lateral bounds of CTV were defined as an extension of 2 cm outside the lateral bounds of GTV. Planning target volume (PTV) was obtained by expanding CTV by 1 cm in all directions. Immobilization was done by thermoplastic mold. In phase I plan, 36 Gy in 20 fractions and in phase II plan up to the total dose of 50.4 Gy was delivered by conventional fractionation. AP-PA portals were used during phase I treatment. For phase II, two anterior oblique wedge portals in upper 1/3rd tumor and one anterior and two posterior oblique portals in middle and lower 1/3rd tumor were used, respectively.
For administering chemotherapy, all patients were premedicated with ranitidine 50 mg IV, dexamethasone 8 mg IV and palonosetron 0.25 mg IV slowly. Cisplatin was administered IV on day 1 mixed in normal saline with proper hydration and mannitol infusion. 5-FU was (1000 mg m–2) administered by continuous IV infusion on days 1-4, and given after 28 days.
The primary study end-point was tumor response, assessed by Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1). Secondary endpoints were acute and delayed organ toxicities and disease-free survival (DFS). During radiotherapy, weekly toxicity assessment was done using Radiation Therapy Oncology Group (RTOG) radiation morbidity scheme. Acute toxicity assessment continued for an additional 8 weeks from the last date of radiation. During the follow-up period, patients were assessed for the appearance of any late toxicity. Chemotherapy-associated adverse reactions were assessed based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4 (CTCAE 4.0). For follow-up, patients were advised to visit every month in the first year, and then at 3 month intervals. In addition to clinical examination during the follow-up visits, CT scan of thorax and abdomen, chest X-ray, endoscopic and other relevant investigations were done when needed. The median follow-up period of the study was 12.5 months.
Statistical analysis was done using Statistica version 6 [Tulsa, Oklahoma: StatSoft Inc., 2001] and MedCalc version 11.6 [Mariakerke, Belgium: MedCalc Software 11.6] software. Numerical variables were normally distributed and were compared between groups by Student's independent samples t test. Fisher's exact probability test (with Freeman-Halton extension if necessary) was employed for comparing categorical variables. 95% confidence interval (CI) values were calculated where deemed relevant. DFS was subjected to Kaplan-Meier analysis and the survival plots were compared by the log rank test. All analyses were two-tailed and P < 0.05 was considered statistically significant.
RESULTS
Between January 2011 and June 2012, a total of 45 patients were screened. Out of these, 41 patients were randomized using a computer-generated random number list. Simple randomization was done for 50 subjects beforehand and the randomization list was kept in the custody of the senior-most investigator, who was not otherwise involved in actual administration of treatment. The allocation sequence was obtained from this investigator once a subject completed all inclusion formalities, including the signing of the informed consent form. The first 41 allocations were used serially from the randomization list.
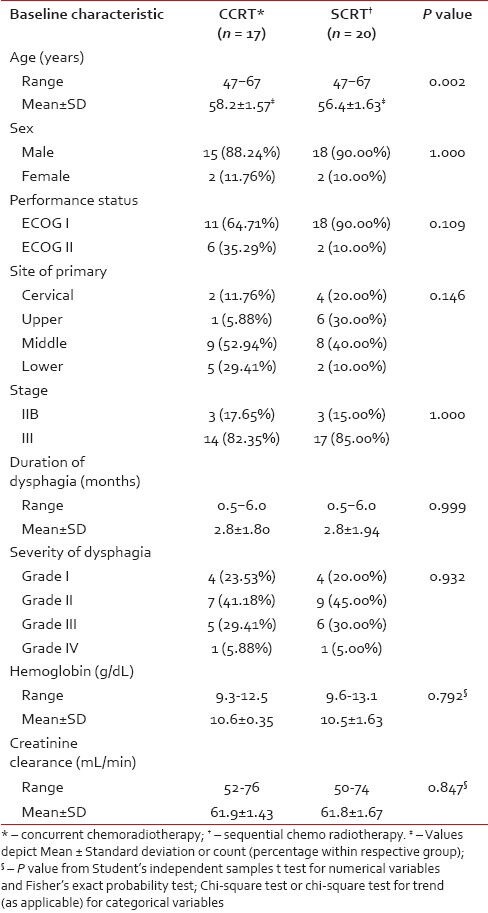
Although 41 subjects were randomized, at the end of the study 37 patients — 17 in Arm A and 20 in Arm B — completed the treatment without interruption and were eligible for per protocol analysis. Three patients died within the study period due to non-oncological causes without returning for any follow-up visit and one patient did not receive allocated intervention due to withdrawal of consent. There was no major treatment delay due to chemotherapy-related complications. Figure 1 depicts the flow of patients in the two study arms. Table 1 presents the baseline demographic and clinical features of the patients. Patients in the CCRT arm were older than those in the SCRT arm by 1.8 years on average. Other than this all baseline parameters were comparable between the two study groups.
Figure 1.
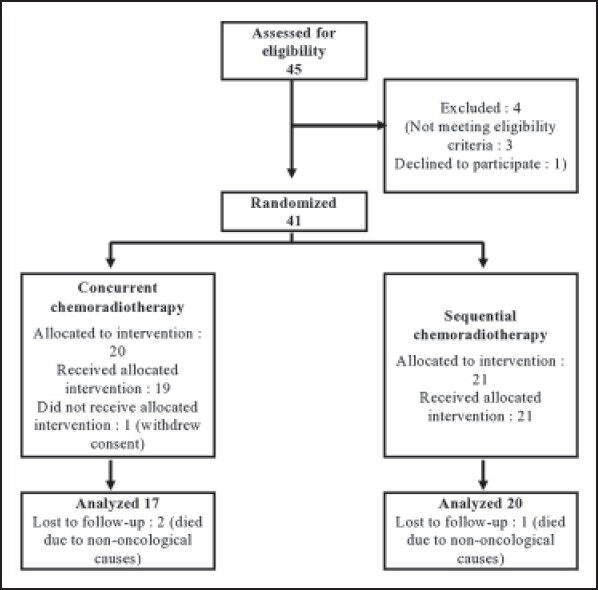
Diagram depicting flow of patients in the study.
Table 1.
Comparison of baseline demographic and clinical characteristics of the study arms
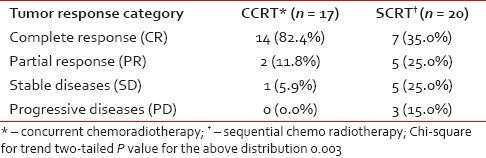
Subjects were evaluated for tumor response based on detailed clinical and relevant radiological findings at stipulated 4 weeks post-treatment and were then categorized as per RECIST criteria. As seen from Table 2, the complete response rate was 82.4% in the CCRT arm and 35.0% in the SCRT arm; 11.8% and 25.0%, respectively, in the two arms, achieved partial response. One patient in arm A and 5 in arm B had stable diseases. Among the 20 patients in arm B, 3 were found to have progressive disease. This response distribution showed statistically significant difference (P = 0.003) in favor of the CCRT arm.
Table 2.
Tumor response evaluation by Response Evaluation Criteria in Solid Tumors (RECIST)
Patients were evaluated for dysphagia before the treatment was started, during the treatment period and in subsequent follow up. The lowest grade of dysphagia following treatment was considered as the improvement in dysphagia over the pre-treatment grade. In our study 82.3% patients in the CCRT arm and 60% in the SCRT arm had improvement in dysphagia.
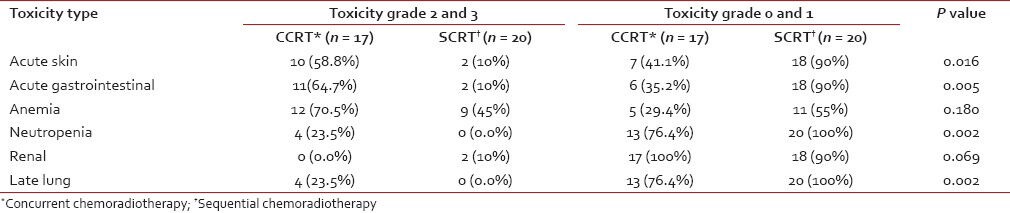
The toxicity profile is presented in Table 3. Combined grade 2 and 3 acute skin toxicity was 58.8% in the CCRT arm and 10% in the SCRT arm, whereas combined grade 0 and 1 acute skin toxicity was 41.1% in the CCRT arm and 90% in the SCRT arm (P = 0.016). Statistically significant differences were also encountered in the frequencies of acute gastrointestinal toxicity of combined grades 2 and 3 and grades 0 and 1. Anemia and renal toxicity incidences were comparable in both arms, whereas grade 2 or 3 neutropenia was encountered only in the CCRT arm. Combined grade 2 and 3 late pulmonary toxicity frequencies were 23.5% and 0%, respectively in CCRT and SCRT groups, and combined grade 0 and 1; toxicities were 76.4% and 100% respectively in the two groups.
Table 3.
Frequency of acute and late treatment related toxicities in the two study arms
With a median follow-up of 12.5 months, recurrence occurred in 5 out of 14 complete responders (35.7%) in the CCRT arm and 2 out of 7 complete responders (28.6%) in the SCRT arm. This difference was not statistically significant. The median time to recurrence was comparable in the two arms [Table 4]. In both arms, the median DFS exceeded the maximum follow-up duration of 18 months. As seen from Figure 2, a comparison of the Kaplan-Meier plots by log-rank test indicated a non-significant difference in DFS (P = 0.641).
Table 4.
Comparison of frequency of recurrence and median survival between the two study arms
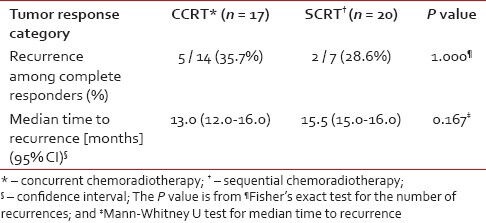
Figure 2.
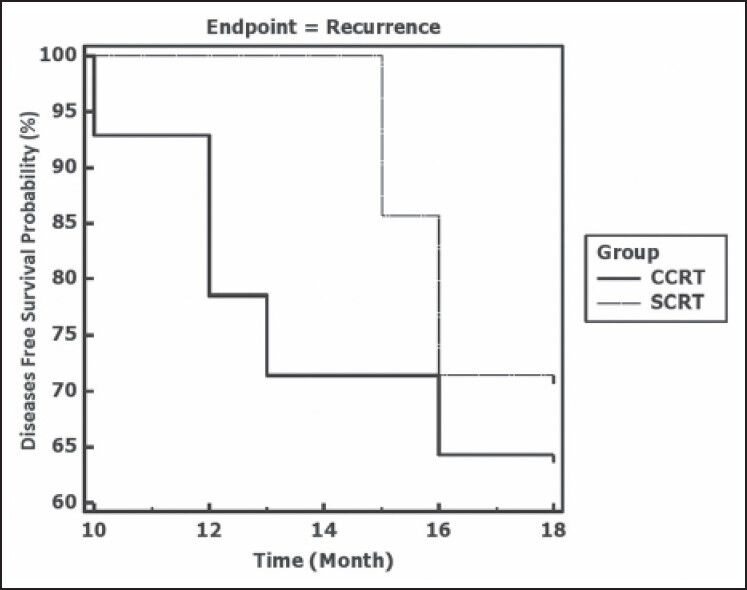
Kaplan-Meier plots depicting disease free survival in the two study groups CCRT (concurrent chemoradiotherapy) and SCRT (sequential chemoradiotherapy). Log rank test indicates a nonsignificant difference (P = 0.641)
DISCUSSION
Esophageal carcinoma accounts for approximately 6% of all gastrointestinal malignancies with a male:female ratio of 3.7:1.[10] Most cases occur in elderly males and those below 55 years are rarely affected. Dysphagia is the most common presenting symptom, occurring in more than 90% of patients.[10] These features were also encountered in our study population.
Primary therapy for esophageal cancer can be surgical or nonsurgical. Patients with unfavorable prognostic features are more commonly selected for nonsurgical therapy. These features include medical contraindications and primary unresectable or metastatic disease. The use of radiotherapy as a potentially curative modality requires doses of at least 50 Gy at 1.8 to 2.0 Gy per fraction. Radiation therapy alone should be reserved for palliation or for patients who are medically unable to receive chemotherapy. Radiotherapy has evolved over the years from conventional to altered fractionation.[11,12] Improved local control and relatively long-term survival has been reported in patients receiving late-course accelerated hyper-fractionated radiotherapy compared with conventional fractionation.[13]
In the ECOG EST-1282 trial, patients who received combined modality therapy had a significantly increased median survival compared with those receiving radiotherapy alone (15 months versus 9 months) but experienced no improvement in 5-year survival (9% versus 7%). However, this was not a pure non-surgical trial because approximately 50% of patients in each arm underwent surgery after receiving 40 Gy of radiation.[14] The trial that was designed to deliver adequate doses of systemic chemotherapy with concurrent radiation therapy was the RTOG 85-01 trial[15] reported by Vigneswaran et al.,[16] Herskovic et al.[17] and Al-Sarraf et al.[18] This intergroup trial primarily included patients with squamous cell carcinoma. Patients received four cycles of 5-FU (1000 mg m–2 by continuous infusion on days 1-4) and cisplatin (75 mg m–2 on day 1). Radiation therapy (50 Gy at 2 Gy per fraction) was given concurrently with day 1 of chemotherapy. The control arm was given radiotherapy alone, albeit at a higher dose (64 Gy) than the CCRT arm. Patients who received CCRT had a significant improvement in median survival (14 months versus 9 months).
The German Esophageal Cancer Study Group compared preoperative chemoradiation followed by surgery versus chemoradiation alone.[6,19] In this trial, 172 eligible patients aged 70 years or more with T3-4N0-1M0 squamous cell cancers of the esophagus were randomized to pre-operative therapy (three cycles of 5-FU, leucovorin, etoposide, and cisplatin, followed by concurrent etoposide, cisplatin, plus 40 Gy) followed by surgery versus chemoradiation alone (the same chemotherapy but the RT dose was increased to 60 to 65 Gy +/- brachytherapy). The initial analysis after median follow-up of 6 years showed non-inferiority of definitive chemoradiation regarding survival, though survival curves somewhat diverged after 3 years.[6] Subsequent analysis after a median follow-up of 10.1 years also failed to show any clear survival difference between operative and conservative treatment.[19] Thus, in the management of locally advanced ESCC, chemoradiation will continue to play an important role. It is therefore worthwhile to explore the modalities of combining chemotherapy and radiotherapy in terms of their efficacy and toxicity.
Our results showed that the response rate of esophageal cancer patients to CCRT was relatively high and reached 82.4%, indicating it can effectively kill tumor cells and rapidly reduce tumor size. This is consistent with the clinical observation that CCRT can quickly relieve the symptom of dysphagia in these patients. The response profile was better with CCRT than SCRT.
Although chemoradiation improves tumor outcome results compared with radiotherapy alone, it may be associated with higher incidence of adverse reactions, including life-threatening toxicities. In the 1997 report of the RTOG 85-01 trial, patients who received chemoradiation had higher incidence acute grade 3 toxicity (44% versus 25%) and grade 4 toxicity (20% versus 3%) compared with those who received radiotherapy alone. Zhao et al. showed CCRT resulted in severe treatment-associated adverse events: 46% of patients developed Grade III-IV adverse events and 6% of patients died, only 43% of patients completed the planned treatment.[20] However, the 5-year survival rate and local control rate were not improved. These results highlight the importance of paying attention to the toxicities when offering concurrent treatment.
With encouraging early results, the RTOG initiated protocol E-0113, a randomized phase II study assessing non-operative therapies. The randomization included induction chemotherapy consisting of 5-FU, cisplatin, and paclitaxel versus induction paclitaxel and cisplatin only. Both arms received continuous infusion of 5-FU with concurrent radiation therapy 1.8 Gy per day to 50.4 Gy.[21] Preliminary results showed increased toxicity in both arms compared to historical controls without significant improvements in outcomes. Additionally, the RTOG is also performing a phase II study (RTOG 0246) using induction paclitaxel, 5-FU, and cisplatin followed by concurrent 5-FU and cisplatin with external irradiation. In our study we also found acute and late toxicities were more common in the CCRT arm.
Local control failure or recurrence is the major reason for treatment failure in esophageal cancer patients. Chen et al. found that in-field recurrence is the major failure mode in a cohort of 132 patients who responded to the concurrent chemoradiation therapy.[22] When irradiated with 50-60 Gy, 60.1-69.9 Gy, ≥70 Gy, the local failure rates of the patients were 69%, 61%, and 52%, while the mean time to recurrence being 5.3, 9.1, and 10.3 months, respectively. In our study, recurrence occurred in 35.7% of the complete responders in the CCRT arm and 28.6% in the SCRT arm. Although this difference was not statistically significant, in view of the small numbers, it would not be correct to draw any definite conclusions regarding local recurrence rate from the present study.
To conclude we can state that, with the caveats of relatively small sample size and limited follow-up, our study has shown that in combining chemotherapy with radiotherapy in the management of locally advanced esophageal cancer, concurrent chemoradiation can provide better tumor response than chemotherapy followed in sequence by radiotherapy. However, this is likely to come at a higher risk of both acute and delayed toxicities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. J Am Med Assoc. 1991;265:1287–9. [PubMed] [Google Scholar]
- 3.Talback M, Rosen M, Stenbeck M, Dickman PW. Cancer patient survival in Sweden at the beginning of the third millennium - predictions using period analysis. Cancer Causes Control. 2004;15:967–6. doi: 10.1007/s10522-004-2475-1. [DOI] [PubMed] [Google Scholar]
- 4.Brenner B, Ilson DH, Minsky BD. Treatment of localized esophageal cancer. Semin Oncol. 2004;31:554–5. doi: 10.1053/j.seminoncol.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Seitz JF, Giovannini M, Padaut-Cesana J, Fuentes P, Giudicelli R, Gauthier AP, et al. Inoperable nonmetastatic squamous cell carcinoma of the esophagus managed by concomitant chemotherapy (5-fluorouracil and cisplatin) and radiation therapy. Cancer. 1990;66:214–9. doi: 10.1002/1097-0142(19900715)66:2<214::aid-cncr2820660204>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7. doi: 10.1200/JCO.2005.00.034. [DOI] [PubMed] [Google Scholar]
- 7.Ruhstaller T, Widmer L, Schuller JC, Roth A, Hess V, Mingrone W, et al. Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02) Ann Oncol. 2009;20:1522–8. doi: 10.1093/annonc/mdp045. [DOI] [PubMed] [Google Scholar]
- 8.Kolaric K, Maricic Z, Roth A, Dujmovic I. Combination of bleomycin and adriamycin with and without radiation on the treatment of inoperable esophageal cancer: A randomized study. Cancer. 1980;45:2265–73. doi: 10.1002/1097-0142(19800501)45:9<2265::aid-cncr2820450908>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Byfield JE, Barone R, Mendelsohn J, Frankel S, Quinol L, Sharp T, et al. Infusional 5-fluorouracil and X-ray therapy for non-resectable esophageal cancer. Cancer. 1980;45:703–8. doi: 10.1002/1097-0142(19800215)45:4<703::aid-cncr2820450415>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 11.Fowler JF. Modelling altered fractionation schedules. BJR Suppl. 1992;24:187–92. [PubMed] [Google Scholar]
- 12.Kikuchi Y. Study on clinical application of multiple fractions per day radiation therapy with concomitant boost technique for esophageal cancer. Hokkaido Igaku Zasshi. 1993;68:537–56. [PubMed] [Google Scholar]
- 13.Zhang YW, Chen L, Bai Y, Zheng X. Long-term outcomes of late course accelerated hyper-fractionated radiotherapy for localized esophageal carcinoma in Mainland China: A meta-analysis. Dis Esophagus. 2011;24:495–501. doi: 10.1111/j.1442-2050.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Ryan LM, Douglass HO, Jr, Haller DG, Dayal Y, Kirkwood J, et al. Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: A study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:269–6. doi: 10.1016/s0360-3016(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Materson JA, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. J Am Med Assoc. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 16.Vigneswaran WT, Trastek VF, Pairolero PC, Deschamps C, Daly RC, Allen MS, et al. Transhiatal esophagectomy for carcinoma of the esophagus. Ann Thorac Surg. 1993;56:838–4. doi: 10.1016/0003-4975(93)90341-e. [DOI] [PubMed] [Google Scholar]
- 17.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–8. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sarraf M, Martz K, Herskovic A, Leichman L, Brindle JS, Vaitkevicius VK, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: An intergroup study. J Clin Oncol. 1997;15:277–4. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 19.Stahl M, Wilke H, Lehmann N, Stuschke M. German Esophageal Cancer Study Group Long-term results of a phase III study investigating chemoradiation with and without surgery in locally advanced squamous cell carcinoma (LA-SCC) of the esophagus. Journal of Clinical Oncology. 2008 May;26 no.15_supplement.4530. [Google Scholar]
- 20.Zhao KL, Shi XH, Jiang GL, Yao WQ, Guo XM, Wu GD, et al. Late course accelerated hyperfractionated radiotherapy plus concurrent chemotherapy for squamous cell carcinoma of the esophagus: A phase III randomized study. Int J Radiat Oncol Biol Phys. 2005;62:1014–20. doi: 10.1016/j.ijrobp.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, Winter K, Komaki R, Kelsen DP, Minsky BD, Liao Z, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemoradiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551–6. doi: 10.1200/JCO.2008.16.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen EC, Liu MZ, Hu YH, Liu H, Li QQ, Cai L, et al. Local failure-related factors of esophageal carcinoma after concurrent chemoradiotherapy [in Chinese] Ai Zheng. 2005;24:498–01. [PubMed] [Google Scholar]


