Abstract
Spermiation—the release of mature spermatozoa from Sertoli cells into the seminiferous tubule lumen—occurs by the disruption of an anchoring device known as the apical ectoplasmic specialization (apical ES). At the same time, the blood–testis barrier (BTB) undergoes extensive restructuring to facilitate the transit of preleptotene spermatocytes. While these two cellular events take place at opposite ends of the Sertoli cell epithelium, the events are in fact tightly coordinated, as any disruption in either process will lead to infertility. A local regulatory axis exists between the apical ES and the BTB in which biologically active laminin fragments produced at the apical ES by the action of matrix metalloproteinase 2 can regulate BTB restructuring directly or indirectly via the hemidesmosome. Equally important, polarity proteins play a crucial part in coordinating cellular events within this apical ES–BTB–hemidesmosome axis. Additionally, testosterone and cytokines work in concert to facilitate BTB restructuring, which enables the transit of spermatocytes while maintaining immunological barrier function. Herein, we will discuss this important autocrine-based cellular axis that parallels the hormonal-based hypothalamic–pituitary–testicular axis that regulates spermatogenesis. This local regulatory axis is the emerging target for male contraception.
Introduction
During spermatogenesis—the male process of gameto-genesis that takes place in the seminiferous tubules of the mammalian testis—the number of chromosomes that are present in spermatogonia (diploid, 2n) are halved when they develop into spermatozoa (haploid, 1n), so that the genetic materials can be reconstituted to diploid (2n) during fertilization with the egg. Spermatogenesis comprises mitosis for renewal of spermatogonial cells and meiosis I, followed immediately by meiosis II to form haploid round spermatids. Spermatids then undergo a process known as spermiogenesis, typified by the condensation of genetic materials in the nucleus and their packaging into the spermatid head with the formation of the acrosome above the head, and the elongation of the spermatid tail. Lastly, spermiation occurs, during which fully developed spermatids detach from the seminiferous epithelium, enter the tubule lumen, and are transported to the epididymis for maturation (Figure 1). The entire process takes about 1–2 months to complete.
Figure 1.
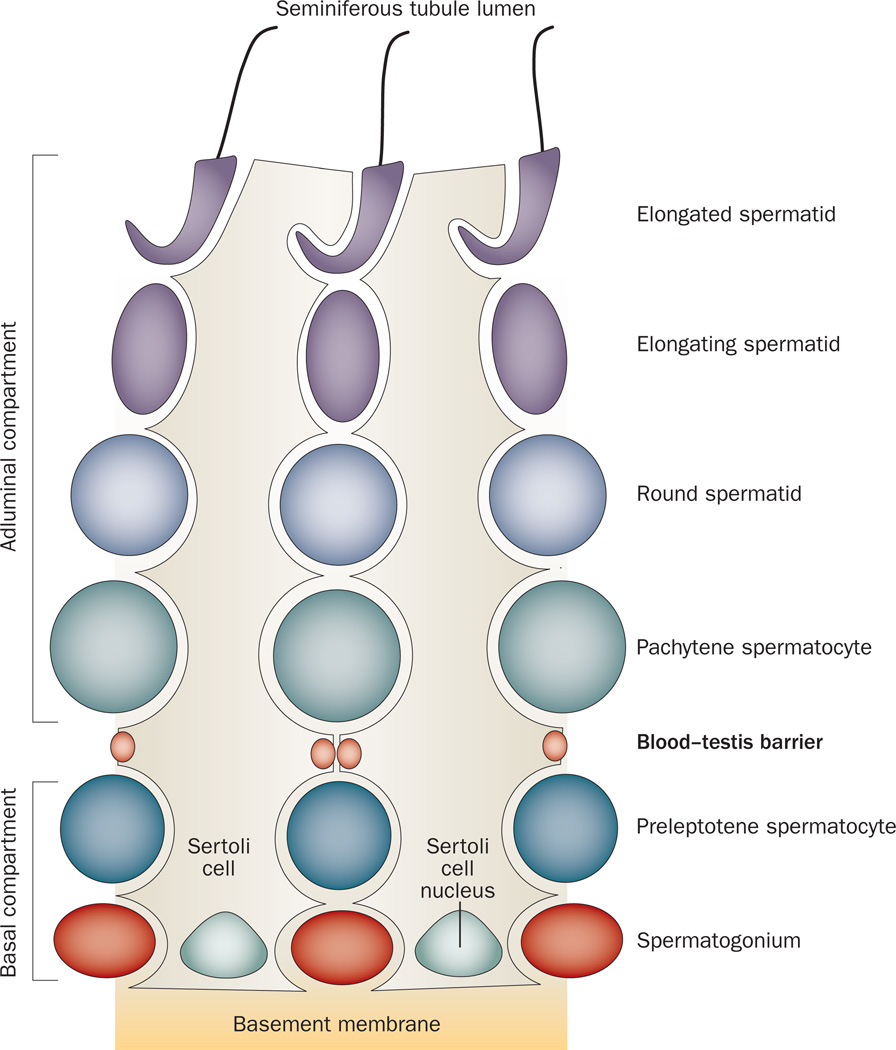
Cross-section of a mammalian seminiferous tubule, which is composed of the seminiferous epithelium constituted by Sertoli cells and germ cells at different stages of their development. During spermiation, fully developed spermatids detach from the seminiferous epithelium, enter the tubule lumen and are transported to the epididymis for maturation. if an investigator is viewing a specific section of a seminiferous tubule under a stereomicroscope, the unique association of developing spermatids (round spermatids, elongating spermatids, and elongated spermatids) in the seminiferous epithelium relative to Sertoli cells and other germ cells, namely spermatogonia and spermatocytes, occur in cycles (or waves), and these cycles are classified into stages, with each stage composed of some unique cellular events pertinent to spermatogenesis.45 For instance, in adult rats, at stage VIII of the epithelial cycle, spermiation takes place simultaneously with the transit of preleptotene spermatocytes at the blood–testis barrier, and at stage XIV, meiosis I and II occur.
Spermatogenesis is regulated by the hypothalamic–pituitary–testicular axis, which functionally connects the brain with the testis; dysfunction of this axis leads to infertility.1,2 In this functional axis, gonadotropin-releasing hormone (GnRH) produced by the hypothalamus stimulates the production of follicle stimulating hormone (FSH) and luteinizing hormone (LH) by the pituitary gland.1,3 In turn, FSH regulates Sertoli cell function in the testes, which supports germ cell development in the epithelium of the seminiferous tubules, whereas LH regulates Leydig cell steroidogenic activity, which leads to the production of testosterone and estradiol-17β in the interstitium.1,4,5
The production of inhibin by Sertoli cells, as well as testosterone and estradiol-17β by Leydig cells, provides a negative feedback loop that reduces the secretion of FSH and LH in the hypothalamus in order to maintain the strict homeostasis of GnRH, FSH, LH, inhibin, testosterone and estradiol-17β within the hypothalamic–pituitary–testicular axis. This strict regulation supports spermatogenesis.1,4,6 The function of testosterone in the maintenance of spermatogenesis, development of accessory reproductive organs and secondary sex characteristics in mammals was already well established,7 when it was reported that deletion of the androgen receptor (AR) in mice via either natural mutation8 or genomic manipulation9 resulted in infertility. In adult mammals, AR is expressed by Sertoli, Leydig and peritubular myoid cells, where it regulates androgen-responsive genes upon binding by testosterone. In essence, knockout of AR in either Sertoli cells9–11 or myoid cells12 in mice resulted in male infertility because of the loss of spermatids from the seminiferous epithelium. Specifically, meiosis was shown to be blocked in mice with a knockout of AR in Sertoli cells, which led to the depletion of elongating and elongated spermatids from the epithelium.
By contrast, mice lacking FSH or the FSH receptor remain fertile, even though the number of germ cells within their seminiferous epithelium is considerably reduced compared with that in wild type animals.13–16 Possibly, this finding is the result of a decline in Sertoli cell function, as the FSH receptor is restricted to Sertoli cells and FSH is known to regulate the secretory function of the Sertoli cell.1,17 Thus, these findings illustrate that FSH acts primarily to optimize spermatogenesis and to support germ cell development by acting on the Sertoli cell and that testosterone is crucial for the completion of meiosis and spermatid development, and both are important for fertility.
Studies have also illustrated that estradiol-17β produced by Leydig, Sertoli and germ cells18–20 is crucial for spermatogenesis, most notably by affecting germ cell apoptosis.4,19–21 For instance, estrogen given to adult rats subcutaneously reduces germ cell apoptosis.22 Likewise, estradiol-17β also effectively inhibits germ cell apoptosis that had been induced by depriving human seminiferous tubules cultured in vitro of survival and/or growth factors.23 estrogen appeared to promote germ cell survival via G-protein-coupled receptor 30 (a novel estrogen receptor) and the signal transduction pathway involving epidermal growth factor receptor and Fos.24 In short, estrogen is a crucial regulator of germ cell apoptosis during spermatogenesis.
In mammals, spermatogenesis, which takes place in the seminiferous epithelium,25 can be functionally divided into three phases. First, self-renewal of type A spermatogonia via mitosis and their differentiation into type B spermatogonia is followed by their transformation into primary spermatocytes (diploid). This transformation begins with preleptotene spermatocytes and leptotene spermatocytes, which are the only primary spermatocytes that traverse the blood–testis barrier (BTB) to enter the adluminal compartment for further development (Figure 2). Second, cell cycle progression takes place behind the BTB: zygotene and pachytene spermatocytes transform into diplotene spermatocytes, which undergo diakinesis and enter meiosis I, followed shortly after by meiosis II to form spermatids (haploid). Third, spermatids develop into spermatozoa via spermio genesis. During this process, round spermatids (step 1 spermatids) differentiate into elongated spermatids via 19 steps in rats and 12 steps in humans, until spermiation, at which time spermatozoa leave the seminiferous epithelium to continue their maturation in the epididymis (Figures 1 and 2).
Figure 2.
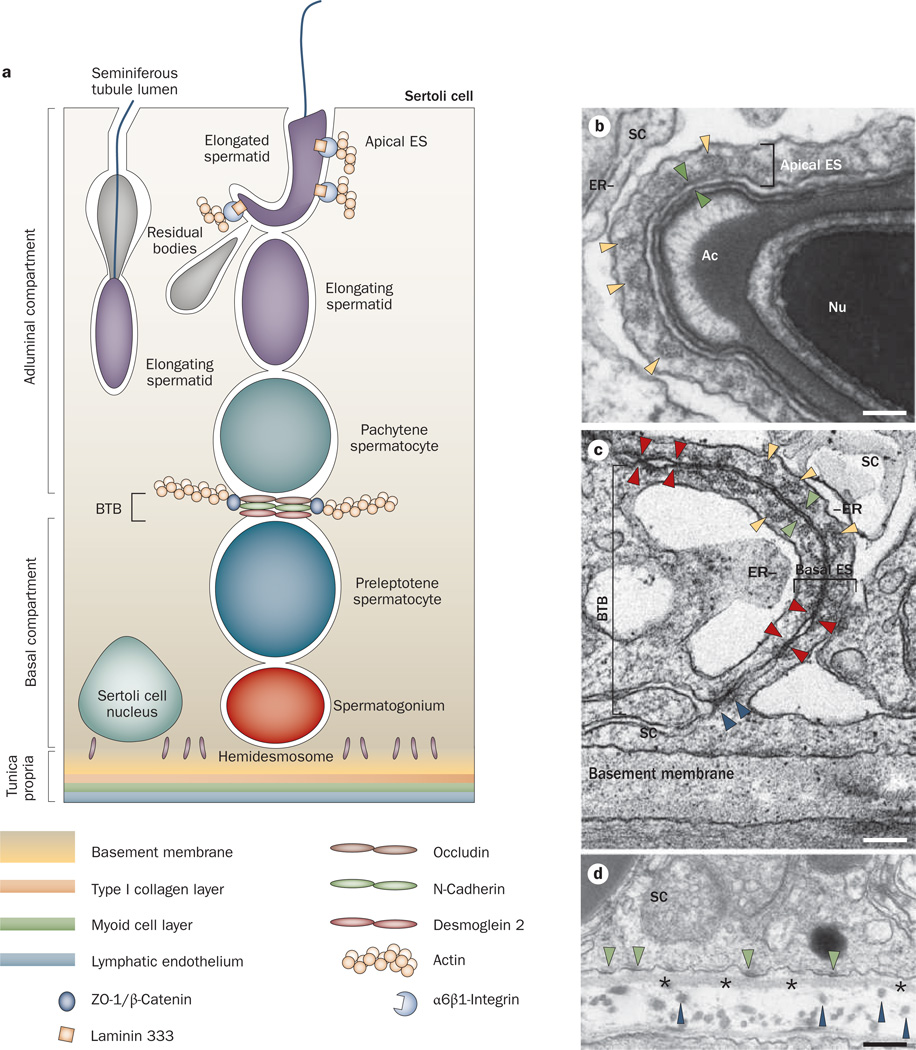
Anatomy of the seminiferous epithelium in the mammalian testis. a | Anatomical features and the spatial relationship of the apical ES, the BTB and the hemidesmosome in adult rat testes. The BTB with coexisting tight junctions (occludin), basal ES (N-cadherin), and desmosome–gap junctions (desmoglein 2) physically divides the seminiferous epithelium into basal and apical (adluminal) compartments. The apical ES is constituted by different protein complexes, such as α6β1-integrin–laminin 333. b | The apical ES at the elongated spermatid–Sertoli cell interface is typified by the presence of actin filament bundles (yellow arrowheads), which are sandwiched in between cisternae of ER and the apposing plasma membrane (see apposing green arrowheads) of the spermatid and the Sertoli cell. These ultrastructures are restricted to the Sertoli cell. c | The BTB is present at the base of two adjacent Sertoli cells near the basement membrane. it is constituted by coexisting tight junctions (denoted by apposing red arrowheads), basal ES and desmosome–gap junctions (noted by the electron-dense material between two adjacent Sertoli cells, see blue arrowheads). The basal ES is typified by the presence of actin filament bundles (yellow arrowheads) sandwiched in between cisternae of ER and the apposing Sertoli cell plasma membrane (see apposing green arrowheads), which is similar to the apical ES except that these ultrastructural features are present within both Sertoli cells. d | The hemidesmosome is found at the interface between the Sertoli cell and the basement membrane (asterisks) typified by patches of electron-dense material (see green arrowheads). Also noted are type I collagen fibrils (blue arrowheads) found below the basement membrane. Bar in panel b, 0.1 µm; panel c, 0.12 µm; panel d, 0.15 µm. Abbreviations: Ac, acrosome; BTB, blood–testis barrier; ER, endoplasmic reticulum; ES, ectoplasmic specialization; Nu, spermatid nucleus; SC, Sertoli cell.
The seminiferous epithelium comprises a layer of polarized Sertoli cells with nuclei located near the basement membrane—a modified form of the extra cellular matrix.26 Sertoli cells cease to divide by ~15 days post partum in rats27 and by birth in humans,28 with their number remaining relatively constant in the testis throughout adulthood at about 40 million per testis in rats,27 and ~130 million per testis in humans.28 each Sertoli cell has the ability to support ~30–50 germ cells by providing nutrients and other biomolecules required for their development without exhausting their capacity (Figure 2).17,29 As such, ~75% of germ cells (including spermatogonia, spermatocytes and spermatids in humans30) are destined to undergo spontaneous degeneration31 in the seminiferous epithelium via an extrinsic pathway of apoptosis that involves Fas and the Fas ligand or an intrinsic mitochondrial pathway that involves the Bcl-2 family of proteins,21 so that the Sertoli:germ cell ratio can be maintained at ~1:30–1:50.20,27,29
Specialized junctions between Sertoli cells that are located near the basement membrane create an immunological barrier known as the BTB. The function of this barrier is to segregate the events of meiosis and post-meiotic germ cell development from the systemic circulation to avoid the production of antisperm antibodies. Autoantigens that reside in late spermatocytes and spermatids (some of which that are expressed transiently during meiosis and spermiogenesis) arise after immune tolerance against self-antigens is established; therefore, the BTB is crucial to confer immune privilege to the testis (Figure 1 and Box 1).32,33 The BTB is established by 15 days postpartum in rats and at puberty in humans (~11–12 years of age) before the first wave of spermiation that occurs by day 45 in rats and after puberty in humans, which illustrates that immune tolerance is established when spermatogenesis occurs continuously. This situation creates the necessity for the segregation, in order to confer immune privilege to the testis, such that localized immune responses against endogenous (gametogenic) antigens are avoided.
Box 1 | The testis is an immune-privileged organ.
An immune-privileged organ, such as the testis, eye, brain, and the uterus in pregnancy, refers to an organ in which graft rejection responses are absent or delayed.153 in these organs, localized immune responses against endogenous antigens (for example, the fetus or germ cells) or exogenous antigens (for example, a graft or tumor) are actively suppressed via different levels of immune control, including immune tolerance, reduced immune activation, localized immunosuppression (conferred in Sertoli cells via their secretory products, such as bioactive proteins and lipids and cytokines, and in Leydig cells by androgens and estrogens) and separation of antigens behind the blood–tissue barrier, such as the BTB in the testis or the blood–brain barrier in the brain.109
Interestingly, spermiation—the release of mature spermatozoa from Sertoli cells—occurs simultaneously with the transit of preleptotene spermatocytes across the BTB while they transform into leptotene, zygotene and pachytene spermatocytes. In other words, the ‘restructuring’ of the BTB that occurs at stage VIII of the seminiferous epithelial cycle, to facilitate the transit of spermatocytes, coincides with the dissolution of the elongated spermatid–Sertoli cell junction, which is known as the apical ectoplasmic specialization (ES). moreover, while the immunological barrier function must be maintained during spermatocyte transit, studies in the past decade have shown that cytokines, such as transforming growth factor (TGF)-β3 and tumor necrosis factor (TNF), and testosterone have opposing effects on BTB integrity: cytokines perturb,34–37 whereas testosterone promotes38–40 the permeability of the Sertoli cell tight junction.
This Review describes a model by which the concerted effects of testosterone and cytokines promote primary spermatocyte migration across the BTB while enabling the immunological barrier to remain intact. This model is further supported by findings in the past few years that the desmosome–gap junction, which coexists with tight junction and basal ES at the BTB, also has a crucial role in the coordination of these events.41,42 In short, this Review will discuss the current status of research regarding the cellular and molecular regulation of the BTB during spermatogenesis. we will also present findings to support the existence of a local functional axis known as ‘the apical ES-BTB-hemidesmosome axis’ that co ordinates these two cellular events, namely BTB restructuring and spermiation, as well as briefly discuss the feedback loop that regulates junction restructuring to prevent unnecessary damage in the seminiferous epithelium during spermatogenesis. while the discussion below is based on studies in rats unless specified otherwise, this information is applicable to other mammals, including humans. This functional axis is an emerging target for male contraceptive development, since a disruption of this axis will lead to male infertility. Also, environ mental toxicants (such as cadmium) that have an impact on male infertility may exert their effects by perturbing this functional axis. Thus, one may be able to therapeutically ‘manage’ male infertility by blocking a toxicant-induced disruption of this functional axis.
The apical ES, BTB and hemidesmosome
The ES (ectoplasmic specialization) is an actin-based anchoring junction found uniquely in the testis at two distinct sites (Figure 2). The first location is between postmeiotic spermatids and Sertoli cells in the seminiferous epithelium. This type of ES is known as the apical ES, and its structure surrounds the entire head of elongating and elongated spermatids43–45 (Figure 2a,b). once it appears between elongating or elongated spermatids and Sertoli cells, the apical ES is the only anchoring device for the developing spermatids that is present, because desmosome–gap junctions, which are found between earlier stage spermatids and Sertoli cells, have all disappeared. The second location of the ES is between adjacent Sertoli cells at the BTB (known as the basal ES; Figure 2c).
Unlike the apical ES, which exists alone, the basal ES coexists with tight junctions, desmosomes and gap junctions, and all of these junctions constitute the BTB and contribute to its immunological barrier function44,45 (Figure 2c). Indeed, the coexistence or intermingling of different types of junctions at the BTB makes this barrier unique from all other blood–tissue barriers, such as the blood–brain barrier and the blood–retina barrier, because these other barriers consist almost exclusively of tight junctions between endothelial cells of the micro vessels.46 By contrast, the BTB in rodents and humans is constituted by adjacent Sertoli cells near the basement membrane, and microvessels in the interstitium contribute relatively little to barrier function.47 while the peritubular myoid cell layer in the tunica propria (Figure 2a) confers some barrier function in rodent testes,48,49 it is less effective in primate and possibly human testes (Figure 2).50
At present, the physiological significance of coexisting junctions at the Sertoli–Sertoli cell interface that constitute the BTB is not completely understood. However, studies in the past few years have demonstrated that desmosomes and gap junctions work together (hence the name desmosome–gap junctions) to regulate tight junction proteins and basal ES proteins at the BTB. This regulation perhaps provides the necessary crosstalk between different junction types, and thereby maintains the tight junction permeability barrier.41,42 For instance, in RNA interference (RNAi) experiments, simultaneous knockdown (decreased expression) of connexin 43 (a gap junction protein, also known as gap junction α1 protein) and plakophilin 2 (a desmosome protein) in Sertoli cells was shown to perturb barrier function.41 This perturbation was concomitant with a redistribution of tight junction proteins occludin and ZO-1 in the cell epithelium, with both proteins partially relocated from the cell–cell interface into the cytosol via a mechanism that involved protein endocytosis.41 Likewise, simultaneous reduction in the expression of desmosomal proteins desmoglein 2 and desmocollin 2 in Sertoli cells via RNAi knockdown also resulted in the internalization of the coxsackievirus and adenovirus receptor (CAR)-c-Src protein complex by a similar mechanism.42 This observation is important, since CAR is an integral membrane protein at the BTB, which contributes to the barrier’s integrity together with c-Src—a nonreceptor protein kinase, which is CAR’s regulatory partner.
Crucially, these findings illustrate that the coexistence of different junctions at the BTB contributes directly to the dynamic nature of this barrier, and this facilitates the transit of preleptotene spermatocytes (many of which are connected by intercellular bridges and in clones)51,52 across the BTB at stage VIII of the seminiferous epithelial cycle. Specifically, integral membrane proteins must be delivered below the preleptotene spermatocytes in transit across the BTB, in order to form ‘new’ tight junction fibrils before proteins are removed (in part by a mechanism involving endocytosis) and ‘old’ tight junction fibrils are broken down above the migrating spermatocytes. This unique sequence of assembly and disassembly of tight junction fibrils thus maintains the integrity of the immunological barrier during the transit of preleptotene spermatocytes across the BTB while they differentiate into leptotene and zygotene spermatocytes.
These observations are also consistent with an earlier report, which demonstrated that under normal physiological conditions, tight junction proteins (such as occludin) and basal ES proteins (such as N-cadherin) ‘engage’ via adaptor proteins (for example, ZO-1 and β-catenin). In essence, an association between ZO-1 and β-catenin can ‘pull’ occludin closer to N-cadherin to produce an ‘engaged’ state that reinforces BTB integrity; this process possibly occurs below the migrating spermatocytes.53 However, tight junction proteins and basal ES–protein complexes were also shown to ‘disengage’ during junction restructuring (possibly above the migrating spermatocytes), and this loss of association between ZO-1 and β-catenin probably occurs at stage VIII of the seminiferous epithelial cycle to facilitate spermatocyte transit. Hence, occludin is ‘pulled away’ from N-cadherin to produce a ‘disengaged’ state that, thereby, perturbs barrier function.53,54 Identification of other adaptor proteins that function similarly in the seminiferous epithelium is an important goal in future studies.
Ultrastructurally, the ES is typified by the presence of actin filament bundles sandwiched in between cisternae of endoplasmic reticulum and the plasma membrane (Figure 2b,c). These ultrastructural features are only detected within Sertoli cells at the apical ES (Figure 2b), and at the basal ES they are found within both Sertoli cells (Figure 2c).55 notably, these ultrastructural features are unique to the ES and to the testis; that is, they are not detected in any other cell type in any organ in the mammalian body. nevertheless, at the molecular level, the ES comprises proteins that are identical to those found in conventional anchoring junctions in other epithelia (Table 1).
Table 1.
Proteins found at the apical ES, BTB and hemidesmosome in the seminiferous epithelium of the rat testis
| Junction type or junction site | Component proteins |
|---|---|
| Apical ES (a cell–cell actin-based testis-specific atypical adherens junction found between Sertoli cells and step 8–19 spermatids) | Integral membrane or structural proteins: α6β1-integrin, laminin α3, laminin β3, laminin γ3, N-cadherin, E-cadherin, nectin-2, nectin-3, CAR, JAM-B, JAM-C, CRB3 Adaptors or scaffolding proteins: catenins, afadin, espin, fimbrin, p120ctn, vinculin, paxillin, axin, zyxin, WASP, p130 Cas, α-actinin, collagen α3(VI), actin Regulatory or signaling proteins: FAK, c-Src, c-yes, testin, ILK, dynamin 1, dynamin 2, Csk, Rab4A, guanylate cyclase, laminin fragments, PI 3-kinase, PTEN, PKB, MTMR2, Eps8, PAK, ERK1/2, CK2, aPKC, Fer kinase, ROCK, LIMK Proteases or protease inhibitors: gelsolin, matrix metalloproteinase 2, matrix metalloproteinase 14, TIMP-2, α2-macroglobulin Polarity proteins: PAR6, PALS1, CRB-3, PATJ, 14-3-3 (Par5) Trafficking proteins: Rab8B, Rab13, RhoB Motor or motor-related proteins: myosin VIIa, Keap1, kinesin, dynein Cytokines: TNF, TGF-β3, IL-1α |
| Blood–testis barrier (found between adjacent Sertoli cells near the basement membrane) | |
| Tight junction | Integral membrane proteins: occludin, claudins, JAM-A, JAM-B Adaptors or scaffolding proteins: ZO-1, ZO-2, actin, collagen α3(IV) Regulatory or signaling proteins: FAK, c-Src, c-yes, p38 MAPK, ERK1/2, JNK, PKB, PAK, dynamin 2 Proteases or protease inhibitors: matrix metalloproteinase 9, TIMP-1, α2-macroglobulin Polarity proteins: PAR3, PAR6, PALS1, PATJ, 14-3-3 Trafficking proteins: Rab8B Cytokines: TNF, TGF-β3, IL-1α |
| Basal ES | Integral membrane proteins: N-cadherin, nectin-2 Adaptors or scaffolding proteins: β-catenin, α-catenin, γ-catenin, afadin Regulatory or signaling proteins: dynamin 2, c-Src, c-yes |
| Desmosome-like junction | Integral membrane proteins: desmoglein 2, desmocollin 2, desmocollin 3 Adaptors or scaffolding proteins: plakoglobin (γ-catenin), plakophilin 1, 2, 4, desmoplakin, vimentin Regulatory or signaling proteins: c-Src |
| Gap junction | Integral membrane proteins: connexin 43, connectin 33 Adaptors: plakophilin 2, β-catenin Regulatory or signaling proteins: c-Src |
| Hemidesmosome (found between Sertoli cells and the basement membrane) | Integral membrane and/or structural proteins: laminin α2, β1-integrin Adaptors: paxillin |
This table was prepared based on earlier reports and recent reviews,41,42,45,60,112,115,132,140–150 and is not intended to be exhaustive. Abbreviations: aPKC, atypical protein kinase C; BTB, blood–testis barrier; CAR, coxsackie and adenovirus receptor; CRB, Crumbs; ERK, extracellular signal-regulated kinase; ES, ectoplasmic specialization; FAK, focal adhesion kinase; IL-1α, interleukin 1α; ILK, integrin-linked kinase; JAM, junction adhesion molecule; JNK, c-Jun amino terminal kinase; LIMK, LIM domain kinase; MMP-14, matrix metalloproteinase 14; MTMR2, myotubularin-related protein 2; PALS1, protein-associated with Lin-7; p38 MAPK, p38 mitogen-activated protein kinase; PAR protein, partitioning defective protein; PATJ, PALS1-associated tight junction protein; ROCK, Rho-associated protein kinase; TGF-β3, transforming growth factor β3; TIMP, tissue inhibitor of metalloproteinase; TNF, tumor necrosis factor; WASP, neural Wiskott Aldrich syndrome protein.
Interestingly, the ES also consists of proteins that are restricted to tight junctions, gap junctions, desmosomes, hemidesmosomes and focal contacts in other epithelia (Table 1), making the ES a hybrid junction.56,57 equally important, some proteins found at the apical ES—β1-integrin, laminin α3, β3, γ3 and N-cadherin—are only expressed transiently (Table 1).58–61 moreover, while the apical and basal ES are identical ultrastructurally (Figure 2), not all proteins found at the apical ES (such as β1-integrin and laminin α3, β3, γ3) are detected at the basal ES, although some proteins are present at both sites (for example N-cadherin; Table 1).
The apical ES (and not the desmosome) was shown to be the strongest anchoring junction in the seminiferous epithelium when adhesion was quantified by a micro pipette pressure-transducing system that essentially measured the force required to pull germ cells (either prestep 8 or step 8 spermatids) away from the Sertoli cell epithelium in vitro.62 Surprisingly, adjudin, a potential male contraceptive,63 was shown to be more effective in disrupting the apical ES than the desmosome both in vitro64 and in vivo.65
The hemidesmosome is an intermediate filament-based cell-matrix anchoring junction present at the interface between the Sertoli cell and the basement membrane (Figure 2). up until now, its molecular composition in the testis has not yet been investigated, except for a study in 2008 that identified laminin α2 and β1-integrin as two constituent proteins of the hemidesmosome.66 unlike other epithelia, in which the tight junction is farthest away from the hemidesmosome, in the seminiferous epithelium, the BTB lies in proximity to the hemidesmosome, which illustrates that tight junctions, basal ES, desmosomes and gap junctions may be interacting with hemidesmosomes to maintain BTB integrity.
Studies in the field of cancer biology have, indeed, described a physiological link between tight junctions and hemidesmosomes in mammalian epithelial cells in vitro.67 oncogenesis, which results in the loss of cell polarity and the disruption of tight junctions, was shown to enhance interactions between ErbB (an epidermal growth factor receptor variant, which is a tight junction-associated protein) and α6β4-integrin (a hemi desmosome protein).67 Together, these proteins seemed to serve as a platform for signal transduction, thereby promoting cell proliferation—a key event in tumorigenesis.67 Thus, these findings suggest that hemidesmosomes might have an important role in the regulation of BTB function during spermatogenesis and possibly vice versa.
The apical ES–BTB–hemidesmosome axis
Laminins and metalloproteinases
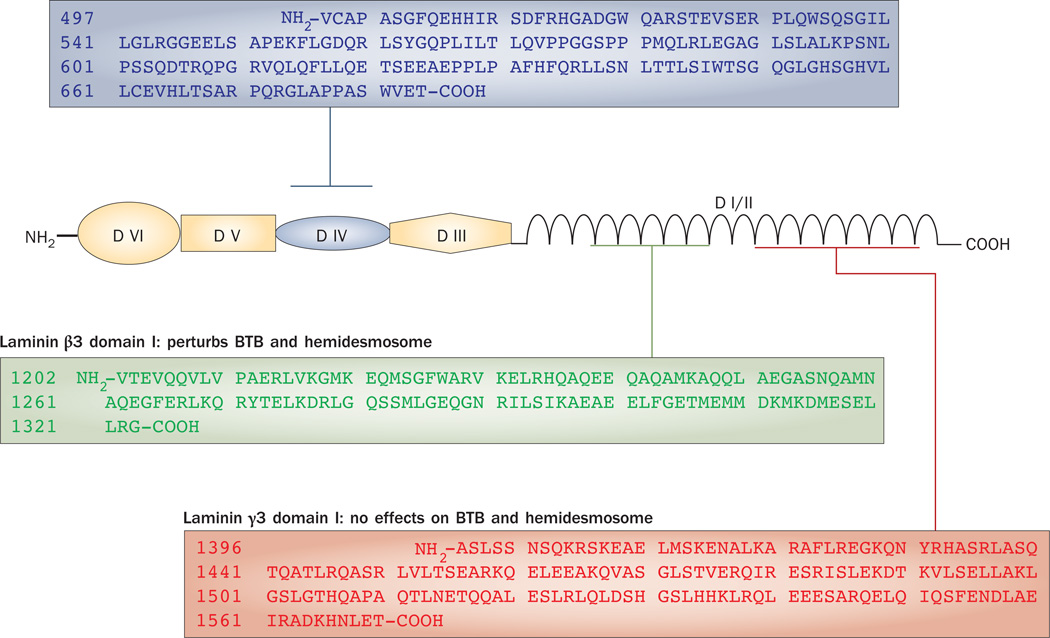
Laminins are restricted to the interface between the epithelial cell and the matrix at focal contacts and at hemidesmosomes, and they are putative substrates of matrix metalloproteinases.68–70 each laminin chain can be divided into six functional domains, namely I–VI (Figure 3). Laminins are ligands for integrins, and each functional laminin is a heterotrimeric glycoprotein composed of one α, one β and one γ chain.56 Five different α chains (α1–α5), three different β chains (β1–β3) and three different γ chains (γ1–γ3) have been identified to date, most of which are restricted to the basement membrane in cell epithelia.56 Fragments of laminin chains are known to be biologically active peptides capable of regulating cell adhesion, migration, inflammatory response and blood–brain barrier function.68,71,72 Interestingly, laminin α3, β3 and γ3 chains are restricted to elongating or elongated spermatids58,59,73 and form bona fide adhesion with α6β1-integrin found on Sertoli cells58 at the apical ES.
Figure 3.
Structural domains of a typical laminin chain. Laminin β3 and γ3 are found at the apical ES in the rat testis. Three laminin fragments have been examined. Laminin β3 domain I and γ3 domain IV, but not laminin γ3 domain I, were shown to be biologically active fragments that affected Sertoli cell BTB dynamics and hemidesmosome function. Abbreviation: BTB, blood–testis barrier.
Matrix metalloproteinase 2 is expressed transiently at the apical ES at late stage VII to early stage VIII of the seminiferous epithelial cycle, and co-localizes precisely with laminin α3 and γ3 chains.59 Thus, matrix metalloproteinase 2 is believed to cleave laminin chains into biologically active fragments, and thereby perturb the adhesion conferred by the interaction between laminin and integrin. This de-adhesion is needed for spermiation, but also for BTB restructuring, so that spermatocytes can cross the BTB at stage VIII of the epithelial cycle—two important cellular events in the seminiferous epithelium that require coordination.
This regulatory mechanism was confirmed in an in vitro study that used Sertoli cells with an established tight junction barrier that mimicked the BTB in vivo.66 In this study, the addition of purified recombinant laminin fragments (laminin β3 domain I or laminin γ3 domain IV) to these Sertoli cells perturbed barrier function in a dose-dependent fashion. This effect was concomitant with a dose-dependent decline in the steady-state levels of integral membrane proteins, such as occludin and junction adhesion molecule A (JAM-A), at the BTB. The disruptive effects of these laminin fragments on BTB function seemed to be specific since a fragment corresponding to recombinant laminin γ3 domain I had no effects.66 moreover, these findings were confirmed when these laminin fragments were over expressed in Sertoli cells, which do not express any laminin β3 and γ3 chains, and their overexpression affected the tight junction permeability barrier of the Sertoli cell concomitant with a statistically significant decline in the levels of BTB integral membrane proteins.66
Interestingly, the β1-integrin level was also significantly reduced in this study,66 but since apical ES was absent in these Sertoli cell cultures, the origin of β1-integrin was initially unknown. Surprisingly, β1-integrin was later found to be a component of the hemidesmosome.66 This finding seemingly suggested that biologically active laminin fragments could also perturb hemidesmosome function by disrupting its β1-integrin level. To better understand the physiological role of β1-integrin in BTB dynamics, we silenced this gene by RNAi in Sertoli cells to investigate if this would affect barrier function. Indeed, a disruption of the hemidesmosome by β1-integrin knockdown led to a disruption of tight junction barrier function mediated by redistributing occludin and N-cadherin from the cell surface to cytosol via an increase in protein endocytosis.66
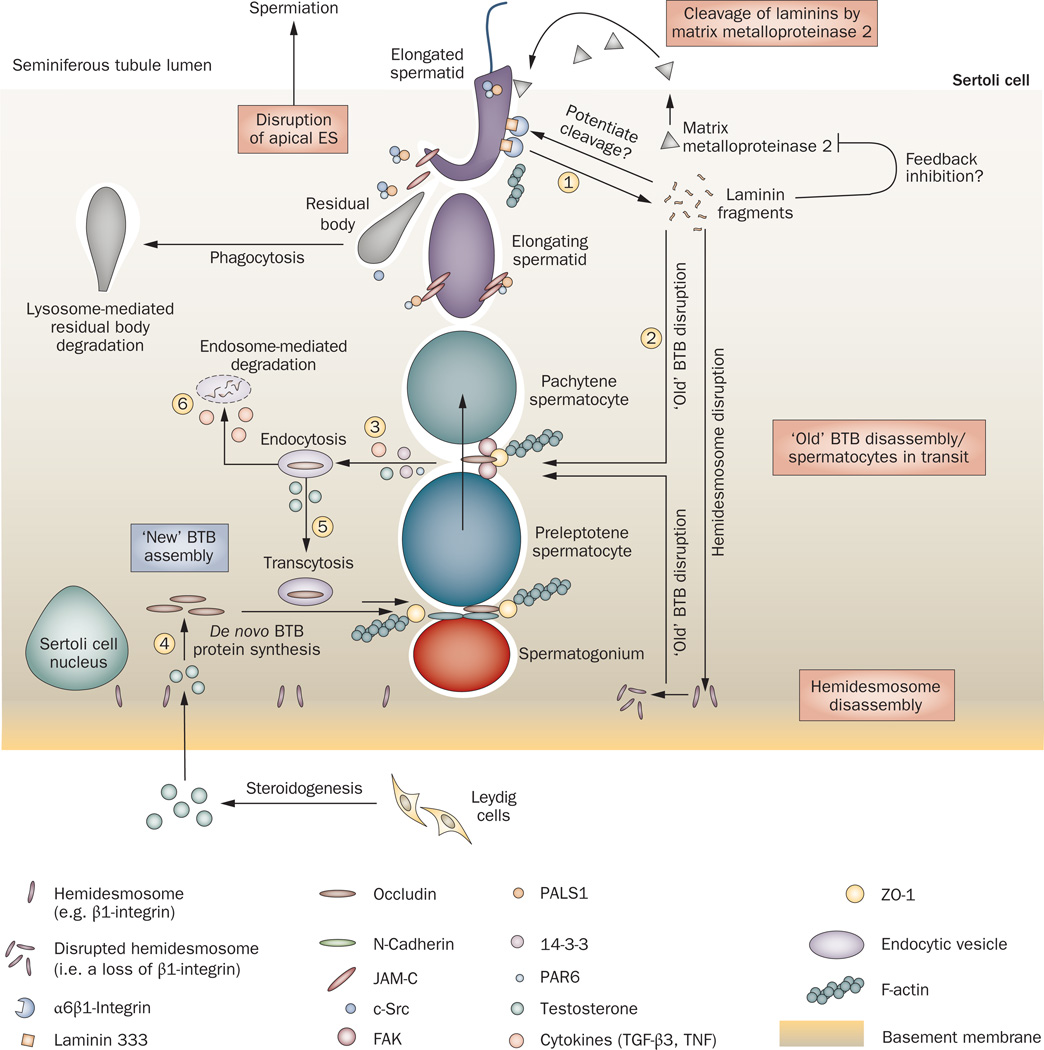
Collectively, these findings point to the existence of a functional axis between the apical ES, BTB and hemidesmosome, in which laminin fragments released from the disassembling apical ES prepare the seminiferous epithelium for spermiation. These biologically active laminin fragments then regulate BTB dynamics directly or in directly by affecting β1-integrin at the hemi desmosome, thereby creating a feedback loop that modulates BTB restructuring (Figure 4). In other words, this functional axis provides a unique physiological link so that spermiation and BTB restructuring can be coordinated at stage VIII of the epithelial cycle. whether a feedback loop exists that involves the BTB and/or the hemi desmosome, in which biologically active laminin fragments limit restructuring of the seminiferous epithelium during the final phases of spermiation, remains to be seen. In addition, the identity of the molecule(s) that triggers matrix metalloproteinase 2 to cleave laminin chains at the apical ES is not known. A signaling molecule known as FNDC3A has been shown to be critical for adhesion between the Sertoli cell and the spermatid at the apical ES.74 Certainly, FnDC3A may be one of the molecules that regulates proteins required for spermatid adhesion, but at this point more functional studies are needed to better understand the role of FnDC3A in this axis.
Figure 4.
The apical ES–BTB–hemidesmosome functional axis in the testis. The cascade of events begins at the apical ES before spermiation. Matrix metalloproteinase 2, which is expressed predominantly before spermiation,59 cleaves the integrin–laminin protein complex at the apical ES to facilitate spermiation, generating the biologically active laminin fragments (1). Whether biologically active laminin fragments induce further proteolytic events to disrupt the apical ES locally and/or a feedback mechanism prevents further damage to the epithelium resulting from matrix metalloproteinase 2 activation is not known. Once biologically active laminin fragments are formed, they induce disruption of tight junction fibrils at the ‘old’ BTB site above preleptotene spermatocytes in transit across the BTB (2)66 and also disrupt the hemidesmosome. This loss of hemidesmosome function, as manifested by a decline in β1-integrin at the hemidesmosome,66 potentiates disruption at the ‘old’ BTB site to facilitate the transit of spermatocytes,66 which is mediated by an increase in protein endocytosis at the site40,66 (3); this endocytosis is facilitated by cytokines (TGF-β3 and TNF),40,113 testosterone,40 PAR polarity proteins (14-3-3 and PAR6)80,84 and kinases (such as FAK and c-Src).116,132 To maintain the immunological barrier during the transit of primary spermatocytes, ‘new’ tight junction fibrils are probably first formed behind migrating spermatocytes, mediated by the de novo synthesis of BTB integral membrane proteins induced by testosterone38–40,151 (4) possibly via both classical and nongenomic pathways,152 so that a ‘new’ BTB can be assembled before the ‘old’ BTB located above transiting spermatocytes is disassembled. Additionally, endocytosed proteins from the ‘old’ BTB site can be transcytosed (5) to the ‘new’ BTB site via protein transcytosis and recycling, which is facilitated by testosterone40 and/or TNF.125 Unwanted integral membrane proteins are degraded via endosome-mediated or ubiquitin-mediated intracellular degradation (6), which is regulated by TGF-β2 and/or TGF-β3.40 Thus, this functional axis provides an efficient system to facilitate spermiation and BTB restructuring that occur simultaneously at stage VIII of the epithelial cycle, and at the same time, the immunological barrier function of the BTB is maintained. Abbreviations: BTB, blood–testis barrier; ES, ectoplasmic specialization; FAK, focal adhesion kinase; PAR polarity proteins, partitioning defective polarity proteins; TGF-β3, transforming growth factor β3; TNF, tumor necrosis factor.
Regulators of the axis
As discussed above, spermiation and BTB restructuring are coordinated in part by the apical ES–BTB–hemidesmosome axis. Studies in the past decade have shown that at least four different groups of proteins and/ or biomolecules regulate or affect this functional axis— polarity proteins, cytokines, testosterone and nonreceptor protein kinases.
Polarity proteins
Early studies in Drosophila melanogaster and Caenorhabditis elegans have identified three conserved protein complexes that control cell polarity and confer cellular asymmetry in multiple types of epithelia in mammals:75–79 the Crumbs (CRB), Scribble, and partitioning defective (PAR) protein complexes. In response to extrinsic (for example, gradients of growth factors) or intrinsic (for example, microtubule cytoskeleton) polarity cues, these complexes recruit additional proteins to form multiprotein complexes within a cell epithelium to induce downstream signaling to trigger the establishment of cell polarity and cellular asymmetry.
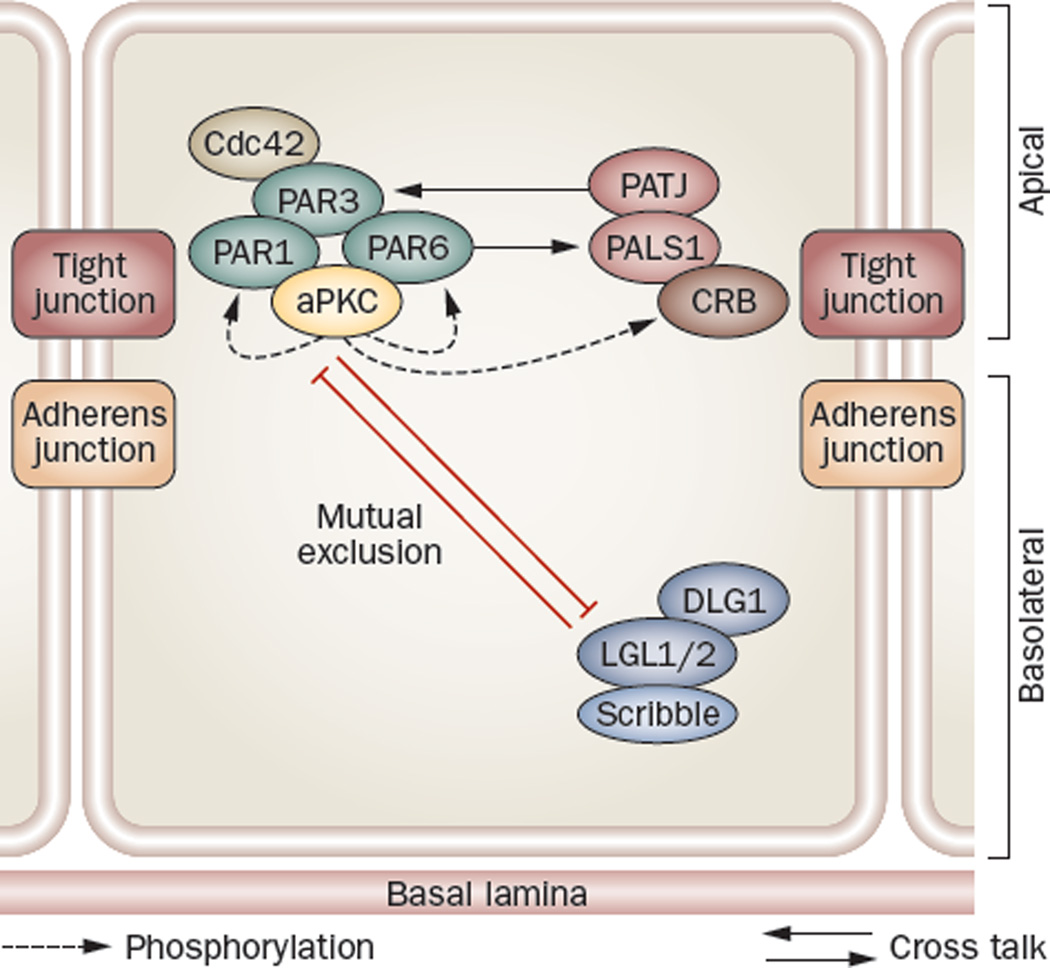
In multiple types of epithelia, the CRB and PAR protein complexes are restricted to tight junctions, whereas the Scribble protein complex is found at the basolateral domain (Figure 5). In these epithelia, mutual exclusion of the PAR and Scribble complex confers apico basal polarity, whereas phosphorylation of LGL of the Scribble complex and PAR1 of the PAR complex mediated by atypical protein kinase C (aPKC) confers asymmetric distribution of polarity proteins.75–77 However, in the testis, besides being found at the BTB in the seminiferous epithelium, CRB and PAR protein complexes are also localized to the apical ES, and these proteins are crucial to the regulation of the functional axis in the testis (Figure 6).
Figure 5.
Polarity protein complexes establish cell polarity. in polarized epithelial cells, the Crumbs and PAR protein complexes localize predominantly to tight junctions (except in the testis, where they are also found at the apical ectoplasmic specialization, see Figure 6), with the Scribble complex restricted to the basolateral region. Mutual exclusion of the PAR and Scribble complex confers apicobasal polarity, whereas phosphorylation of LGL2 and PAR1 mediated by atypical protein kinase C confers asymmetric distribution of polarity proteins.75–77 Abbreviations: aPKC, atypical protein kinase C; CRB, Crumbs; DLG1, discs large 1; LGL1, lethal giant larvae 1; PALS1, protein-associated with Lin-7; PAR protein, partitioning defective protein; PATJ, PALS1-associated tight junction protein.
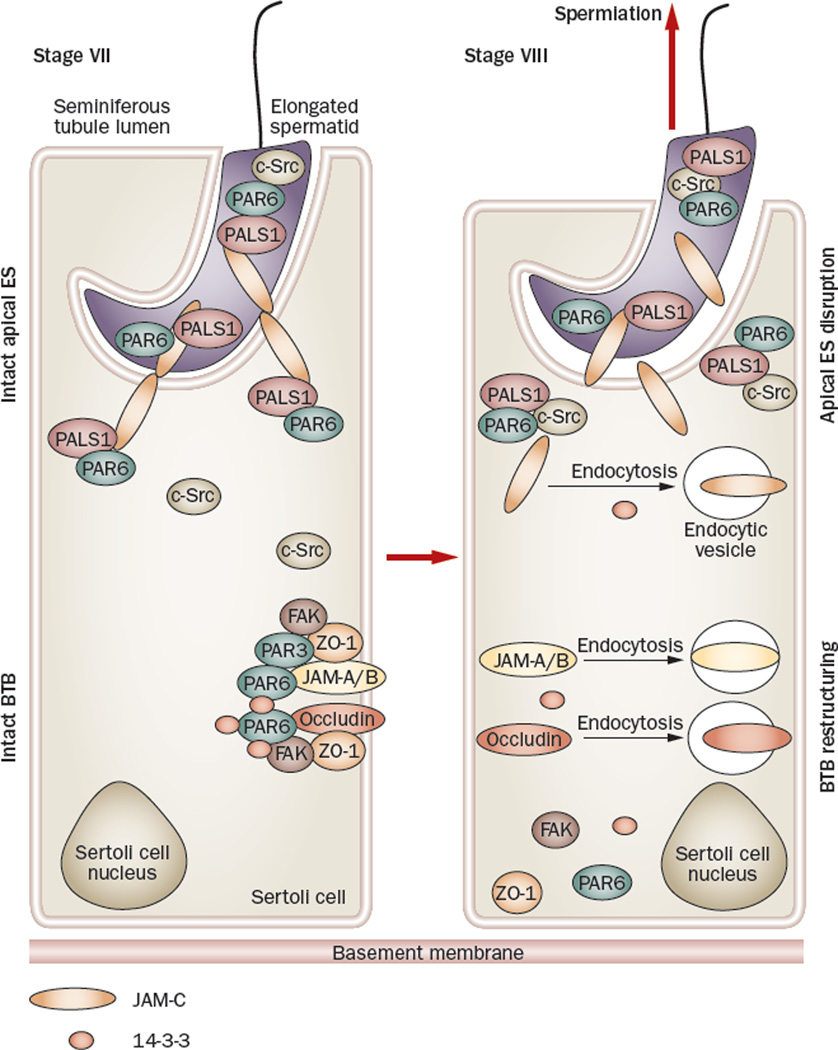
Figure 6.
Role of polarity proteins and nonreceptor protein kinases in the apical ES–BTB functional axis. Polarity proteins, such as PAR6 and PALS1, form a stable protein complex with JAM-C at the apical ES, as well as with integral membrane proteins (for example, occludin) at the BTB to confer cell adhesion and maintain their integrity, such as in a stage VII tubule80 (left panel). Also, c-Src does not bind to the PAR6–PALS1 protein complex to avoid unwanted phosphorylation of the polarity proteins by c-Src so as to maintain the apical ES integrity,80 but FAK strongly associates with occludin to maintain its proper phosphorylation status and the adhesive function of the occludin–ZO-1 complex to confer BTB integrity,116 since occludin is known to require proper phosphorylation in order to be assembled to the tight junction fibrils.138,139 However, during spermiation (right panel), which occurs at stage VIII of the epithelial cycle of spermatogenesis, c-Src associates tightly with the PAR6–PALS1 complex, pulling these protein complexes away from JAM-C and destabilizing the apical ES, facilitating spermiation. The loss of association of FAK and 14-3-3 with protein complexes at the BTB causes reduced phosphorylation of occludin and also induces an increase in the kinetics of protein endocytosis at the site, respectively, destabilizing BTB integrity.84,116 The sum of these events is believed to destabilize BTB integrity, inducing BTB restructuring to facilitate spermatocyte movement across the BTB and to facilitate spermiation. Abbreviations: BTB, blood–testis barrier; ES, ectoplasmic specialization; FAK, focal adhesion kinase; JAM, junction adhesion molecule; PALS1, protein-associated with Lin-7; PAR protein, partitioning defective protein.
The CRB protein complex is composed of CRB, PALS1 (protein-associated with Lin-7) and PATJ (PALS1-associated tight junction protein). CRB is an integral membrane protein with three isoforms: CRB1, CRB2 and CRB3, but only CRB3 is expressed by Sertoli and germ cells,80 and its overexpression induces tight junction assembly in epithelial cells.81 PALS1 is an adaptor protein that recruits proteins such as CRB3 and PAR6 to the tight junctions. In essence, crosstalk between CRB and PAR protein complexes is mediated by interactions between PALS1 and PAR6 via aPKC and/or c-Src in the testis (Figure 6). Finally, PATJ is another adaptor that is known to interact with other tight junction proteins, such as claudin-1, ZO-381 and JAM-C.80 Similar to CRB3, both PALS1 and PATJ are expressed by both Sertoli and germ cells, localizing to the ES in the testis.80
The Scribble protein complex was first described in D. melanogaster. This protein complex is composed of Scribble, Discs large (DLG, with DLGs 1–5 known to exist) and Lethal giant larvae (LGL1/2). Scribble binds directly to LGL2 and indirectly to DLG1 in vertebrates,76 which are tumor suppressors in flies and mammals.82 of these, LGL2 was shown to be expressed by the testis;76 however, the function of the Scribble protein complex in the testis is unknown.
The PAR protein complex is composed of PAR3, PAR6, aPKC and Cdc42. PAR3 and PAR6 were reported to regulate anterior–posterior polarity in C. elegans zygotes.83 These proteins also interact with aPKC and Cdc42.76,79,81 aPKC is a very important regulatory component in this complex. First, it regulates PAR3 intrinsically via phosphorylation, and second, it regulates cell polarity by mediating crosstalk among the different protein complexes via its effects on CRB3 and LGL1/2.79
As mentioned above, the PAR protein complex is not restricted to the BTB in the testis. PAR6 is an integrated component of the apical ES and is expressed pre dominately at this site at stages VII and VIII of the epithelial cycle.80 Adult rats treated with adjudin were used as a study model with which to assess the function of polarity proteins in the testis, because adjudin is known to selectively disrupt apical ES to induce spermatid loss (to mimic spermiation).63–65 In this study, PAR6 was shown to confer polarity to spermatids throughout spermiogenesis by maintaining proper orientation of developing spermatids in the seminiferous epithelium, that is, with their heads pointing towards the basement membrane.80
The study findings also indicated that PAR6 may regulate spermatid adhesion at the apical ES, because PAR6 was found to structurally interact with PALS1 and JAM-C (an integral membrane protein at the apical ES) to form a stable protein complex to confer Sertoli cell–spermatid adhesion (Figure 6). During adjudin-induced spermatid loss,45 c-Src was shown to become tightly associated with the PAR6–PALS1 complex, possibly phosphorylating JAM-C and/or PAR6, and caused dissociation of PAR6–PALS1 from JAM-C.80 Thus, this action de stabilizes the PAR6–PALS1–JAM-C protein complex, and leads to detachment of spermatids from the seminiferous epithelium (Figure 6).80 whether c-Src’s association with the PAR6–PALS1 protein complex can mediate junction disassembly at the BTB as well is not yet known.
The role of PAR3 and PAR6 in regulating cell adhesion at the BTB has also been investigated by studies using the RNAi technique.80 when Sertoli cells were cultured in vitro and allowed to form an intact cell epithelium that mimicked the BTB in vivo, a knockdown of PAR3 led to a loss of JAM-A, ZO-1 and α-catenin from the cell–cell interface as these proteins relocated into the cell cytosol, whereas knockdown of PAR6 led to a redistribution of JAM-A, N-cadherin and α-catenin from the cell surface to the cytosol possibly as a result of an increase in endocytosis.80 In short, these findings illustrate that PAR proteins, together with PALS1 and c-Src, are crucial regulators of cell adhesion. A subsequent study has shown that PAR proteins are also regulators of protein endocytosis. 84 For instance, silencing of PAR5, which is also known as 14-3-3, by RNAi was shown to accelerate the endocytosis of JAM-A and N-cadherin in Sertoli cells, perturbing the tight junction barrier.84 The biochemical study using an endocytosis assay,84 therefore, confirms the results of dual immunofluorescence analysis80 that PAR proteins are regulators of protein endocytosis.
At late stage VIII of the epithelial cycle, when PAR6 and 14-3-3 are localized to the apical ES,84 the apical ES is transformed into an ultrastructure known as the apical tubulobulbar complex (apical TBC). This ultrastructure anchors spermatids to Sertoli cells in the epithelium44,85 and possibly also serves as a device to eliminate unwanted cytoplasmic substances, also known as the residual body, during spermiogenesis (Figure 7).86 The structure is typified by the presence of membranous and tubular-like invaginations in Sertoli cells which run alongside the head of the elongated spermatid and are reminiscent of large endocytic vesicles.44,86,87 Indeed, findings in the past few years seemingly suggest that the apical TBC may be a ‘late-stage’ apical ES whose primary function is to internalize and recycle junctional complex components, 88,89 possibly from the ‘old’ apical ES to be used for the assembly of the ‘new’ apical ES via protein re cycling and trans cytosis during spermiogenesis (Figure 7), analogous to BTB restructuring as depicted in Figure 6.
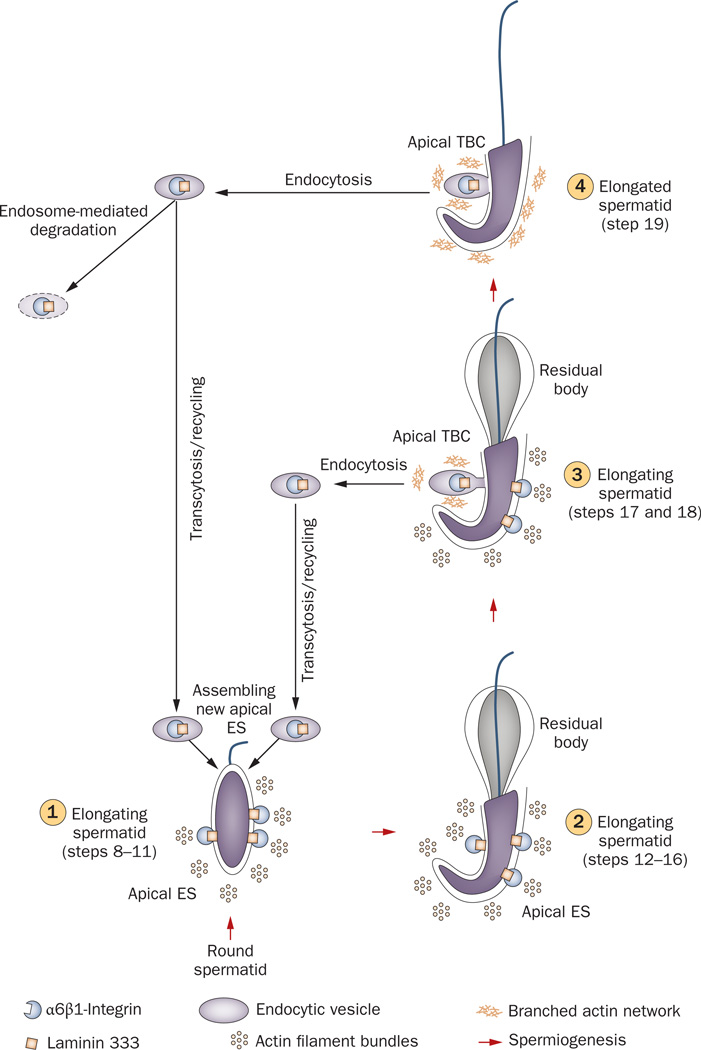
Figure 7.
Role of the TBC in apical ES dynamics during spermiogenesis. At stage XIV of the epithelial cycle in rat testes, round spermatids (step 1) derived from meiosis II, begin the maturation process known as spermiogenesis. This is typified by condensation of the genetic material in the spermatid head, elongation of the tail, the development of the acrosome and the shedding of unwanted cytoplasmic material (residual body). At the step 8 spermatid stage (1), the apical ES appears, which is constituted predominantly by α6β1-integrin–laminin 333, nectin–afadin and cadherin–catenin protein complexes45,93 (2). in late step 18 spermatids (3), apical ES proteins at the concave side of the spermatid head begin to undergo endocytic vesicle-mediated protein internalization creating an ultrastructure known as TBC, and the TBC replaces all apical ES at step 19 spermatids (4), destabilizing the apical ES to facilitate spermiation. Endocytosed apical ES proteins can either be transcytosed and recycled back to cell surface to form ‘new’ apical ES in newly formed step 8 spermatids or be degraded via endosome-mediated or ubiquitin-mediated pathways. Abbreviations: ES, ectoplasmic specialization; TBC, tubulobulbar complex.
Internalization of proteins at the apical TBC88 is facilitated by proteins such as clathrin, N-WASP and cortactin that are components of the apical TBC89 and are known to participate in endocytic vesicle-mediated protein endocytosis.90,91 These findings demonstrate that protein endocytosis is crucial for the disassembly of the apical ES to facilitate spermiation, and that these cellular events may be mediated in part by PAR6 and 14-3-3, as the expression of both is rapidly lost from the apical ES before spermiation. In addition, 14-3-3 regulates protein endocytosis at the BTB, and the level of this protein also decreases there before spermiation.84 Together, these observations suggest that a transient loss of these proteins at the apical ES and at the BTB is needed to facilitate junction restructuring at both sites, and this is probably mediated via changes in the kinetics of protein endocytosis that destabilize cell adhesion.
Potentially, polarity proteins such as PAR6 and 14-3-3 function as molecular switches in the apical ES–BTB–hemidesmosome functional axis to ‘turn on’ or ‘turn off’ protein endocytosis during the epithelial cycle to facilitate junction restructuring, thereby coordinating spermiation and primary spermatocyte transit across the BTB (Figure 6). In other words, the transient loss of PAR6 and/or 14-3-3 at the apical ES at stage VIII of the epithelial cycle may activate the cleavage of laminin chains by matrix metalloproteinase 2 to produce biologically active laminin fragments. This transient loss of PAR6 and 14-3-3 at both the apical ES and BTB also destabilizes cell orientation and cell adhesion via an enhancement in protein endocytosis, thus inducing spermiation and BTB restructuring (Figures 4, 6 and 7).80,84
Cytokines and testosterone
Cytokines, such as TGF-β, epidermal growth factor (EGF), interleukin (IL)-1α, IL-13 and TNF, have all been clearly implicated in the regulation of the function of the tight junction barrier in different epithelial cells.92–97 For example, TNF can perturb intestinal barrier function by inducing endocytosis of occludin.94,98 However, the precise role of cytokines in BTB dynamics was not known until a decade ago, when it was first reported that TGF-β and TNF can affect the assembly of the tight junction permeability barrier of the Sertoli cell.99–101 Since this time, additional in vivo studies in rats have demonstrated that the BTB is regulated by p38 mitogen-activated protein kinase34,36,37,102 and c-Jun n-terminal kinase35,101,103 signaling pathways that are activated by TGF-β and TNF, respectively.
These findings illustrate that cytokines not only affect germ cell apoptosis104,105 (in order to maintain the Sertoli:germ cell ratio at ~1:30–1:5029,106 in the adult rat testis),107–109 but also induce junction restructuring at the apical ES and BTB.110–112 In essence, these studies demonstrate that cytokines can perturb adhesion at the apical ES, as well as at the BTB, by reducing the steady-state levels of key integral membrane proteins, such as N-cadherin at the apical ES and occludin at the BTB.93,111 In support of these observations, two subsequent reports illustrated that the downregulation of integral membrane BTB proteins observed after treating Sertoli cells with cytokines was mediated in part by an increase in protein endocytosis40,113 and endosome-mediated intracellular degradation.40 Interestingly, phagocytosis of residual bodies in spermatids by Sertoli cells that occurs before spermiation induces Sertoli cells to secrete IL-1α in vitro, consistent with the observation in vivo that the production of this cytokine is highest in stage VIII tubules.114 In fact, IL-1α administered intratesticularly to adult rats induces spermatid depletion from the epithelium that mimics spermiation and a disruption of the barrier that mimics BTB restructuring.115 Collectively, these findings suggest that the timely production of IL-1α by Sertoli cells following phagocytosis of residual bodies before spermiation may coordinate spermiation and BTB restructuring at stage VIII of the epithelial cycle.
Testosterone, on the other hand, promotes the integrity of the BTB and the assembly of junctional complexes.7,38,39 In vitro studies have demonstrated that testosterone can facilitate the production of tight junction proteins required for the assembly and maintenance of the barrier function of the Sertoli cell tight junction.116–118 moreover, the high level of intratesticular testosterone, which in both rodents and humans is about ~50–100-fold higher in this organ than in the systemic circulation,119,120 is critical for apical ES integrity.121–124 As such, it was concluded that cytokines and testosterone elicit opposing effects on cell junction integrity in the seminiferous epithelium.
At this point, we might question how cytokines and testosterone, which essentially function within the same microenvironment, can have opposing effects on junction dynamics yet still maintain the homeostasis of the seminiferous epithelium? Studies in the past few years that examined the effects of cytokines, such as TGF-β2, and testosterone on protein endocytosis and recycling have provided much needed insight. For example, both cytokines and testosterone enhanced the kinetics of endocytosis of integral membrane proteins of the BTB in vitro in Sertoli cells with an established tight junction barrier.40,113 However, only testosterone induced the recycling of endocytosed proteins back to the Sertoli cell surface,40 and hence promoted the barrier function of the tight junction, whereas cytokine-induced endocytosed proteins were destined for intracellular degradation. These findings explain how immunological barrier function can be maintained during the transit of preleptotene primary spermatocytes across the BTB at stage VIII of the epithelial cycle (Figure 4).
Testosterone also seems to facilitate the assembly of ‘new’ tight junction fibrils below migrating primary spermatocytes by de novo synthesis of new tight junction proteins and also by the transcytosis and re cycling of internalized tight junction proteins from above spermatocytes to below them. At the same time, cytokines produced by Sertoli and germ cells trigger the disassembly of ‘old’ tight junction fibrils situated above migrating spermatocytes as this would enable germ cells to cross the barrier. while this model seems to be supported by the observation that TNF can induce the expression of AR in the Sertoli cell,125 additional studies are needed to determine whether a separate but related mechanism exists in which cytokines can also work with testosterone to promote barrier function. Certainly, not all cytokines induce junction disassembly so that additional functional studies would be needed.
Taken collectively, these findings illustrate that polarity proteins, testosterone and cytokines coordinate junction restructuring at the apical ES and BTB during spermatogenesis within the apical ES–BTB–hemidesmosome functional axis. Possibly, biologically active laminin fragments released at the apical ES before spermiation affect polarity proteins, the AR or cytokine receptors. These laminin fragments may also affect the de novo synthesis of cytokines and/or polarity proteins (or their spatial localization) within the seminiferous epithelium. Alternatively, biologically active laminin fragments may affect the kinetics of protein endocytosis, recycling, transcytosis and/or endosome-mediated degradation. Again, additional functional studies are needed before these cellular events are better understood.
Interestingly, treatment of mice with mono-(2-ethylhexyl) phthalate (MEHP) induced Sertoli cell injury that led to a disruption of apical ES and lead to premature sloughing of elongating spermatids from the epithelium.126 These phenotypic changes were shown to be associated with an increase in the levels of matrix metalloproteinase 2126 and TNF,127 concomitant with a significant loss of proteins at the apical ES (laminin γ3 and β1-integrin) and BTB (occludin and ZO-1), which illustrates that BTB function was also compromised. Intriguingly, these effects were blocked by treating mice with specific matrix metalloproteinase 2 inhibitors.126 Furthermore, the MEHP-induced TNF production that caused the BTB disruption was regulated by matrix metalloproteinase 2.127 These findings, based on a toxicity model, thus support the existence of a functional apical ES–BTB axis in the seminiferous epithelium,126 they also suggest that matrix metalloproteinase 2 may have a crucial role in the induction of BTB restructuring mediated by TNF,127 which illustrates a physiological relationship between matrix metalloproteinase 2 and cytokines in the axis.
Additionally, matrix metalloproteinases (such as matrix metalloproteinase 9) at the basement membrane128,129 are known to be capable of generating bio logically active fragments from collagens130,131 to modulate tight junction barrier function in different epithelia. In fact, TNF stimulates the production of matrix metalloproteinase 9 by Sertoli cells, which in turn, cleaves collagen chains to perturb the Sertoli cell tight junction function at the BTB.101 In short, these findings illustrate that cytokines can also mediate their effects in the basement membrane via proteases that generate biologically active collagen fragments, which, in turn, modulate the BTB function, to support the presence of a functional axis between BTB and the basement membrane.
Nonreceptor protein kinases
As described above, the nonreceptor protein kinase, c-Src, is an important regulator of the PAR6 protein complex that confers adhesion at the apical ES (Figure 6).80 Focal adhesion kinase (FAK), another non receptor protein tyrosine kinase, is an integrated component of the occludin–ZO-1 protein complex132 of the BTB of the adult rat testis.133 Interestingly, the phosphorylated or activated forms of this protein seem to localize predominantly to the apical ES instead of the BTB.121,133 However, these earlier findings were not actively pursued at the time because FAK was known to have other, unrelated roles in most epithelia.134 Following the identification of c-Src80 and other non receptor protein kinases135 at the apical ES and BTB, the importance of FAK in the apical ES–BTB functional axis became more obvious.
Besides being a component of the occludin–ZO-1 protein complex at the BTB,132 FAK was shown to phosphorylate occludin in this protein complex to determine its adhesive status.116 For instance, FAK was recruited to the occludin-based tight junction fibrils to induce occludin phosphorylation116 in order to protect the BTB from the damaging effects of cadmium, which is known to disrupt barrier integrity.136,137 This finding is consistent with earlier reports that occludin associated with tight junctions is phosphorylated more extensively than occludin located basally within epithelial cells.138,139 Furthermore, a knockdown of FAK by RNAi in Sertoli cells, which have an established tight junction barrier, induces a loss in occludin phosphorylation, which disrupts the occludin–ZO-1 protein complex,116 and results in relocation of occludin and ZO-1 from the cell surface to the cell cytosol116 via an increase in protein endocytosis.132 Thus, these findings demonstrate the significance of FAK in BTB dynamics. In essence, c-Src and FAK, besides regulating adhesion function at the apical ES (Figure 4) and BTB (Figure 6), may also mediate the effects of biologically active laminin fragments, and thereby regulate the apical ES and BTB along the apical ES-BTB-hemidesmosome axis (Figure 4).
Conclusions
Herein, we have reviewed findings in the past decade that support the presence of a functional apical ES–BTB–hemidesmosome axis in the seminiferous epithelium that regulates important cellular events occurring throughout spermatogenesis, such as junction restructuring at the apical ES and BTB via autocrine factors (for example, biologically active laminin fragments), testosterone, cytokines and kinases. Polarity proteins also play an important part in protein endocytosis, recycling and perhaps transcytosis to provide an efficient mechanism for restructuring of the seminiferous epithelium during germ cell migration, which also serve as molecular ‘switches’ in the functional axis. This apical ES–BTB–hemidesmosome axis functions in parallel with the hypothalamic–pituitary–testicular axis in that the former axis coordinates cellular events across opposite ends of the seminiferous epithelium, whereas the latter axis co ordinates hormonal events between the brain and testis. nevertheless, both regulate spermatogenesis. Hopefully, functional studies in the future will expand our understanding of the apical ES, BTB and hemi desmosome, as many regulatory components within this axis may become viable targets for male contraceptive development. For instance, if laminin fragments can disrupt spermatogenesis, then an antagonist to the biologically active fragment may block BTB restructuring and thus deny entry of primary pre leptotene spermatocytes into the apical compartment for meiosis and subsequent spermatid differentiation via spermiogenesis. Further more, some components of this axis may also be the targets of environmental toxicants (for example, cadmium) that cause male infertility. If the mechanism(s) is known, certain toxicant-induced infertility can be therapeutically managed.
Key points.
-
■
The apical ectoplasmic specialization–BTB (blood–testis barrier)–hemidesmosome axis, a novel functional axis in the testis, coordinates events occurring at opposite ends of the seminiferous epithelium during spermatogenesis
-
■
To maintain immunological barrier function at the BTB during the transit of spermatocytes, ‘new’ tight junction fibrils are assembled below migrating spermatocytes before ‘old’ tight junction fibrils are disassembled
-
■
Protein endocytosis, recycling, transcytosis and endosome-mediated or ubiquitin-mediated protein degradation play a critical part in the homeostasis of this apical ectoplasmic specialization–BTB–hemidesmosome functional axis
Polarity proteins are important regulators of endocytic vesicle-mediated protein trafficking events in the seminiferous epithelium during spermatogenesis
Review criteria.
The selected articles on which this Review is based were searched on PubMed using the following key terms: “spermatogenesis”, “testis”, “blood–testis barrier”, “ectoplasmic specialization”, “hemidesmosome”, “protein endocytosis, “tight junction, “transcytosis”, “protein recycling”, “endosome”, “ubiquitin”, “cytokines”, “testosterone”, “androgen receptor”, “Sertoli cell”, “Sertoli-germ cell interactions” and “seminiferous epithelial cycle”. Searches were limited to years 1995–2010. All of the selected papers are full-text papers that were written in English.
Acknowledgments
We thank current and past members of our laboratory for their investigations and discussions which have helped to form the basis of this article. Studies performed in the authors’ laboratory were supported by grants from the National institutes of Health (NICHD, R01 HD056034 and U54 HD029990 Project 5 to C. Y. C; and R03 HD061401 to D. D. M).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Sharpe RM. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- 2.Sokol RZ. Endocrinology of male infertility: evaluation and treatment. Semin. Reprod. Med. 2009;27:149–158. doi: 10.1055/s-0029-1202303. [DOI] [PubMed] [Google Scholar]
- 3.Kakar SS, Malik MT, Winters SJ, Mazhawidza W. Gonadotropin-releasing hormone receptors: structure, expression, and signaling transduction. Vitam. Horm. 2004;69:151–207. doi: 10.1016/S0083-6729(04)69006-6. [DOI] [PubMed] [Google Scholar]
- 4.Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1571–1579. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLachlan RI, et al. Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J. Androl. 2002;23:149–162. [PubMed] [Google Scholar]
- 6.Mather JP, Moore A, Li RH. Activins, inhibins, and follistatins: further thoughts on a growing family of regulators. Proc. Soc. Exp. Biol. Med. 1997;215:209–222. doi: 10.3181/00379727-215-44130. [DOI] [PubMed] [Google Scholar]
- 7.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 2009;30:119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 9.De Gendt K, et al. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl Acad. Sci. USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C, et al. infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl Acad. Sci. USA. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 12.Welsh M, Saunders PT, Atanassova N, Sharpe RM, Smith LB. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009;23:4218–4230. doi: 10.1096/fj.09-138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel MH, et al. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 14.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knock-out (FORKO) mouse. Biol. Reprod. 2000;62:1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- 16.Abel MH, et al. Spermatogenesis and Sertoli cell activity in mice lacking Sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology. 2008;149:3279–3285. doi: 10.1210/en.2008-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Kretser DM, Kerr JB. In: The Physiology of Reproduction. Knobil E, Neill J, editors. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 18.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carreau S. Estrogen: Roles in spermatogenesis. Immun. Endocr. Metab. Agents Med. Chem. 2008;8:59–65. [Google Scholar]
- 20.Shaha C. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Landes Bioscience and Springer Science+Business Media; Austin, TX: 2008. pp. 42–64. [Google Scholar]
- 21.Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J. Reprod. Immunol. 2009;83:31–35. doi: 10.1016/j.jri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza R, et al. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol. Cell. Endocrinol. 2005;241:41–48. doi: 10.1016/j.mce.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J. Clin. Endocrinol. Metab. 2000;85:2057–2067. doi: 10.1210/jcem.85.5.6600. [DOI] [PubMed] [Google Scholar]
- 24.Sirianni R, et al. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149:5043–5051. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- 25.Lie PP, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: A biochemical model. Trends Biochem. Sci. 2009;34:366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dym M. Basement membrane regulation of Sertoli cells. Endocr. Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 27.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat. Rec. 1982;203:485–492. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 28.Nistal M, Abaurrea MA, Paniagua R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J. Anat. 1982;14:351–363. [PMC free article] [PubMed] [Google Scholar]
- 29.Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am. J. Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- 30.Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev. Reprod. 1999;4:38–47. doi: 10.1530/ror.0.0040038. [DOI] [PubMed] [Google Scholar]
- 31.Bartke A. Apoptosis of male germ cells, a generalized or cell type-specific phenomenon? Endocrinology. 1995;136:3–4. doi: 10.1210/endo.136.1.7828545. [DOI] [PubMed] [Google Scholar]
- 32.Setchell BP. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Landes Bioscience/Springer Science+Business Media; Austin, TX: 2008. pp. 212–233. [Google Scholar]
- 33.Dym M, Cavicchia JC. Functional morphology of the testis. Biol. Reprod. 1978;18:1–15. doi: 10.1095/biolreprod18.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood–testis barrier and Sertoli-germ cell adhesion. J. Biol. Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 35.Li MW, et al. TNFalpha reversibly disrupts the blood–testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 36.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-beta3 regulates the blood–testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 37.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood–testis barrier dynamics: an in vivo study. J. Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 38.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood–testis barrier. Proc. Natl Acad. Sci. USA. 2005;102:16696–16670. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang RS, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 40.Yan HH, Mruk DD, Lee WM, Cheng CY. Blood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood–testis barrier dynamics. Proc. Natl Acad. Sci. USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ ZO-1 protein complexes, regulates blood–testis barrier dynamics. Int. J. Biochem. Cell Biol. 2010;42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell L. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 44.Vogl A, Vaid K, Guttman J. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Landes Bioscience/ Springer Science+Business Media; Austin, TX: 2008. pp. 186–211. [Google Scholar]
- 45.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong CH, Cheng CY. The blood–testis barrier: its biology, regulation and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 47.Setchell BP, Waites GMB. In: The Handbook of Physiology. Hamilton DW, Greep RO, editors. Vol. 5. Washington, D. C.: American Physiological Society; 1975. pp. 143–172. Section 7. [Google Scholar]
- 48.Fawcett DW, Leak LV, Heidger PM., Jr Electron microscopic observations on the structural components of the blood–testis barrier. J. Reprod. Fertil. Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- 49.Dym M, Fawcett DW. The blood–testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol. Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 50.Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood–testis barrier. Anat. Rec. 1973;175:639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- 51.Fawcett DW. intercellular bridges. Exp. Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- 52.Mruk DD, Cheng CY. Delivering non-hormonal contraceptives to men: advances and obstacles. Trends Biotechnol. 2008;26:90–99. doi: 10.1016/j.tibtech.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan HH, Cheng CY. Blood–testis barrier dynamics are regulated by an engagement/ disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl Acad. Sci. USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan HH, Mruk DD, Lee WM, Cheng CY. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Landes Bioscience and Springer Science+Business Media; Austin, TX: 2008. pp. 234–254. [Google Scholar]
- 55.Lie PP, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim. Biophys. Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan HH, Cheng CY. Laminin alpha3 forms a complex with beta3 and gamma3 chains that serves as the ligand for alpha6beta1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 59.Siu MK, Cheng CY. interactions of proteases, protease inhibitors, and the beta1 integrin/laminin gamma3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 60.Lee NP, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol. Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 61.Johnson K, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol. Reprod. 2002;66:992–1000. doi: 10.1095/biolreprod66.4.992. [DOI] [PubMed] [Google Scholar]
- 62.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J. Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- 63.Cheng CY, et al. AF-2364 [1-(2,4- dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Wolski KM, Mruk DD, Cameron DF. The Sertoli-spermatid junctional complex adhesion strength is affected in vitro by adjudin. J. Androl. 2006;27:790–794. doi: 10.2164/jandrol.106.000422. [DOI] [PubMed] [Google Scholar]
- 65.Chen YM, Lee NP, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol. Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- 66.Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood–testis barrier restructuring during spermatogenesis. Proc. Natl Acad. Sci. USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carraway CA, Carraway KL. Sequestration segregation of receptor kinases in epithelial cells: implications for ErbB2 oncogenesis. Sci. STKE. 2007;2007:re3. doi: 10.1126/stke.3812007re3. [DOI] [PubMed] [Google Scholar]
- 68.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 69.Siu MK, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 70.Tzu J, Marinkovich MP. Bridging structure with function: Structure, regulatory, and developmental role of laminins. Int. J. Biochem. Cell Biol. 2008;40:199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan HH, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male contraceptive development. Curr. Top. Dev. Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 72.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koch M, et al. Characterization and expression of the laminin gamma3 chain: a novel, non-basement membrane-associated, laminin chain. J. Cell Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Obholz KL, Akopyan A, Waymire KG, MacGregor GR. FNDC3A is required for adhesion between spermatids and Sertoli cells. Dev. Biol. 2006;298:498–513. doi: 10.1016/j.ydbio.2006.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulgakova NA, Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- 76.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 77.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 80.Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood–testis barrier restructuring during spermatogenesis. Proc. Natl Acad. Sci. USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong EW, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int. Rev. Cell. Mol. Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M, Vasioukhin V. Cell polarity and cancer-cell and tissue polarity as a non-canonical tumor suppressor. J. Cell Sci. 2008;121:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 83.Kemphues KJ, Priess JR, Morton DG, Cheng NS. identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 84.Wong EW, Sun S, Li MW, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat. Rec. 1979;194:213–232. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- 86.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat. Rec. 1979;194:233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 87.Russell LD. Observations on the interrelationships of Sertoli cells at the level of the blood–testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am. J. Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 88.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol. Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 89.Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol. Reprod. 2009;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 90.Fletcher SJ, Rappoport JZ. The role of vesicle trafficking in epithelial cell motility. Biochem. Soc. Trans. 2009;37:1072–1076. doi: 10.1042/BST0371072. [DOI] [PubMed] [Google Scholar]
- 91.Derivery E, Gautreau A. Generation of branched actin networks: assembly and regulation of the N-WASP and WAVE molecular machines. Bioessays. 2010;32:119–131. doi: 10.1002/bies.200900123. [DOI] [PubMed] [Google Scholar]
- 92.Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv. Drug Deliv. Rev. 2000;41:303–313. doi: 10.1016/s0169-409x(00)00048-x. [DOI] [PubMed] [Google Scholar]
- 93.Xia W, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring during spermatogenesis—a lesson to learn from the testis. Cytokine Growth Factor Rev. 2005;16:469–493. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 94.Turner JR. intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 95.Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009;20:329–338. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koch S, Nusrat A. Dynamic regulation of epithelial cell fate and barrier function by intercellular junctions. Ann. NY Acad. Sci. 2009;1165:220–227. doi: 10.1111/j.1749-6632.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 97.Kirschner N, et al. Alteration of tight junction proteins is an early event in psoriasis: Putative involvement of proinflammatory cytokines. Am. J. Pathol. 2009;175:1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clayburgh DR, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lui WY, Lee WM, Cheng CY. Transforming growth factor-b3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 100.Lui WY, Lee WM, Cheng CY. Transforming growth factor-beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol. Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 101.Siu MK, Lee WM, Cheng CY. The interplay of collagen iv, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 102.Xia W, Cheng CY. TGF-beta3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev. Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 103.Wong CH, Mruk DD, Siu MK, Cheng CY. Blood–testis barrier dynamics are regulated by alpha2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 104.Pentikainen V, et al. TNF-alpha down-regulated the Fas ligand and inhibits germ cell apoptosis in the human testis. J. Clin. Endocrinol. Metab. 2001;86:4480–4488. doi: 10.1210/jcem.86.9.7861. [DOI] [PubMed] [Google Scholar]
- 105.Suominen JS, Wang Y, Kaipia A, Toppari J. Tumor necrosis factor-alpha (TNF-alpha) promotes cell survival during spermatogenesis, and this effect can be blocked by infliximab, a TNF-alpha antagonist. Eur. J. Endocrinol. 2004;151:629–640. doi: 10.1530/eje.0.1510629. [DOI] [PubMed] [Google Scholar]
- 106.Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am. J. Anat. 1983;167:143–161. doi: 10.1002/aja.1001670202. [DOI] [PubMed] [Google Scholar]
- 107.Lui WY, Lee WM, Cheng CY. TGF-betas: their role in testicular function and Sertoli cell tight junction dynamics. Int. J. Androl. 2003;26:147–160. doi: 10.1046/j.1365-2605.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 108.Loveland KL, Dias V, Meachem S, Rajpert-De Meyts E. The transforming growth factor-beta superfamily in early spermatogenesis: potential relevance to testicular dysgenesis. Int. J. Androl. 2007;30:377–384. doi: 10.1111/j.1365-2605.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 109.O’Bryan MK, Hedger MP. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Austin, TX: Landes Bioscience and Springer Science+Business Media; 2008. pp. 92–114. [Google Scholar]
- 110.Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis—a molecular and biochemical perspective. Cytokine Growth Factor Rev. 2007;18:299–311. doi: 10.1016/j.cytogfr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li MW, Mruk DD, Cheng CY. “Unlocking” the blood–testis barrier and the ectoplasmic specialization by cytokines during spermatogenesis: Emerging targets for male contraception. Immun. Endocr. Metab. Agents Med. Chem. 2008;8:20–27. [Google Scholar]
- 112.Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 2009;15:159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia W, Wong EW, Mruk DD, Cheng CY. TGF-beta3 and TNFalpha perturb blood–testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Syed V, et al. Residual bodies activate Sertoli cell interleukin-1alpha (IL-1alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology. 1995;136:3070–3078. doi: 10.1210/endo.136.7.7789334. [DOI] [PubMed] [Google Scholar]
- 115.Sarkar O, Mathur PP, Cheng CY, Mruk DD. interleukin 1 alpha (IL1A) is a novel regulator of the blood–testis barrier in the rat. Biol. Reprod. 2008;78:445–454. doi: 10.1095/biolreprod.107.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood–testis barrier regulator. Proc. Natl Acad. Sci. USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung NP, Cheng CY. is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 118.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures—a new model for toxicological investigations of the “blood–testis” barrier in vitro. Toxicol. Appl. Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- 119.Turner TT, et al. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 120.Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann. NY Acad. Sci. 2005;1061:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 121.Beardsley A, Robertson DM, O’Donnell L. A complex containing alpha6beta1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J. Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, et al. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-beta-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: an in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–1284. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- 123.Wong CH, et al. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology. 2005;146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- 124.Xia W, Wong CH, Lee NP, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood–testis barrier integrity: an in vivo study using an androgen suppression model. J. Cell. Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 125.Delfino FJ, Boustead JN, Fix C, Walker WH. NF-kappaB and TNF-alpha stimulate androgen receptor expression in Sertoli cells. Mol. Cell. Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 126.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol. Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao PL, Lin YC, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol. Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heino J, Kapyla J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 2009;15:1309–1317. doi: 10.2174/138161209787846720. [DOI] [PubMed] [Google Scholar]
- 130.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J. Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Heino J. The collagen family members as cell adhesion proteins. Bioessays. 2007;29:1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- 132.Siu ER, et al. An occludin-focal adhesion kinase protein complex at the blood–testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siu MK, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of beta1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 134.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol. Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 135.Lee NP, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J. Cell. Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- 136.Setchell BP, Waites GM. Changes in the permeability of the testicular capillaries and of the “blood–testis barrier” after injection of cadmium chloride in the rat. J. Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 137.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol. Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 138.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J. Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am. J. Physiol. Renal Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 140.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 141.Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J. Cell Sci. 2000;113:2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- 142.Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol. Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 143.Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood–testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lie PP, et al. Dynamin II interacts with the cadherin- and occludin-based protein complexes at the blood–testis barrier in adult rat testes. J. Endocrinol. 2006;191:571–586. doi: 10.1677/joe.1.06996. [DOI] [PubMed] [Google Scholar]
- 145.Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J. Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 146.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 147.Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique Sertoli/spermatid adherens junctions in rat and mouse testes. Biol. Reprod. 2007;77:1037–1048. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- 148.Mruk DD, Lau AS, Sarkar O, Xia W. Rab4A GTPase-catenin interactions are involved in cell junction dynamics in the testis. J. Androl. 2007;28:742–754. doi: 10.2164/jandrol.106.002204. [DOI] [PubMed] [Google Scholar]
- 149.Mruk DD, Lau AS. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2009;80:590–601. doi: 10.1095/biolreprod.108.071647. [DOI] [PubMed] [Google Scholar]
- 150.Le Magueresse-Battistoni B. Serine proteases and serine protease inhibitors in testicular physiology: the plasminogen activation system. Reproduction. 2007;134:721–729. doi: 10.1530/REP-07-0114. [DOI] [PubMed] [Google Scholar]
- 151.Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 152.Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74:602–607. doi: 10.1016/j.steroids.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 153.Barker CF, Billingham RE. immunologically privileged sites. Adv. Immunol. 1977;25:1–54. [PubMed] [Google Scholar]