Abstract
Allergic asthma is a chronic inflammatory disorder of the airway associated with bronchial obstruction, airway hyper-reactivity (AHR), and mucus production. The epithelium may direct and propagate asthmatic-like responses. Central to this theory is the observation that viruses, air pollution, and allergens promote epithelial damage and trigger the generation of IL-25, IL-33, and TSLP via innate pathways such as TLRs and purinergic receptors. Similarly, engineered nanomaterials promote a Th2-associated pathophysiology. In this study, we tested the hypothesis that instillation of multi-walled carbon nanotubes (MWCNT) impair pulmonary function in C57Bl/6 mice due to the development of IL-33-dependent Th2-associated inflammation. MWCNT exposure resulted in elevated levels of IL-33 in the lavage fluid (likely originating from airway epithelial cells), enhanced AHR, eosinophil recruitment, and production of Th2-associated cytokines and chemokines. Moreover, these events were dependent on IL-13 signaling and the IL-33/ST2 axis, but independent of T and B cells. Finally, MWCNT exposure resulted in the recruitment of innate lymphoid cells. Collectively, our data suggest that MWCNT induce epithelial damage that results in release of IL-33, which in turn promotes innate lymphoid cell recruitment and the development of IL-13-dependent inflammatory response.
Keywords: Nanotoxicology, nanotubes, mechanistic toxicology
Introduction
Asthma is a major public health concern that affects ~10% of the general population within the United States and ~300 million people worldwide. Allergic asthma is recognized as a chronic inflammatory disorder of the airway associated with varying degrees of bronchial obstruction, the development of airway hyper-responsiveness (AHR), and increased mucus production. Asthma has classically been viewed as a Th2-mediated disease with therapeutic interventions directed toward blocking Th2 cytokines and preventing eosinophil recruitment (Wegmann 2011). However, this view is rapidly changing due to intensive research efforts, accumulating evidence indicating that distinct clinical phenotypes of asthma exist, and the general failure of therapies targeting the Th2 response (Lloyd 2010; Lloyd & Saglani 2010).
Evidence is accumulating that the respiratory epithelium plays a vital role in directing and propagating mucosal immune responses (Lambrecht & Hammad 2012). The pulmonary epithelium is prone to damage from pathogens such as viruses, air pollution, and also the protease activity of allergens. Central to this theory is the observation that these molecules promote epithelial damage and trigger the generation of IL-25, IL-33, and TSLP via innate signals (Hammad et al. 2009; Kouzaki et al. 2011). This group of innate cytokines then exert profound effects on a variety of cell types to promote the Th2-associated immune response and are intimately involved in the development/maintenance of allergic inflammation, as well as host defense against helminth infections (Oboki et al. 2011).
Of these cytokines, IL-33 is constitutively expressed in epithelial cells of the bronchus and small airways in both mouse and humans (Schmitz et al. 2005). IL-33 and its receptor (ST2/IL-1R4) are part of the IL-1 cytokine family, and their interactions promote a variety of actions from numerous cell types. The IL-33/ST2 signaling axis is unique amongst the IL-1 family members in that it is associated with the promotion of predominantly Th2, and not Th1, responses and is intimately involved in the promotion/maintenance of allergic or Th2-associated inflammation, as well as host defense against helminth infections (Oboki et al. 2011). IL-33 may be released by damaged cells during infection or trauma—suggesting it functions as an alarmin (Moussion et al. 2008). Pathogens, allergens, and other environmental agents may also trigger tissue damage that results in IL-33 secretion (Wang et al. 2011; Yamaguchi et al. 2012; Bartemes et al. 2012; Williams et al. 2012; Barlow et al. 2012). Moreover, IL-33 expression in bronchial asthma correlates with disease severity (Prefontaine et al. 2010). When administered directly to the mouse lung, IL-33 induces eosinophilic inflammation and airway hyper-reactivity (AHR) (Kondo et al. 2008; Kurowska-Stolarska et al. 2009). Consistent with this, administration of neutralizing antibodies against ST2 or IL-33 at the time of airway challenge attenuates eosinophilic inflammation and AHR (Lohning et al. 1998; Coyle et al. 1999; Liu et al. 2009). These data indicate that IL-33 has an important role during the development or exacerbation of certain types of Th2-associated airway inflammation.
Engineered nanomaterials (ENM) are anticipated for use in electronics, cosmetics, cleaning materials, coatings, food packaging, and medicines, with ever-increasing and inevitable human exposure. Many consumer products containing ENM will end up as waste, and subsequent degradation may liberate particles into the environment where they may accumulate (Helland et al. 2007). As of yet, little quantitative data on consumer exposure exist; however, as production volumes continue to increase and use becomes commonplace, human exposure will follow suit. Therefore, the potential adverse health effects of ENM are a major concern (Bonner 2010). In particular, pulmonary toxicity remains a foremost health concern as ENM are very small (1–100 nm), and when airborne may be deposited within the alveolar regions (Heyder 2004; Guthrie 1997; Fubini et al. 1999). Indeed, pulmonary exposure to ENM results in oxidative stress, inflammation, granuloma formation, and fibrosis (Pacurari et al. 2010). Likewise, previous studies demonstrated that ENM enhance several hallmarks of Th2-associated inflammation (Inoue et al. 2009; Inoue et al. 2005; Inoue et al. 2006a; Inoue et al. 2006b; Inoue et al. 2007; Inoue et al. 2010; Ryman-Rasmussen et al. 2009), suggesting that ENM may promote an allergic-like pathophysiology. Similarly, recent studies demonstrated altered pulmonary function in response to various forms of ENM (North et al. 2011; Wang et al. 2011). Finally, studies of iron-associated ENM showed IL-33 gene and protein expression were increased relative to vehicle control (Wang et al. 2011; Inoue et al. 2009; Yamaguchi et al. 2012). Owing to its capacity to activate cells of both the innate and adaptive immune system, IL-33 may be an important cytokine in the initiation and perpetuation of nanomaterial-induced inflammation and asthma-like pathophysiology. In the present study, we tested the hypothesis that instillation of multi-walled carbon nanotubes (MWCNT) impairs pulmonary function in C57Bl/6 mice due to the development of IL-33-dependent Th2-associated inflammation. We examined the effects of MWCNT on airway hyper-reactivity (AHR) and acute pulmonary inflammation in vivo and the underlying cellular and molecular mechanisms relative to IL-33/ST2 signaling.
Materials and methods
Mice
Breeding pairs of C57Bl/6 (C57BL/6J, stock #000664) and Rag1−/− (B6.129S7-Rag1tm1Mom/J, stock #002216) mice were originally purchased from The Jackson Laboratory (Bar Harbor, ME). IL-13−/− mice were obtained from Dr. Jack Elias (Yale University School of Medicine, New Haven, CT), who in turn received them from Dr. Andrew McKenzie at the Medical Research Council at Cambridge. IL-33−/− mice were obtained from Dirk Smith (Department of Inflammation Research, Amgen, Seattle, WA). All mice were bred and maintained in the University of Montana SPF facility and used at 6–8 weeks of age. All animal use procedures were in accordance with NIH and University of Montana IACUC guidelines.
Experimental instillations
Previously described FA21 multi-walled carbon nanotubes (MWCNT) (manuscripts in preparation, supplemental Table 1) were determined to be free of endotoxin contamination (data not shown). Mice were anesthetized with 4% isoflurane and instilled via the oropharyngeal exposure route to 25 µl dispersion medium containing 0.6 mg/ml mouse serum albumin and 0.01 mg/ml 1,2-dipalmitoyl-snglycero-3-phosphocholine in sterile saline (vehicle) or 50 µg MWCNT suspended in 25 µl of vehicle to minimize agglomeration. Other mice were anesthetized and instilled with 500 ng recombinant mouse IL-33 (Biolegend, San Diego, CA). For ST2-blocking studies, mice were i) instilled with 2 µg anti-mouse ST2/IL-1R4 antibody or 2 µg normal goat IgG isotype control antibody (R&D Systems, Minneapolis, MN), or ii) injected i.p. with 200 µg anti-mouse ST2 blocking antibody or 200 µg rat IgG1k isotype control antibody (Amgen, Thousand Oaks, CA) 18 h prior to MWCNT instillation.
Pulmonary function assessments
Transpulmonary resistance (RL) and dynamic compliance (Cdyn) were directly measured by pulmonary mechanics on sedated, ventilated subjects as previously described (Wells et al. 2008; Beamer et al. 2010; Beamer et al. 2012). Mice were challenged with vehicle, followed by increasing concentrations of methacholine (1.5, 3, 6, 12, and 24 mg/ml). Aerosols were generated with an ultrasonic nebulizer (Aeroneb Laboratory Nebulizer; Buxco Electronics, Inc., Troy, NY). A computer program (FinePointe; Buxco Electronics) was used to calculate RL and Cdyn.
Assessment of pulmonary inflammation
Mice were euthanized and whole lung lavages (WLL) collected 24 h following the MWCNT instillation. WLL was performed by cannulating the trachea and infusing the lungs with 1 ml sterile 0.5 mM EDTA/PBS. The acellular lavage fluid was collected by centrifugation and frozen at –20°C until further analysis. Total WLL cells were enumerated using a Coulter Counter (Beckman Coulter, Brea, CA), prepared for cytospin slides, and stained with Wright’s Giemsa using a Hematek 2000 autostainer (Miles-Bayer-Siemens Diagnostics, Deerfield, IL). Differential counts (percent and absolute cell number) were determined by two to three independent readers.
Cytokine/chemokine ELISAs
Eotaxin, IL-5, IL-6, and IL-33 were measured in clarified WLL using murine ELISA kits according to the manufacturer’s instructions and assay procedure (RnD Systems). Color development was assessed at 450 nm on a plate reader.
Flow cytometry
ILCs were identified as lineage−CD45+ICOS+IL-7Ra+Sca1+ cells within the WLL of MWCNT-exposed mice (Neill et al. 2010; Barlow & McKenzie 2011; Chang et al. 2011). Cells from five to eight mice were pooled together for analysis, and repeated three times. Monoclonal Abs specific to F4-80 (FITC, clone #BM8)), CD3 (FITC, clone #17A2, catalog #100204), CD4 (FITC, clone #RM4-5, catalog #100510), CD8 (FITC, clone #53-6.7, catalog #100706), CD11b (FITC, clone #M1/70, catalog #101206), CD11c (FITC, clone # N418)), CD19 (FITC, clone #MB19-1, catalog #101506), Gr-1 (FITC, clone #RB6-8C5, clone #108406), TER119 (FITC, clone #TER-119, catalog #116206), FcεR1 (FITC, clone #MAR-1, catalog #134306), CD45 (PE, clone #30-F11, catalog #103106), ICOS (AlexaFluor647, clone #C398.4A, catalog #313516), IL-7Ra (Brilliant Violet 421, clone #A7R34, catalog #135023), and Sca-1 (PerCpCy5.5, clone #D7, catalog #108124) were purchased from Biolegend. Whole lung lavage cells were washed in PBS, stained with Aqua LIVE/DEAD® fixable dead cell stain according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and re-suspended in 50 µl of purified rat anti-mouse CD16/CD32 diluted 1:100 in PBS with 1% bovine serum albumin and 0.1% sodium azide (PAB) for 15 min on ice to block nonspecific Ab binding. 0.125 to 2.0 µg of each Ab was added per 106 total cells and allowed to incubate for 30 min in the dark at room temperature. Finally, cells were washed twice with PBS and re-suspended in 0.3 ml PAB. Cell acquisition (1 × 106 events per sample) analysis was performed on a FACS Aria flow cytometer using FACS Diva software (version 4.1.2, Becton Dickinson). Compensation of the spectral overlap for each fluorochrome was completed by gating positive vs. negative populations using anti-rat/hamster Ig compensation beads or single-stained cell controls (BD Biosciences, San Diego, CA).
Cell lines and culture conditions
Immortalized C10 mouse lung type II alveolar epithelial cells, originally derived from cultured primary BALB/c mouse lung, were obtained from Dr. Galya Orr (Pacific Northwest National Laboratories, Richland, WA). C10 cells were maintained in RPMI medium containing L-glutamine and 25 mM HEPES (Mediatech, Manassas, VA) supplemented with 10% FBS (PAA Laboratories, Dartmouth, MA), 1% penicillin/streptomycin, and 1 mM sodium pyruvate (Mediatech). Stages of cell culture were estimated visually and cells passed every 3–4 days. Cells were removed from the plates by trypsinization for 5–10 min at 37°C. For experiments, cells were seeded 3 h prior to treatment to allow adherence and recovery. Cells were treated with MWCNT (25–200 µg/ml) for 4 h and the acellular tissue culture supernatants collected for ELISA.
Histopathological evaluation of IL-33 expression
Mice were euthanized 24 h following the MWCNT instillation and the lungs inflated with 1 ml of 10% neutral buffered formalin (VWR International, Radnor, PA), subsequently immersed in formalin for 24 h, dehydrated through graded alcohols and then embedded in paraffin. Tissues were sectioned at 5 µm and antigen retrieval accomplished by heating the slides in a steamer with retrieval buffer (Diva Decloaker, Biocare Medical, Concord, CA) for 60 min. After washing in dilute Tris buffer, slides were blocked for nonspecific protein binding (Sniper Block, Biocare Medical, Concord, CA) and nonspecific avidin and biotin binding (Avidin–Biotin kit, Biocare Medical) for 15 min each. Biotinylated anti-mouse IL-33 (ENZO, Farmingdale, NY) or mIgG1 isotype control (Cedarlane, Burlington, Ontario, Canada) antibodies in diluent (Da Vinci Green; Biocare Medical) were subsequently added to the sections and incubated overnight in a humidified chamber at 4°C. After washing in dilute Tris buffer, endogenous peroxidase activity was blocked for 10 min (Peroxidazed-1, Biocare Medical), sections were washed in dilute Tris buffer and incubated with streptavidin/horseradish peroxidase complexes (Vectastain ABC kit, Vector labs, Burlingame, CA) at room temperature for 30 min. After washing in dilute Tris buffer, slides were developed with DAB for 5 min (Cardassian DAB, Biocare Medical), washed in dilute Tris buffer, counterstained with hematoxylin and coverslipped. Alternate tissue sections were stained for the Periodic acid-Schiff (PAS), however, we were unable to detect any appreciable increase in the presence of mucus within the epithelial lining (data not shown).
RNA isolation/PCR array analysis
The Mouse Allergy & Asthma RT2 Profiler PCR Array (Qiagen, Valencia, CA) outlines the expression of 84 key genes central to allergic responses. Total RNA was isolated from snap frozen lungs using the RNeasy Mini Kit (Qiagen). RNA samples were quantified using the NanoDrop and quality was assessed using Agilent RNA 6000 Nano Kit (Agilent, Santa Clara, CA). Reverse transcription was performed (RT2 First Strand Kit, Qiagen), and all samples were run on the PCR array (PAMM-067, Qiagen). The raw CT data were imported into the Web-based PCR Array Data Analysis Software (http://pcrdataanalysis.sabiosciences. com/pcr/arrayanalysis.php). The data were normalized to housekeeping genes on the array and the CT cutoff was 35 for all genes. All treated groups were compared to the control group to obtain lists of dysregulated genes and the fold changes. The scatter plot was used to compare the normalized expression of every gene on the array between two groups (control vs. MWCNT and MWCNT vs. αST-2 mAb + MWCNT) by plotting them against one another to quickly visualize large gene expression changes. The boundary (fold regulation cut-off) was set to 3.
Statistical analysis
For each parameter, the values for individual mice were averaged and the standard deviation and standard error calculated. The significance of the differences between the exposure groups was determined by t-test, one-way, or two-way ANOVA, in conjunction with Tukey’s test for variance, where appropriate. All ANOVA models were performed with Prism software, version 4. A p value of < 0.05 was considered significant.
Results
MWCNTs induce secretion of IL-33 by epithelial cells
IL-33 is a key player in the initiation and exacerbation of inflammation acting as a bridge between innate and adaptive immune responses (Kim et al. 2012). Furthermore, studies with epithelial cells revealed that IL-33 plays an important role in inducing Th2-associated responses against allergens and helminth infections (Eiwegger & Akdis 2011; Lloyd 2010). To investigate the potential mechanisms involved in FA21 MWCNT-induced airway inflammation, we first assessed protein levels of IL-33 in the WLL fluid of vehicle and MWCNT-exposed mice. Twenty-four hours post-instillation, FA21 MWCNT-exposed C57Bl/6 mice exhibited increased levels of IL-33 protein present in the WLL compared with vehicle control (Figure 1A). In addition, C10 mouse alveolar type II epithelial cells showed a dose-dependent increase in total IL-33 protein and LDH in response to FA21 MWCNT in vitro (Figure 1B). Microscopic examination of lungs from mice exposed to MWCNT revealed that much of the material was localized to small airways at the level of the respiratory bronchiole and alveolar duct. The majority of the material had been phagocytosed by alveolar macrophages, but rare, clumps or individual fibers were present in these airways and adjacent alveolar spaces in the region. The pulmonary interstitium surrounding the small airways containing MWCNT material was expanded by eosinophils, neutrophils and lesser numbers of monocyte/macrophages. Interestingly, immunohistochemistry demonstrated robust intranuclear IL-33 protein expression by epithelial cells phenotypically consistent with type II pneumocytes (Figure 1C, green arrows) located in the vicinity of alveolar macrophages that have phagocytosed MWCNTs (Figure 1C, red arrows) or MWCNT free in the alveolar space (Figure 1C, blue arrows), but not in similarsized airways elsewhere in the lung that were devoid of MWCNTs. Isotype controls showed no immunoreactive cells within lung sections from either vehicle or FA21 MWCNT-exposed mice (data not shown). Together, these results indicate that alveolar type II epithelial cells within and adjacent to airways containing MWCNTs are a likely source of IL-33 following MWCNT exposure.
Figure 1. Elevated IL-33 expression following MWCNT exposure.
IL-33 expression was increased in the whole lung lavage fluid of MWCNT-exposed C57Bl/6 mice (A), the tissue cultures supernatants of C10 type II epithelial cells following MWCNT exposure (B), and by type II pneumocytes (inset, green arrows) in C57Bl/6 mouse lung sections after MWCNT exposure (C) 24 h following exposure. MWCNT material was localized to scattered small airways at the level of the respiratory bronchiole and alveolar duct and the majority of the material had been phagocytosed by alveolar macrophages. The pulmonary interstitium surrounding the small airways containing MWCNT material was expanded by eosinophils, neutrophils, and lesser numbers of monocyte/macrophages. Immunohistochemistry revealed IL-33+ type II pneumocytes located in the vicinity of alveolar macrophages that have phagocytosed MWCNTs (inset, red arrows) or MWCNT free in the alveolar space (inset, blue arrows), but not in regions of the lung devoid of MWCNT material. Isotype controls from vehicle and MWCNT-exposed mice were negative for IL-33 staining (data not shown). Magnification 200× and 1000× (inset). n = 4–10, values are means ± SEM; *p < 0.05 compared to vehicle control.
MWCNTs provoke IL-33-dependent airway hyper-reactivity and inflammation
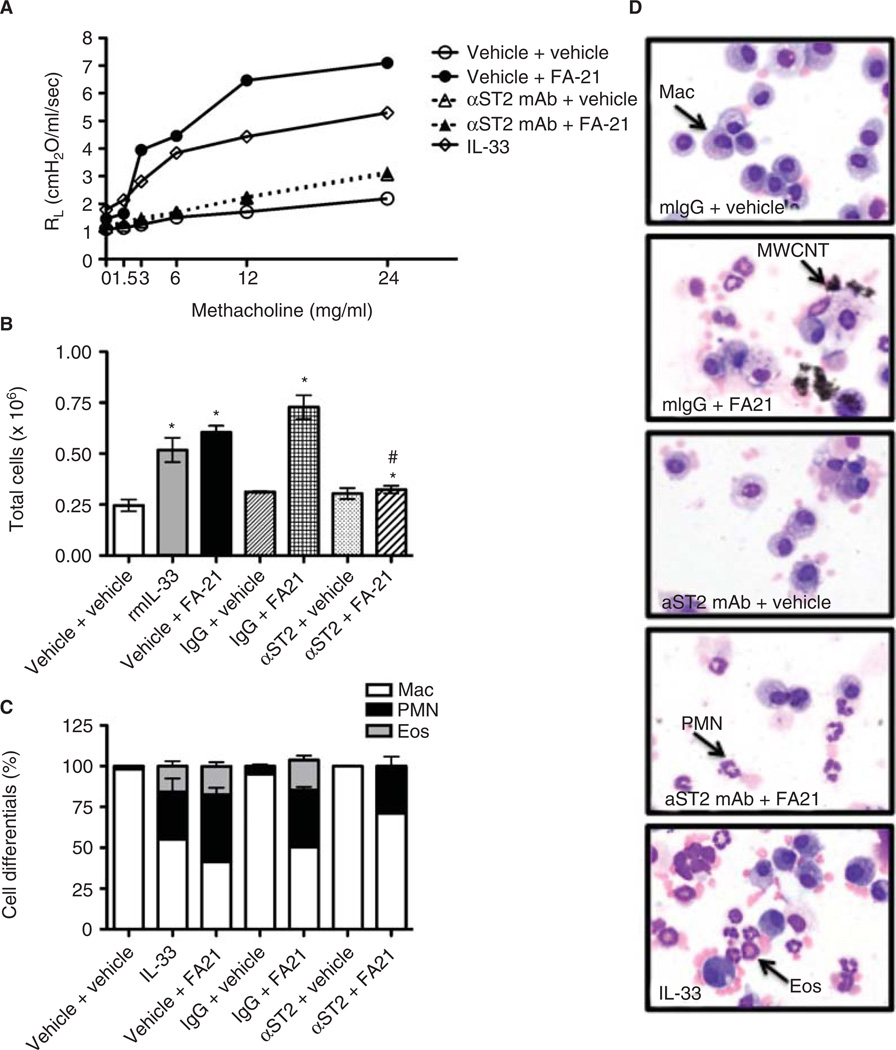
To investigate the association between MWCNT-induced inflammation and pulmonary function in C57Bl/6 mice and assess whether these responses are dependent on IL-33, airway responsiveness to inhaled methacholine was assessed via transpulmonary resistance (RL) and dynamic compliance (Cdyn) 24 h after exposure. Together, these parameters quantify changes in lung capacity and compliance in disease models (Glaab et al. 2007; Vanoirbeek et al. 2009). As anticipated, methacholine challenge resulted in dose-dependent increases in RL and decreases in Cdyn, with FA21 MWCNT exposure significantly affecting both measures (Figure 2, Cdyn data not shown). These results were largely recapitulated with the instillation of 500 ng recombinant mouse IL-33, and inhibited by 18 h pretreatment with 200 µg monoclonal αST2-blocking antibody (Figure 2). No effects on RL or CDyn were observed in response to pre-treatment with control antibody (data not shown). Area under the curve (AUC) analysis showed that RL increased from 7.87 ± 0.6 (vehicle) to 64.45 ± 10.3 (IL-33) or 97.96 ± 15.1 (FA-21). Pre-treatment with αST2 blocking antibody resulted in a significant reduction in the RL that was caused by MWCNT (AUC = 18.68 ± 2.4) (Figure 2A). Similar results were observed with localized delivery of a commercially available αST2 polyclonal and control antibody (data not shown).
Figure 2. Inflammation and airway hyper-reactivity in MWCNT-exposed C57Bl/6 mice is IL-33 dependent.
Acute MWCNT exposure increased airway hyper-reactivity (AHR) (A), the total number of lavageable cells (B), and neutrophil and eosinophil recruitment to the airways (C) in a manner similar to the addition of exogenous recombinant murine IL-33. Inhibiting the IL-33-ST2 signaling axis with systemic delivery of a monoclonal anti- ST2 blocking antibody completely ablated the observed increase in AHR, cellularity, and eosinophilia, but not neutrophilia. Representative images of cell differentials reflect the quantitative data and show the presence of free and alveolar macrophage-associated MWCNT, as well as recruitment of neutrophils and eosinophils (D). Magnification 1000×. n = 5–6, values are means ± SEM; *p < 0.05 compared to vehicle.
To determine the inflammatory effects of FA21 MWCNT exposure that may be associated with the observed changes in pulmonary function, whole lung lavage differential analysis was performed. FA21 MWCNT exposure results in acute inflammation as measured by increases in the total number of lavage cells recovered (Figure 2B) and the increased presence of neutrophils (PMN) and eosinophils (Eos) (Figure 2C). Exposure to rmIL-33 resulted in a slight elevation in the number of PMN and Eos, whereas pretreatment with αST2-blocking antibody prior to MWCNT exposure resulted in significantly reduced numbers of total cells recovered (Figure 2B), and Eos recruitment (Figure 2C). This effect appeared to be specific to Eos recruitment, because the absolute number of PMN infiltrating the airways did not change. No effects were observed in response to pre-treatment with control antibody (Figure 2B and C). Representative images of Wright Giemsa-stained lavage cells support these quantitative data (Figure 2D).
To assess the potential mechanisms involved in FA21 MWCNT-induced changes in pulmonary function and inflammation, we examined the protein levels of three cytokines/chemokines that are of importance to the pathophysiology of allergic asthma. Twenty-four hours after exposure, FA21 MWCNT and rmIL-33 result in elevated levels of eotaxin, IL-5, and IL-6. While pre-treatment with anti-mouse ST2/IL-1R4 blocking antibody attenuated the induction of eotaxin and IL-5, it appeared to marginally enhance the secretion of IL-6 in response to FA21 MWCNT. Treatment with anti-mouse ST2/IL-1R4 blocking antibody alone did not appear to affect levels of eotaxin, IL-5, and IL-6 compared with vehicle control (Figure 3). No changes were observed in response to 18 h pre-treatment with Ig control antibody (data not shown).
Figure 3. Expression of cytokines and chemokines in MWCNT-exposed C57Bl/6 mice.
Levels of IL-5 (A), IL-6 (B), and Eotaxin (C) were increased in the WLL fluid after MWCNT exposure in C57Bl/6 mice. The increased expression of IL-5 and Eotaxin was attenuated by pre-treatment anti- ST2 polyclonal antibody, whereas the expression of IL-6 was augmented. n = 5–8, values are means ± SEM; *p < 0.05 compared to vehicle control, # p < 0.05 compared to MWCNT alone.
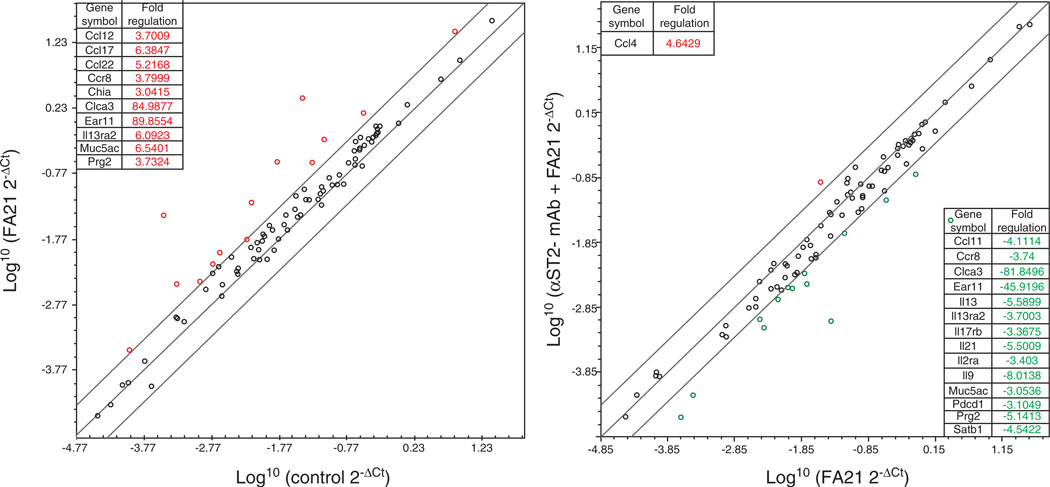
Effects on the expression of candidate genes relevant to FA21 MWCNT exposure and downstream of the IL-33/ST2 signaling axis were screened using a RT2 Profiler Allergy and Asthma Mouse PCR Array. Of the 84 mRNA transcripts represented, the largest changes following exposure to FA21 (relative to control) were noted in the expression of Clca3 (~85-fold increase) and Ear11 (~90-fold increase), and in the expression of a select number of other genes using a threefold cutoff (Figure 4). Pre-treatment with anti-mouse ST2/IL-1R4-blocking antibody inhibited the increase in Clca3 and Ear11 mRNA expression, and reduced the expression of select number of other mRNAs (Figure 4). Interestingly, only Ccl4 mRNA was upregulated following pre-treatment with αST2/IL-1R4-blocking antibody (Figure 4). Finally, no differences were observed between the untreated and vehicle control mice confirming a lack of response to the vehicle (data not shown). Collectively, Figures 2, 3, 4 suggest that MWCNTs provoke an IL-33/ST2 Th2-associated signaling axis, particularly via Clca3 and Ear11, which is prominently involved in FA21 MWCNT-induced AHR and Th2-associated inflammation.
Figure 4. Scatter plots of mouse lung gene expression in response to FA21 MWCNT exposure in the presence and absence of anti-ST2-blocking antibody.
For each experiment (untreated, vehicle, FA21 MWCNT, or pre-treatment with anti-ST2-blocking Ab plus FA21 MWCNT), six mice were exposed and the resulting total lung RNA extracted. The four best quality RNA samples were then submitted to a mouse Allergy and Asthma PCR array. The scatter plots show the comparison of log2 fold change between the biological replicates listing of up- and down-regulated genes and their respective fold induction.
MWCNTs elicit recruitment of innate lymphoid cells into the airways
IL-33 and IL-13 are associated with the promotion of Th2 responses and allergic-like inflammation through their actions on both lymphocytes and innate cells, including alternatively activated macrophages, mast cells, and eosinophils (Lloyd 2010; Eiwegger & Akdis 2011; Barlow & McKenzie 2011; Koyasu & Moro 2011). To evaluate the contribution of IL-33 and IL-13, as well as lymphocytes to MWCNT-induced AHR, we assessed lung function in IL-33−/−, IL-13−/−, and Rag1−/− mice. As expected, methacholine challenge in C57Bl/6 wild-type mice resulted in dose-dependent increases in RL and decreases in Cdyn, with FA21 MWCNT pre-exposure exerting significant effects on both measures. These results were not dependent on the presence of T and B cells since Rag1−/− mice showed similar dose-dependent increases in RL and decreases in Cdyn. However, MWCNT-induced AHR was dependent on IL-13 and IL-33, as it was reduced in both IL-13−/− and IL-33−/− mice (Figure 5). Subsequently, we assessed MWCNT-induced airway inflammation in Rag1−/−, IL-13−/−, and IL-33−/− mice. As anticipated, FA21 MWCNT exposure in C57Bl/6 wild-type mice resulted in increases in the total number of lavage cells recovered (Figure 6A) and the recruitment of PMNs and Eos (Figure 6B). Similar results were observed in Rag1−/− mice exposed to FA21 MWCNT; however, in contrast, in the absence of IL-13 or IL-33, PMNs, but not Eos were recruited to the airways. Instillation of rmIL-33 in C57Bl/6 wild-type or Rag1−/− mice resulted in increases in the total number of lavage cells recovered and the recruitment of PMNs and Eos, indicating IL-33 can promote lymphocyte-independent airway inflammation. Furthermore, the ability of IL-33 to induce granulocyte infiltration was reduced in the IL-13−/− mice. Taken together, these data suggest that lymphocytes are not an integral component of the AHR observed following acute MWCNT exposure, but that IL-33 and IL-13 signaling is critical. Furthermore, these data suggest that an innate cell type might be responsible for the production of IL-13 and the enhanced AHR observed in response to MWCNT exposure.
Figure 5. MWCNT-induced airway hyper-reactivity occurs independently of T and B cells, but is dependent on IL-33 and IL-13.
As anticipated, exposure to either rmIL-33 or MWCNT resulted in increased RL and decreased Cdyn 24 h after instillation in C57Bl/6 mice. Similar results were observed in Rag1−/− mice; whereas these responses were abolished in IL-33−/− and IL-13−/− mice. Values for RL and Cdyn were obtained in vehicle (solid line, open circles), rmIL-33 (solid line, diamonds) and MWCNT-exposed mice (dashed line, stars) in response to increasing concentrations of methacholine. n = 4–6 mice/group; repeated 2–3 times, values are means.
Figure 6. Cellular response to MWCNT exposure in Rag1−/−, IL-13−/−, IL-33−/−, and C57Bl/6 mice.
Exposure to either rmIL-33 or MWCNT resulted in elevated number of cells recovered via WLL 24 h after instillation in all strains of mice (A). This response was exaggerated in IL-33−/− mice and appeared to be largely due to a massive influx of neutrophils (B). Cell differential analysis revealed the recruitment of neutrophils and eosinophils in response to either rmIL-33 or MWCNT in Rag1−/− and C57Bl/6 mice. In contrast, neutrophil but not eosinophil recruitment was observed in IL-13−/− and IL-33−/− mice. n = 4–6 mice/group; repeated 2–3 times, values are means ± SEM; *p < 0.05 compared with vehicle control.
Recent studies identified a previously unrecognized innate cell population(s) capable of producing large amounts of IL-5 and IL-13 in response to IL-33, which plays a significant role in mediating AHR and immune responses independent of adaptive immunity (Kim et al. 2012; Moro et al. 2010; Neill et al. 2010; Saenz et al. 2010; Price et al. 2010). These innate lymphoid cells (ILCs) are identified as lineage−CD45+ICOS+IL-7Ra+Sca1+ cells (Neill et al. 2010; Barlow & McKenzie 2011; Chang et al. 2011; Kim et al. 2012). We identified these ILCs, or nuocytes, in C57Bl/6 and Rag1−/− mice as lineage negative (F4-80, CD3, CD4, CD8, CD11b, CD11c, CD19, Gr-1, TER119, FcεR1), CD45+, Sca-1+, IL-7Ra+, and ICOS+ (Figure 7A and B). Flow cytometric analysis showed that although nuocytes represent ~1% of the total number of cells in the whole lung lavage, their numbers are significantly increased 24 h following FA21 MWCNT exposure in both C57Bl/6 and Rag1−/− mice (Figure 7C). Taken together, our results suggest that MWCNT activate epithelial cells to produce IL-33, which in turn recruits and stimulates ILCs to produce IL-13, a known inducer of AHR (Kim et al. 2012; Wills-Karp et al. 1998; Padilla et al. 2005; Grunig et al. 1998).
Figure 7. Innate lymphoid cells are recruited to the airways in response to MWCNT exposure.
WLL were obtained from vehicle or MWCNT-exposed mice and innate lymphoid cells gated from CD45+ live cells, and then assessed based on the expression of Sca-1 on lineage negative cells in C57Bl/6 (A) and Rag1−/− (B) mice. The graph represents the number of Lineage−Sca-1+ natural helper cells in the airways (C). n = 5–10 mice/group; repeated two times, values are means ± SEM; *p < 0.05 compared with vehicle control.
Discussion
Recent advances in Nanoscience have created a multitude of innovative ENM with wide-ranging applications. Because we cannot predict how ENM will interact with biological systems, they represent an emerging risk for both environmental and occupational lung diseases. The diversity of ENM being produced includes those that are carbon-based, metal-based, and those of a biological nature (Bonner 2010). Recent studies indicated that carbon nanotubes (CNTs), when delivered to the lungs of mice and rats, result in fibrosis, changes in immune function, and exacerbation of allergic responses in both the presence and absence of allergen (Wang et al. 2011; Aschberger et al. 2010; Inoue et al. 2009; Inoue et al. 2010; Porter et al. 2010; Park et al. 2009). Collectively, these studies suggest that individuals with allergic asthma may be very susceptible to immune activation and airway remodeling in response to CNTs exposure, and that CNTs alone may trigger Th2-associated immune responses in the airways. Although a vast array of unique carbon derivatives (e.g. nanotubes, nanowires, fullerenes) fall under the category of “carbon nanotubes,” this study focused on understanding the mechanisms responsible for Th2-associated immune responses in the respiratory tract. In response to a single oropharyngeal exposure to FA21 MWCNT in the absence of allergen sensitization, we observed a Th2-associated inflammatory response characterized by enhanced airway hyper-reactivity (AHR), increased cytokine/chemokine production (e.g. IL-33, IL-5, IL-6, and eotaxin), and recruitment of inflammatory cells (e.g. neutrophils and eosinophils). These responses were not dependent on the presence of T or B lymphocytes, but were dependent on the IL-33/ST2 signaling axis and IL-13. Finally, ILCs or nuocytes were recruited to the airways in response to MWCNT. Taken together, these results support the notion that ILCs, acting in response to IL-33 stimulate AHR and eosinophil recruitment through the release of IL-13 following exposure to MWCNT (Figure 8).
Figure 8. Schematic of the IL-33–ST2 axis in the development of MWCNT-induced AHR.
Upon activation by MWCNT, type II pneumocytes produce IL-33, which in turn activates innate lymphoid cells to produce IL-13, resulting in eosinophil recruitment and development of AHR.
Previous studies of iron (Fe)-associated MWCNT showed IL-33 gene and protein expression were increased relative to vehicle control (Wang et al. 2011; Inoue et al. 2009). Our results demonstrate that acute exposure (e.g. 24 h) to a single instillation of 50 µg FA21 MWCNT caused marked local IL-33 production and release into the airways. Using sub-confluent C10 mouse lung type II alveolar epithelial cells, we showed dose-dependent increases in IL-33 release and decreases in cell viability in response to FA21 MWCNT 4 h post-treatment. Although other groups have shown that IL-33 is constitutively expressed in epithelial cells of the bronchus and small airways in both mouse and humans (Schmitz et al. 2005), we observed very low to non-existent numbers of IL-33 positive cells in naïve C57Bl/6 mice—which may be due to the limits of detection. Similarly, other groups have shown that IL-33 is expressed in epithelial cells in bronchial asthma and correlates with disease severity (Prefontaine et al. 2010; Kim et al. 2012; Barlow et al. 2012). Nonetheless, the conditions that result in IL-33 secretion in the lung remain unclear, although IL-33 is produced by airway epithelial cells activated by TLR4 ligation by dust mite allergens, influenza A infection and alternaria exposure (Chang et al. 2011; Hammad et al. 2009; Kouzaki et al. 2011). Together, our data suggest that type II pneumocytes are the likely source of IL-33 in the airways of mice in response to MWCNT exposure.
IL-33 is the only known ligand for ST2, which is expressed on the cell surface of mast cells, Th2 cells, basophils, eosinophils, dendritic cells, and invariant NKT cells (Kurokawa et al. 2011; Oboki et al. 2011; Lloyd 2010; Smith 2010). IL-33 signals by interacting with the ST2/IL-1R accessory protein (IL-1RAcP) receptor complex resulting in NF-κB and MARK activation (Chackerian et al. 2007; Funakoshi-Tago et al. 2008). Because the IL-33/ST2 signaling axis is associated with mucosal Th2 responses, allergic airway inflammation, and inflammatory cytokine/chemokine production, we addressed the contributions of this pathway to MWCNT-induced changes in lung capacity and pulmonary function via RL and CDyn measures, differential analysis of the cellular infiltrate, and cytokine/chemokine analysis. Together, these parameters quantify changes in lung capacity and compliance in disease models (Glaab et al. 2007; Vanoirbeek et al. 2009). In C57Bl/6 mice, as anticipated, administration of IL-33 lead to enhanced AHR following methacholine challenge, inflammation accompanied by eosinophils, and elevated levels of eotaxin, IL-5, and IL-6 expression relative to vehicle control mice (Kondo et al. 2008; Coyle et al. 1999). In the absence of allergen, FA21 MWCNT exposure resulted in an exaggerated RL and Cdyn, a mixed inflammatory response (neutrophil to eosinophil ratio = 2.3: 1), and elevated levels of eotaxin, IL-5, and IL-6 relative to either IL-33 or vehicle control. Airway hyper-reactivity, eosinophil but not neutrophil inflammation, and levels of eotaxin and IL-5, but not IL-6, were reduced by pre-treatment with monoclonal and polyclonal anti-ST2-blocking antibodies. These results are consistent with previous reports showing development of AHR and changes in lung function in response to environmental triggers such as allergens (Cohn et al. 1998; Corry et al. 1998; Gavett et al. 1994), airborne oxidants (Chen et al. 1995), and irritants (Saunders et al. 2010; Garssen et al. 1990; Matheson et al. 2001). Likewise, these results confirm previous reports showing reductions in airway inflammation in mice treated with anti-ST2 monoclonal and anti- IL-33 polyclonal antibodies (Meisel et al. 2001; Coyle et al. 1999; Liu et al. 2009). Interestingly, although ST2-deficient mice showed normal respiratory functions, eosinophil recruitment and IgE levels, IL-33−/− mice showed attenuated eosinophil influx and reduced inflammation, yet similar levels of IL-5 and IgE to wild-type controls in response to allergens (Oboki et al. 2011; Oboki et al. 2010). The discrepancy between studies using deficient mice and the studies using mice treated with blocking antibodies remains unclear. However, collectively, our data show reduced airway AHR, reduced eosinophilic inflammation, and reduced levels of eotaxin and IL-5 in response to FA21 MWCNT following neutralization of IL-33/ST2 signaling. A focus of our study was to determine those genes that are responsible for the relationship between MWCNT exposure, IL-33/ST2 signaling, and Th2-associated inflammation. Those genes (particularly Ear11 and Clca3) that were regulated by both MWCNT and αST2-blocking Ab experimental conditions were considered of particular interest. Indeed, although the exact mechanisms remain unknown, Ear11 and Clca3 have previously been implicated in asthma pathology (Di Valentin et al. 2009; Zhou et al. 2001; Kondo et al. 2012) and may represent suitable therapeutic targets to mediate Th2-associated inflammation. Experiments are currently underway in our laboratories to better understand the contributions of Ear11 and Clca3 expression to the IL-33/ST2 signaling axis and their importance to the establishment of Th2-associated inflammation following MWCNT exposure. Together, these data suggest that during MWCNT-induced injury to the respiratory tract, upregulation of IL-33 by epithelial cells may significantly contribute to Th2-associated pathophysiological changes.
In summary, this is the first report that identifies an immunological pathway for MWCNT-induced AHR that occurs independently of Th2 cells, but that is dependent on ILCs and IL-13. This pathway is also dependent on the IL-33 receptor ST2 and on IL-33 production presumably by type II pneumocytes. Because this innate pathway may be relatively resistant to current therapeutics, a greater understanding of the role of epithelial cells, ILCs and IL-33 in response to insults such as nanomaterials exposure may result in improved therapies for asthma and other mucosal inflammatory disorders.
Supplementary Material
Acknowledgments
We would like to thank Britten Postma, Mary Buford, Pam Shaw, and Lou Herritt for their expert technical assistance with various aspects of this manuscript. This work is supported by grants F32 ES019816 (TAG), RC2 ES018742 (AH), and COBREs P20 RR017670 from the National Center for Research Resources (NCRR) and the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR, NIGM, or NIH.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Supplementary information available online
Supplementary Table 1.
References
- Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SA, et al. Review of carbon nanotubes toxicity and exposure–appraisal of human health risk assessment based on open literature. Crit Rev Toxicol. 2010;40:759–790. doi: 10.3109/10408444.2010.506638. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e1–e4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Mckenzie AN. Nuocytes: expanding the innate cell repertoire in type-2 immunity. J Leukoc Biol. 2011;90:867–874. doi: 10.1189/jlb.0311160. [DOI] [PubMed] [Google Scholar]
- Bartemes KR, 2nd, Jima K, Kobayashi T, Kephart GM, Mckenzie AN, Kita H. IL-33-Responsive lineage-CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Migliaccio CT, Jessop F, Trapkus M, Yuan D, Holian A. Innate immune processes are sufficient for driving silicosis in mice. J Leukoc Biol. 2010;88:547–557. doi: 10.1189/jlb.0210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Seaver BP, Shepherd DM. Aryl hydrocarbon receptor (AhR) regulates silica-induced inflammation, but not fibrosis. Toxicol Sci. 2012:554–568. doi: 10.1093/toxsci/kfs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc. 2010;7:138–141. doi: 10.1513/pats.200907-061RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, Mckenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gavett SH, Wills-Karp M. CD4+ T lymphocyte modulation of ozone-induced murine pulmonary inflammation. Am J Respir Cell Mol Biol. 1995;12:396–403. doi: 10.1165/ajrcmb.12.4.7695918. [DOI] [PubMed] [Google Scholar]
- Cohn L, Tepper JS, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Valentin E, Crahay C, Garbacki N, Hennuy B, Gueders M, Noel A, et al. New asthma biomarkers: lessons from murine models of acute and chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L185–L197. doi: 10.1152/ajplung.90367.2008. [DOI] [PubMed] [Google Scholar]
- Eiwegger T, Akdis CA. IL-33 links tissue cells, dendritic cells and Th2 cell development in a mouse model of asthma. Eur J Immunol. 2011;41:1535–1538. doi: 10.1002/eji.201141668. [DOI] [PubMed] [Google Scholar]
- Fubini B, Zanetti G, Altilia S, Tiozzo R, Lison D, Saffiotti U. Relationship between surface properties and cellular responses to crystalline silica: studies with heat-treated cristobalite. Chem Res Toxicol. 1999;12:737–745. doi: 10.1021/tx980261a. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Tago K, Hayakawa M, Tominaga S, Ohshio T, Sonoda Y, et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal. 2008;20:1679–1686. doi: 10.1016/j.cellsig.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Garssen J, Van Loveren H, Van Der Vliet H, Nijkamp FP. T cell mediated induction of bronchial hyperreactivity. Br J Clin Pharmacol. 1990;30(Suppl 1):153S–155S. doi: 10.1111/j.1365-2125.1990.tb05491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie GD., Jr Mineral properties and their contributions to particle toxicity. Environ Health Perspect. 1997;105(Suppl 5):1003–1011. doi: 10.1289/ehp.97105s51003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland A, Wick P, Koehler A, Schmid K, Som C. Reviewing the environmental and human health knowledge base of carbon nano-tubes. Environ Health Perspect. 2007;115:1125–1131. doi: 10.1289/ehp.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–316. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Hirano S, Sakurai M, Shimada A, et al. Effects of airway exposure to nanoparticles on lung inflammation induced by bacterial endotoxin in mice. Environ Health Perspect. 2006a;114:1325–1330. doi: 10.1289/ehp.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Ichinose T, Sakurai M, Yoshikawa T. Effects of nano particles on cytokine expression in murine lung in the absence or presence of allergen. Arch Toxicol. 2006b;80:614–619. doi: 10.1007/s00204-006-0075-3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Sakurai M, Abe S, Yoshino S, et al. Effects of nanoparticles on lung physiology in the presence or absence of antigen. Int J Immunopathol Pharmacol. 2007;20:737–744. doi: 10.1177/039463200702000409. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, et al. Effects of nano particles on antigen-related airway inflammation in mice. Respir Res. 2005;6:106. doi: 10.1186/1465-9921-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free Radic Biol Med. 2010;48:924–934. doi: 10.1016/j.freeradbiomed.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216–227. e1–e6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Nakata J, Arai N, Izumo T, Tagaya E, Takeyama K, et al. Niflumic acid inhibits goblet cell degranulation in a Guinea pig asthma model. Allergol Int. 2012;61:133–142. doi: 10.2332/allergolint.11-OA-0307. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- Kouzaki H, 2nd, Jima K, Kobayashi T, O’grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11:109–114. doi: 10.1097/ACI.0b013e3283448808. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Matsukura S, Kawaguchi M, Ieki K, Suzuki S, Odaka M, et al. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Int Arch Allergy Immunol. 2011;155(Suppl 1):12–20. doi: 10.1159/000327259. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–185. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lloyd CM. IL-33 family members and asthma - bridging innate and adaptive immune responses. Curr Opin Immunol. 2010;22:800–806. doi: 10.1016/j.coi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Saglani S. Asthma and allergy: the emerging epithelium. Nat Med. 2010;16:273–274. doi: 10.1038/nm0310-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson JM, Lange RW, Lemus R, Karol MH, Luster MI. Importance of inflammatory and immune components in a mouse model of airway reactivity to toluene diisocyanate (TDI) Clin Exp Allergy. 2001;31:1067–1076. doi: 10.1046/j.1365-2222.2001.01125.x. [DOI] [PubMed] [Google Scholar]
- Meisel C, Bonhagen K, Lohning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001;166:3143–3150. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ’alarmin’? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North ML, Amatullah H, Khanna N, Urch B, Grasemann H, Silverman F, et al. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyper-responsiveness in murine models of asthma. Respir Res. 2011;12:19. doi: 10.1186/1465-9921-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Nakae S, Matsumoto K, Saito H. IL-33 and airway inflammation. Allergy Asthma Immunol Res. 2011;3:81–88. doi: 10.4168/aair.2011.3.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010;73:378–395. doi: 10.1080/15287390903486527. [DOI] [PubMed] [Google Scholar]
- Padilla J, Daley E, Chow A, Robinson K, Parthasarathi K, Mckenzie AN, et al. IL-13 regulates the immune response to inhaled antigens. J Immunol. 2005;174:8097–8105. doi: 10.4049/jimmunol.174.12.8097. [DOI] [PubMed] [Google Scholar]
- Park EJ, Cho WS, Jeong J, Yi J, Choi K, Park K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology. 2009;259:113–121. doi: 10.1016/j.tox.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–147. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40:349–358. doi: 10.1165/rcmb.2008-0276OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter-induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118:640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, Mcclanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2009:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- Wang X, Katwa P, Podila R, Chen P, Ke PC, Rao AM, et al. Multi-walled carbon nanotube instillation impairs pulmonary function in C57BL/6 mice. Part Fibre Toxicol. 2011;8:24. doi: 10.1186/1743-8977-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann M. Targeting eosinophil biology in asthma therapy. Am J Respir Cell Mol Biol. 2011;45:667–674. doi: 10.1165/rcmb.2011-0013TR. [DOI] [PubMed] [Google Scholar]
- Wells SM, Buford MC, Migliaccio CT, Holian A. Elevated asymmetric dimethylarginine alters lung function and induces collagen deposition in mice. Am J Respir Cell Mol Biol. 2008:179–188. doi: 10.1165/rcmb.2008-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Rahman S, Hubeau C, Ma HL. Cytokine pathways in allergic disease. Toxicol Pathol. 2012:205–215. doi: 10.1177/0192623311430694. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Fujitani T, Ohyama K, Nakae D, Hirose A, Nishimura T, et al. Effects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in mice. J Toxicol Sci. 2012;37:177–189. doi: 10.2131/jts.37.177. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dong Q, Louahed J, Dragwa C, Savio D, Huang M, et al. Characterization of a calcium-activated chloride channel as a shared target of Th2 cytokine pathways and its potential involvement in asthma. Am J Respir Cell Mol Biol. 2001;25:486–491. doi: 10.1165/ajrcmb.25.4.4578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.