Summary
The chemokine receptor CCR7 plays a crucial role in the homing of central memory and naïve T cells to peripheral lymphoid organs. Here we show that the HIV-1 accessory protein Vpu downregulates CCR7 on the surface of CD4+ T cells. Vpu and CCR7 were found to specifically interact and co-localize within the trans-Golgi network, where CCR7 is retained. Downmodulation of CCR7 did not involve degradation or endocytosis and was strictly dependent on Vpu expression. Stimulation of HIV-1 infected primary CD4+ T cells with the CCR7 ligand, CCL19, resulted in reduced mobilization of Ca2+, reduced phosphorylation of Erk1/2, and impaired migration towards CCL19. Specific amino acid residues within the transmembrane domain of Vpu, A14, A18 and W22, previously shown to be critical for BST-2 downmodulation, were also necessary for CCR7 downregulation. These results suggest that downregulation of BST-2 and CCR7 may share similar mechanistic aspects.
Introduction
HIV-1 encodes four accessory genes, vpu, nef, vif and vpr, which have a plethora of effects on the host cell. These effects include downregulation of cell surface molecules and evasion of restriction factors and innate immune responses (reviewed in (Kirchhoff, 2010; Malim and Emerman, 2008)). The HIV-1 Vpu protein has a predicted length that ranges from 77 to 86 amino acid residues. Vpu is translated from a vpu-env bicistronic mRNA (Schwartz et al., 1990; Strebel et al., 1988) during a late phase of the viral life cycle and is not thought to be incorporated into budding virions (Nomaguchi et al., 2008). Structurally, Vpu consists of three major domains: a short N-terminal luminal tail (3–12 amino acids), a single hydrophobic transmembrane (TM) domain (27 amino acids) and a C-terminal amphipathic portion (54 residues) that extends into the cytoplasm (Maldarelli et al., 1993; Wray et al., 1995). The C-terminal region consists of two alpha-helices, connected by a short motif in which two conserved serine residues (serine 52 and serine 56) are phosphorylation sites for casein kinase II and are responsible for the recruitment of β-TrCP-1 and −2 (Strebel, 2007).
Vpu sequesters de novo synthesized CD4 in the endoplasmic reticulum, targeting it for proteasomal degradation (Willey et al., 1992). This function is dependent on the binding of β-TrCP to Vpu’s cytoplasmic phosphoserine residues (Butticaz et al., 2007; Margottin et al., 1998). Vpu-mediated downmodulation of BST-2/Tetherin has been shown to be partly dependent on the interaction with β-TrCP (Iwabu et al., 2009), although whether this interaction leads to degradation of BST-2 is still debated (Dube et al., 2010; Mangeat et al., 2009). Vpu interacts with BST-2 within the trans-Golgi network (TGN), and in recycling endosomes (Douglas et al., 2009; Dube et al., 2010; Mitchell et al., 2009), rather than within the ER as is the case with CD4 (Willey et al., 1992). Vpu has been shown to cooperate with Nef in the downregulation of CD1d from the surface of HIV-1 infected DCs, thereby limiting the ability of CD1d to patrol the endocytic system in search of lipid antigens to present to invariant NK T-cells (iNK) (Moll et al., 2010).
Shah and colleagues recently found that Vpu also downmodulates the surface expression of the NK cell co-activating receptor NK-T and B cell antigen (NTB-A) (Shah et al., 2010) on infected CD4+ T cells. As a consequence, degranulation by NK cells, which requires signaling through NTB-A, is impaired. Downregulation of NTB-A by Vpu protects the infected cells from lysis by NK cells (Shah et al., 2010). Interestingly, in both Vpu-mediated CD1d and NTB-A surface downmodulation, recruitment of β-TrCP appears to not be required, suggesting that Vpu acts as a multifunctional viral protein able to interfere in different ways with different host factors (Sandberg et al., 2012).
In this work, we describe the ability of HIV-1 to downregulate the C-C chemokine receptor-7 (CCR7) from the surface of primary CD4+ T cells, in a Vpu-dependent manner. CCR7 belongs to a family of seven-transmembrane spanning chemokine receptors that mediate their signals through the activation of heterotrimeric Goti proteins. CCR7 is mainly expressed by mature dendritic cells (Ohl et al., 2004), naïve B cells (Reif et al., 2002), and naïve and central memory CD4+ T cells (Sallusto et al., 1999). Studies in CCR7-deficient mice underscored the central role of CCR7 as a major homing receptor that directs the migration of cells into the lymph nodes, where priming and assembly of immune responses takes place (Forster et al., 1999). Additionally, recent studies have revealed previously unrecognized functions of CCR7 in promoting T-cell recirculation in peripheral tissues (Debes et al., 2005; Hopken et al., 2010). CCR7 signaling is triggered by the chemokines CCL19 and CCL21 (Rot and von Andrian, 2004), which are constitutively expressed by reticular stromal cells in lymphoid organs (Luther et al., 2000). Binding of either ligand to the receptor culminates in G-protein activation, calcium flux and chemotactic responses (Willimann et al., 1998; Yoshida et al., 1998). We show that HIV-1 infected cells, through the action of Vpu, display reduced expression of CCR7 and reduced ability to signal and migrate in response to CCL19.
Results
HIV-1 downregulates the chemokine receptor CCR7 on the surface of primary CD4+ T-lymphocytes
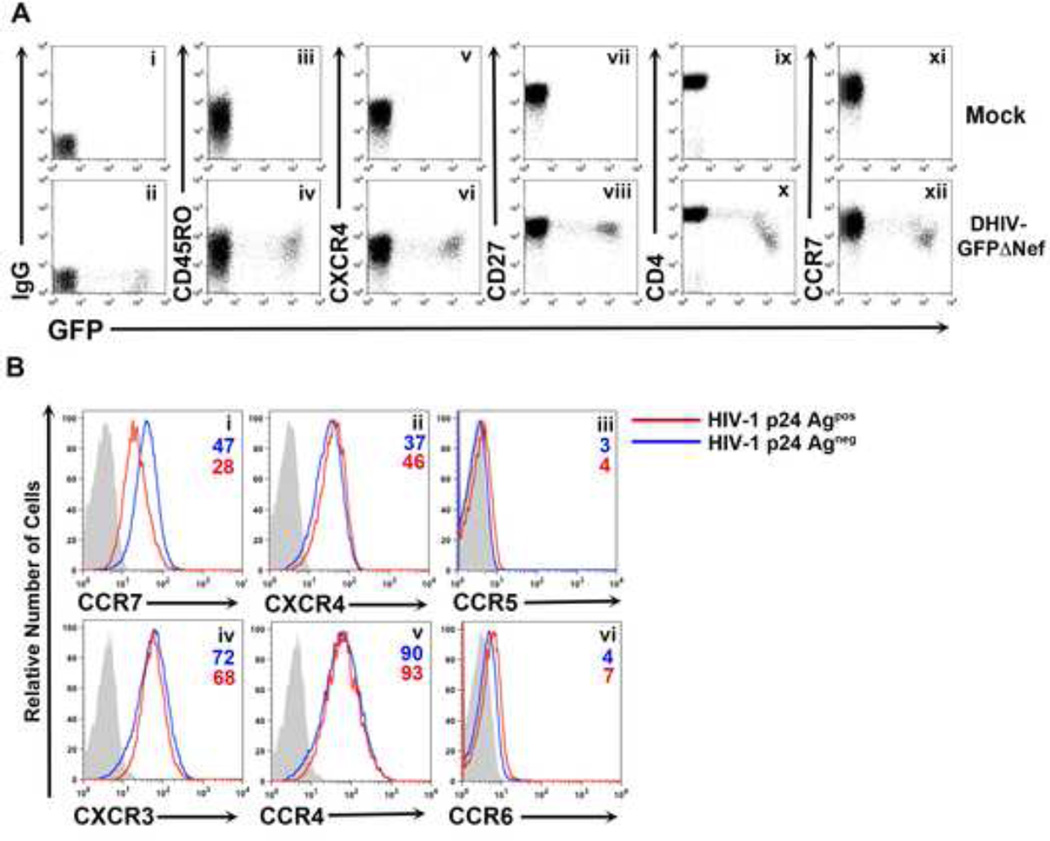
We previously reported that HIV-1 infection of in vitro cultured central memory T cells (TCM) generates a population of productively infected cells (Bosque and Planelles, 2009). We wished to examine whether any phenotypic differences induced by HIV-1 infection occurred in these cells. To that end, we infected primary CD4+ lymphocytes (generated as described in the Experimental Procedures) with a replication deficient (termed DHIV) HIV-1 molecular clone carrying GFP in place of Nef (DHIV-GFPΔNef ; Figure S1) and analyzed the expression of GFP versus different surface markers two days post infection. As shown in Figure 1A, both uninfected and infected cells expressed similar levels of the activation marker CD45RO, the chemokine receptor CXCR4 and the co-stimulatory molecule CD27, all of which are highly expressed on cultured TCM. As expected, infected cells downregulated CD4 as a consequence of Vpu expression (Willey et al., 1992). Unexpectedly, we found that the levels of the chemokine receptor CCR7 were 49% lower (based on mean fluorescence intensity values) in infected cells relative to uninfected cells (Figure 1A).
Figure 1. HIV-1 downregulates the chemokine receptor CCR7 from the surface of infected primary CD4+ T cells.
A) Surface levels of CD45RO (iii, iv), CXCR4 (v, vi), CD27 (vii, viii), CD4 (ix, x) and CCR7 (xi, xii) versus GFP expression were analyzed two days post infection in uninfected (Mock) and infected (DHIV-GFPΔNef) cultured CD4+ TCM cells. An IgG matched control was used for establishing positive surface marker expression (i, ii). Unless otherwise noted, all figures involving primary CD4+ T cells are representative of three separate experiments performed in three different donors.
B) Primary CD4+ T cells were either mock-infected or infected with DHIV. Two days post infection cells were surface stained for the chemokine receptors CCR7 (i), CXCR4 (ii), CCR5 (iii), CXCR3 (iv), CCR4 (v) or CCR6 (vi) followed by intracellular staining for HIV-1 p24Gag. A comparison between p24Gagneg cells (blue line) and p24Gagpos cells (red line) are depicted in each histogram along with an IgG matched isotype control (gray shaded histogram).
We then investigated whether this was a general effect of HIV-1 on chemokine receptors. We infected TCM cells with a molecular clone of HIV-1 that encodes all the accessory genes. In this case, cells were stained for surface expression of the chemokine receptors CCR7, CXCR4, CXCR3, CCR4, CCR6 and CCR5 followed by intracellular staining of p24Gag viral antigen. As shown in Figure 1B, among the tested receptors, HIV-1 was only able to downregulate CCR7. Contrary to previous findings showing that Nef downmodulates the chemokine receptor CXCR4 (Hrecka et al., 2005; Venzke et al., 2006), we did not observe CXCR4 downregulation.
Vpu mediates cell surface CCR7 downregulation in CD4+ T cells
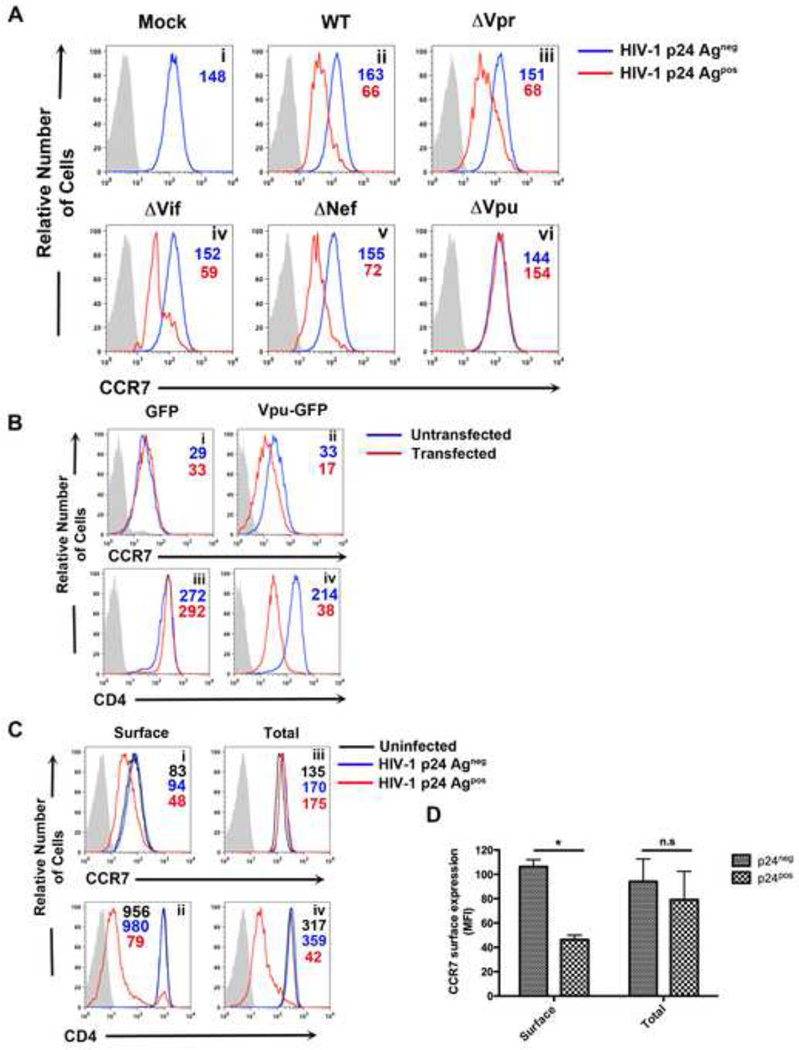
Next, we tested whether any accessory protein had a potential role in manipulating CCR7 expression. To that end, cells were infected with HIV-1 viruses lacking each accessory gene and CCR7 expression analyzed two days post infection. As shown in Figure 2A, CCR7 was downmodulated from the cell surface by HIV-1AVpr, HIV-1AVif and HIV-1ANef to the same extent as it was by wild-type HIV-1 (Panels i-v). However, HIV-1AVpu failed to downregulate CCR7, indicating that Vpu was necessary for this function (Panel vi).
Figure 2. HIV-1 Vpu is necessary and sufficient for surface downmodulation, but not degradation, of CCR7.
A) Primary CD4+ T cells were either mock infected (i) or infected with DHIV (ii), DHIVAVpr (iii), DHIVAVif (iv), DHIVANef (v) or DHIVAVpu (vi). Two days later, cells were assessed for surface levels of CCR7 in p24Gagneg (blue line) and p24Gagpos cells (red line). Gray shaded histograms represent IgG matched isotype controls.
B) CEM-CCRF cells were nucleofected with 2µg of either a GFP or Vpu-GFP expression vector. Twenty-four hours post-transfection, relative surface levels of CCR7 (i and iii) or CD4 (ii and iv) were measured. Histograms depict a comparison between untransfected (blue line) and transfected (red line) cells relative to IgG matched control (gray shaded histogram). Figure is representative of three independent experiments.
C) Relative surface levels of CCR7 (i) and CD4 (ii) were assessed two days post infection with DHIV, as in A. In addition, cells were permeabilized and co-stained with antibodies for either CCR7 (iii) or CD4 (iv) along with antibody for p24Gag. HIV-1 p24Gagpos cells and p24Gagneg cells are represented by red or blue line, respectively. Uninfected cells (black line) were used as a control along with an IgG matched isotype (gray shaded histogram).
D) Data depicts mean fluorescence intensity (MFI) values of surface and total levels of CCR7 from three independent experiments. Data was normalized by setting MFI values from uninfected (mock) cells to 100% and is depicted graphically as +/− mean SEM. (*, P <0.05)
We then examined whether Vpu was sufficient for CCR7 surface downregulation. CEM-CCRF T cells, which constitutively express CCR7 and CD4, were nucleofected with expression vectors encoding either Vpu-GFP or GFP alone (Shah et al., 2010). CCR7 surface expression was reduced in Vpu-GFP, but not GFP transfected cells (Figure 2B, compare Panels i and ii), indicating that Vpu is sufficient to downmodulate CCR7. As expected, CD4 surface levels were also lower in Vpu-GFP, but not GFP, expressing cells (Figure 2B, Panels iii and iv) (Willey et al., 1992).
To address whether HIV-1 infection reduced total levels of CCR7 (as opposed to only surface levels), cells were fixed, permeabilized and co-stained with CCR7 and p24Gag antibodies. As a control, we stained for CD4, whose degradation is triggered by Vpu via the ER-associated degradation (ERAD) pathway (Binette et al., 2007; Magadan et al., 2010; Schubert et al., 1998; Willey et al., 1992). As shown in Figure 2C (Panels i and iii) and 2D, total levels of CCR7 were not significantly different between infected and uninfected cells (see “Total” in Figure 2C and 2D), suggesting that Vpu did not induce CCR7 degradation but, more likely, promoted its redistribution within the cell. In contrast, HIV-1 infection drastically reduced surface and total levels of CD4 (Figure 2B, Panels ii and iv), due to the combined effect of both Vpu and Nef degrading the protein (Kirchhoff, 2010).
To directly assess whether Vpu induces CCR7 degradation, we conducted both a cyclohexamide study as well as pulse-chase analysis. We used cyclohexamide (CHX; a blocker of protein synthesis) so that we could evaluate the fate of total levels of protein in the absence of de novo synthesis. As shown in Figure S2A, when HIV-1 infected primary cells were incubated in the presence of CHX for 24 hours, total levels of CCR7 remained constant between infected and uninfected cells. Therefore, the decrease in surface CCR7 induced by Vpu cannot be explained by protein degradation.
As an independent method to examine the possible degradation of CCR7, we performed pulse-chase analysis in 293T cells by co-transfecting CCR7-Flag with an expression vector encoding GFP or a Vpu-GFP fusion protein (Shah et al., 2010). 24 hours post transfection, cells were pulse-labeled with [35S] for 30 minutes and chased for up to 24 hours. CCR7 was then immunoprecipitated using an anti-Flag antibody and the lysates separated by SDS-PAGE, followed by autoradiography. We observed a band at 43 kDa, corresponding to CCR7. We only detected a minor difference in CCR7 protein levels in the absence or presence of Vpu (100% versus 86%, respectively) after 24 hrs (Figure S2B and S2C). Therefore, although these results do not exclude a minor contribution of degradation of CCR7, we conclude that degradation is not the major mechanism by which Vpu induces downregulation of CCR7 from the cell surface.
Downmodulation of CCR7 by Vpu occurs with replication-competent HIV-1
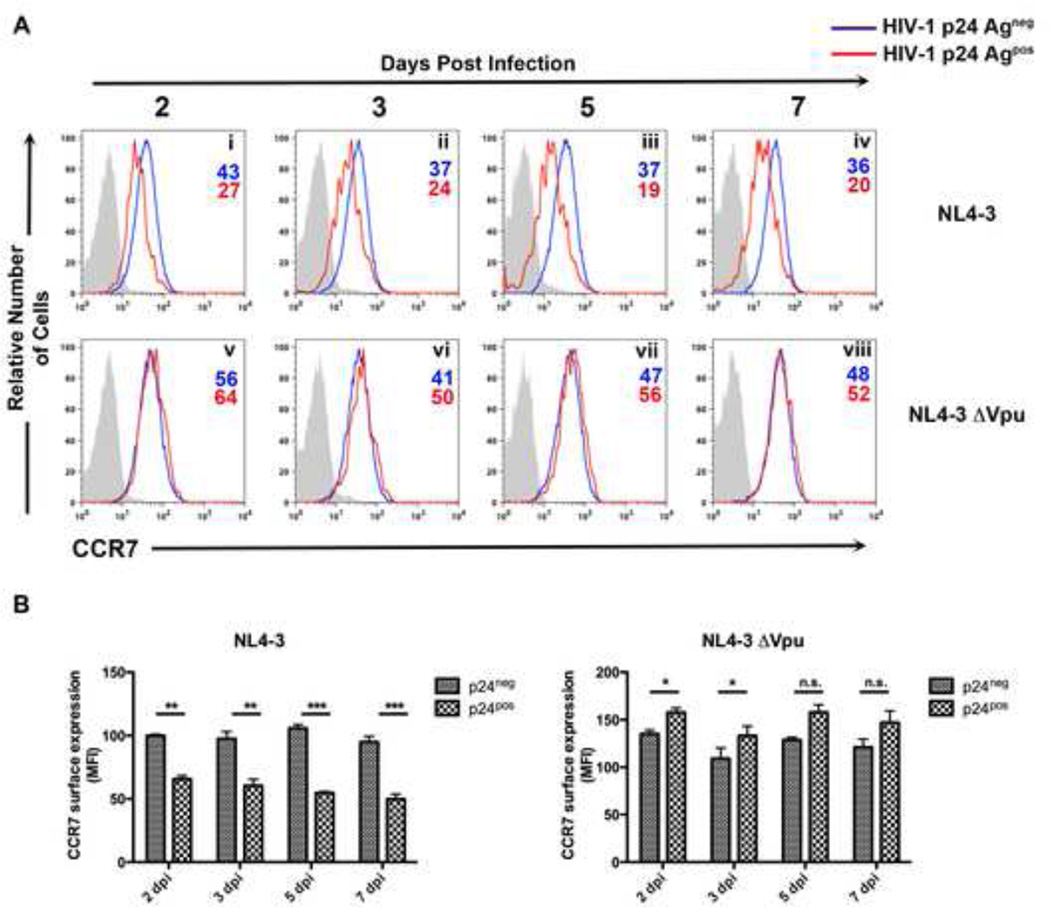
To directly examine under more physiological conditions (i.e., in a spreading infection) whether Vpu could downmodulate CCR7, primary CD4+ T cells were infected with either HIV-1NL4-3 or the mutant HIV-1NL4-3ΔVpu, in which the start codon of Vpu was mutated to a stop codon. At 2, 3, 5 and 7 days post infection, cells were surface-stained for CCR7 or BST-2 followed by intracellular staining for p24 Gag. Replication-competent HIV-1 efficiently downregulated CCR7 from the cell surface, with this effect becoming more significant as the time of infection increased (Figure 3 A, Panels i-iv; Figure 3B). By comparison, infection of cells with HIV-1NL4-3ΔVpu was unable to induce CCR7 surface downmodulation (Figure 3A, Panels v-viii; Figure 3B). As a control, Vpu also efficiently downregulated BST-2 from the cell surface (Figure S3).
Figure 3. Vpu downregulates CCR7 in the context of a spreading infection.
A) Primary CD4+ T cells were infected with either HIV-1NL4-3 or HIV-1NL4-3ΔVpu at an MOI=.1. At 2, 3, 5 and 7 days post infection, cells were surface stained for CCR7 and permeabilized for detection of p24Gag. Histograms represent either p24Gagneg cells (blue line), p24Gagpos cells (red line) or an IgG matched isotype (gray shaded histogram).
B) Data depicts mean fluorescence intensity (MFI) values of surface CCR7 levels from either HIV-1NL4-3 (left) or HIV-1NL4-3ΔVpu (right) infected cells. Normalization was achieved by setting MFI values from uninfected (mock) cells to 100% and is depicted graphically as +/− mean SEM. Data representative of three independent experiments from three separate donors (*, P < 0.05; **, P < 0.01; ***, P < 0.001)
CCR7 and Vpu co-localize within the trans-Golgi network
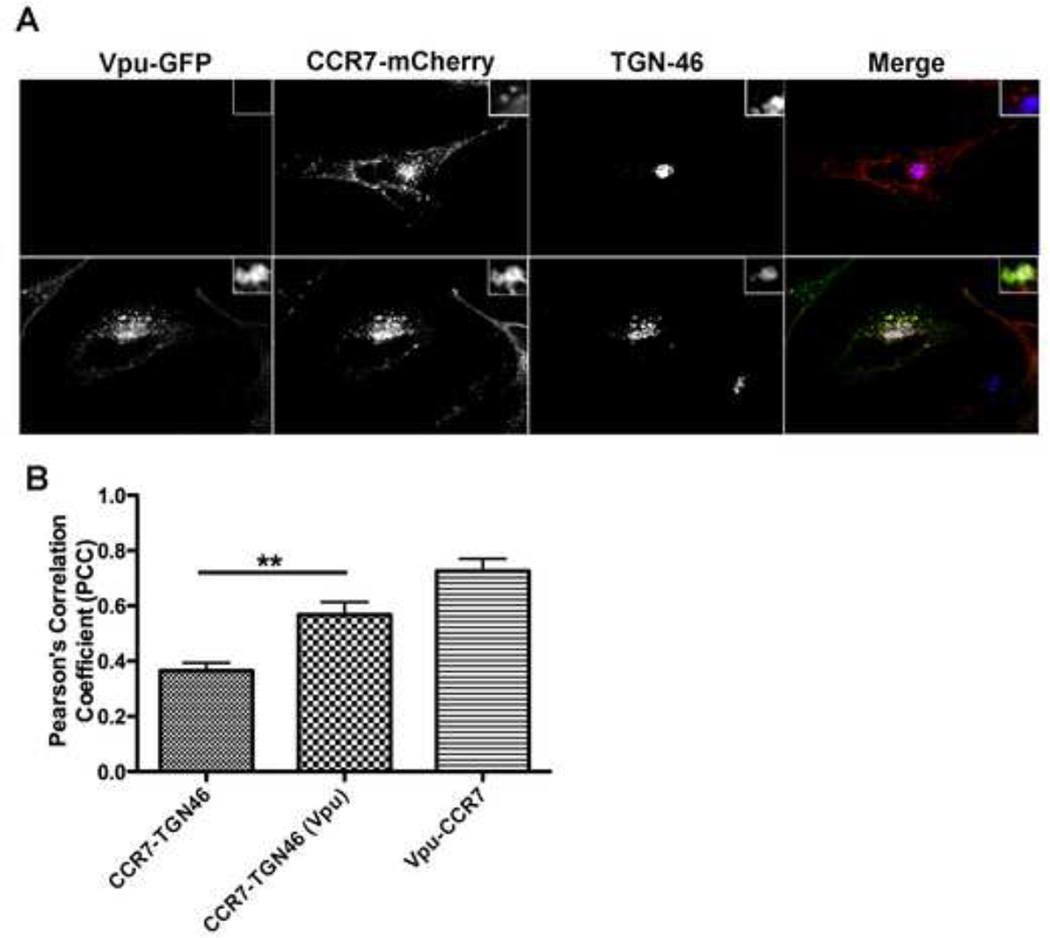
Vpu has been previously shown to co-localize with BST-2 (Van Damme et al., 2008) and preferentially sequester the host protein within a perinuclear compartment, specifically the trans-Golgi network (TGN) (Dube et al., 2010; Dube et al., 2009; Hauser et al., 2010; Vigan and Neil, 2010). Therefore, to determine whether Vpu co-localizes and/or sequesters CCR7, HeLa cells were transfected with a CCR7 fusion construct bearing a C-terminal mCherry-tag (CCR7-mCherry) along with Vpu-GFP. In the absence of Vpu-GFP, CCR7-mCherry localized both at the cell surface and intracellularly (Figure 4A, top row). The amount of co-localization between CCR7-mCherry and TGN46, a TGN marker, in the absence of Vpu, was minimal (Figure 4A, upper panels, Figure 4B). However, in cells co-transfected with CCR7-mCherry and Vpu-GFP, both proteins highly co-localized together, as quantified by Pearson’s Correlation Coefficient (PCC ; Figure 4B) and, specifically, within the TGN cellular compartment (Figure 4A, lower panels, Figure 4B) (Barlow et al., 2010). Moreover, the degree of co-localization between CCR7-mCherry and TGN46 (PCC= 0.38), increased when Vpu-GFP was present (PCC= 0.58), suggesting that Vpu sequesters CCR7 within the TGN. These findings are highly reminiscent of how Vpu induces BST-2 surface downregulation (Dube et al., 2010; Vigan and Neil, 2010).
Figure 4. Vpu co-localizes with CCR7 within the trans-Golgi network.
A) HeLa cells were transiently transfected with either CCR7-mcherry alone (top row) or in combination with Vpu-GFP (bottom row). Twenty-four hours post transfection, cells were fixed, permeabilized and stained with a trans-Golgi network (TGN) specific antibody (TGN46). Images were acquired using a spinning disc confocal microscope. CCR7-mcherry (red); Vpu-GFP (green); TGN46 (blue).
B) Relative co-localization levels between CCR7-TGN46, Vpu-TGN46 or Vpu and CCR7 were quantified using Pearson’s correlation coefficient (PCC). Data is graphically depicted as mean +/− SEM PCC and is representative of ten individual cells where ** denotes a p value < 0.01.
Vpu does not increase the endocytosis rate of CCR7 in the presence of CCL19
Since we observed that the steady-state levels of CCR7 remained constant in HIV-1 infected cells, we tested whether Vpu may be increasing the internalization rate of the chemokine receptor. Primary CD4+ T cells infected with HIV-1NL4-3 were stained with an antibody against CCR7 at 4°C and then placed back at 37°C for various time points to allow for internalization. The cells were then stained with an APC-conjugated secondary antibody followed by p24Gag antigen and analyzed by flow cytometry. As shown in Figure S4, we did not observe endocytosis of CCR7 in either uninfected cells (mock; solid black line), p24Gagneg (solid blue line) or p24Gagpos cells (solid red line). This indicates that Vpu does not increase the constitutive endocytosis rate of CCR7. Moreover, the stability of the chemokine receptor on the surface is consistent with previous reports showing that CCR7 is highly stable on the cell membrane unless provided with one of its chemokine ligands, such as CCL19 (Otero et al., 2006). As a positive control for endocytosis of CCR7, we stimulated cells with CCL19 (Figure S4; dashed black, blue and red lines). It is noteworthy that CCL19-induced endocytosis of CCR7 was also unaffected by the presence of Vpu.
The transmembrane domain of Vpu is required for downregulation of CCR7
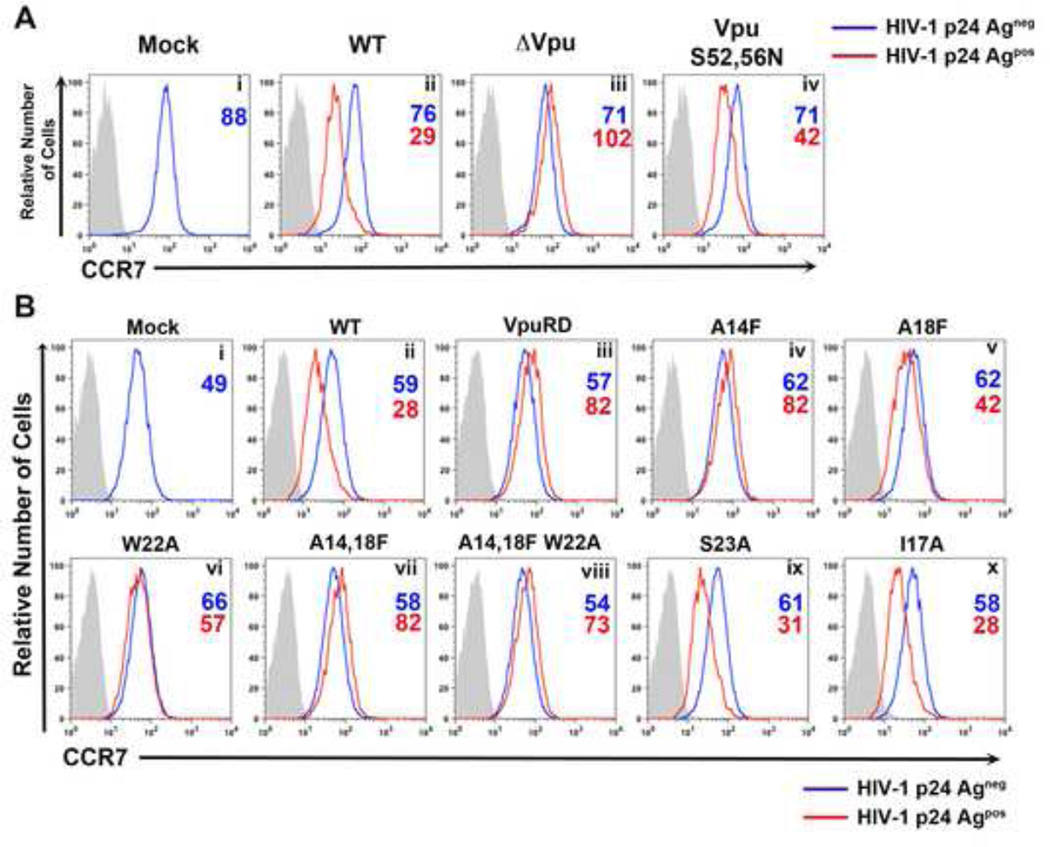
Vpu triggers CD4 proteasomal degradation by linking this protein to the SCF (Skp1-Cullin-F-box)/β-TrCP E3 ubiquitin ligase complex (Kerkau et al., 1997; Margottin et al., 1998). Vpu-mediated BST-2 removal from the surface of HIV-1 infected cells also requires the interaction with β-TrCP (Mitchell et al., 2009). Two phosphorylated serines (52 and 56) in the DpSGXXpS motif (where “p” denotes phosphorylation of the following amino acid residue and “X” any amino acid residue) present at the C-terminus of Vpu recruit p-TrCP (Evrard-Todeschi et al., 2006; Wu et al., 2003). To address whether Vpu interaction with β-TrCP was required for CCR7 downregulation, we used an HIV-1 mutant where both Vpu serine residues were mutated to asparagine (VpuS52,56N). We anticipated that if interaction with the SCF/β-TrCP complex were required for CCR7 downregulation, then mutation of the serine residues would completely abolish this phenotype, as is the case for downregulation of CD4 (Willey et al., 1992). As shown in Figure 5A (Panels ii and iv), VpuS52,56N was still able to downregulate CCR7 (MFI of 71 and 42 for p24- and + cells, respectively; 41% downregulation), although somewhat less efficiently than WT Vpu (MFI of 76 and 29 for p24- and + cells, respectively; 62% downregulation. Therefore, VpuS52,56N retained most of the ability to downregulate surface CCR7. We interpret these results to mean that interaction with the β-TrCP-containing E3 ubiquitin ligase complex is not required for CCR7 surface downmodulation by Vpu.
Figure 5. CCR7 surface downregulation requires Vpu’s transmembrane domain but not its conserved serines.
A) Primary CD4+ T cells were either mock-infected (i) or infected with DHIV (ii), DHIVΔVpu (iii) or DHIV-VpuS52,56N (iv), in which Vpu’s conserved serines were mutated to asparagines. All cells were surface stained for CCR7 expression followed by intracellular p24Gag staining, as in Figure 1B.
B) Primary CD4+ T cells were either mock-infected (i) or infected with DHIV (ii), or DHIV-VpuRD (iii), in which the transmembrane domain of Vpu was scrambled. Additionally, cells were infected with the indicated Vpu TM mutants (iv–x). Cells were stained and analyzed as described in A.
The transmembrane domain (TMD) of Vpu (residues 4–27) is highly conserved among strains of the pandemic HIV-1 group M (Vigan and Neil, 2010, 2011). Moreover, this region is required for downregulation of NTB-A (Shah et al., 2010), BST-2 (McNatt et al., 2013; Van Damme et al., 2008) and CD4 (Magadan and Bonifacino, 2012; Magadan et al., 2010; Tiganos et al., 1998). To determine whether the TMD of Vpu played a role in CCR7 downmodulation, CD4+ T cells were infected with an HIV-1 mutant encoding Vpu with a scrambled TMD (VpuRD) (Schubert et al., 1996). Infection of cells with HIV-1VpuRD virus failed to induce CCR7 downregulation (Figure 5B, Panels ii and iii). We then investigated the specific residues within the TM region that may be critical for Vpu to downmodulate CCR7. Previous studies have shown that the A14, W22 and, to a lesser extent, A18, residues within Vpu’s TMD are important for the downmodulation and interaction of Vpu with BST-2 (Skasko et al., 2012; Vigan and Neil, 2010). Mutation of alanine 14 and of tryptophan 22, to phenylalanine and alanine, respectively, completely abolished Vpu-dependent CCR7 downregulation (Figure 5B, Panels iv and vi). As previously shown for Vpu-dependent downregulation of BST-2 (Vigan and Neil, 2010), the change of residue 18 of Vpu also had an intermediate effect on CCR7 downregulation (Panel v). The A14,18F and A14,18F/W22A mutations also abolished CCR7 downregulation (Panels vii and viii). Mutation of serine 23 to alanine (Panel ix) or isoleucine 17 to alanine (Panel x), however, did not affect CCR7 downmodulation. The above data indicated that CCR7 downregulation requires specific residues in the transmembrane domain of Vpu, and these residues are the same as those previously shown to be important for BST-2 downregulation (Vigan and Neil, 2010).
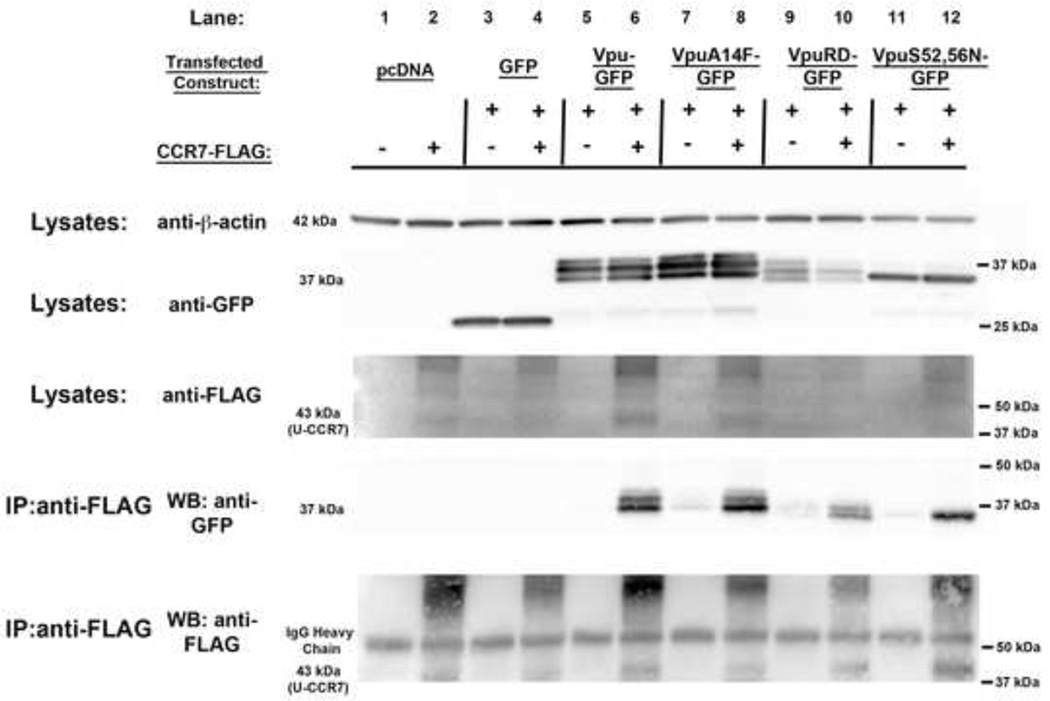
CCR7 co-immunoprecipitates with Vpu
To address whether Vpu-mediated downregulation of CCR7 required physical interaction between the viral protein and the chemokine receptor, 293T cells were transfected with a plasmid expressing either GFP or Vpu-GFP, including mutants (VpuA14F–GFP, VpuRD-GFP, VpuS52,56N–GFP) alone or in combination with CCR7-Flag. Twenty-four hours post transfection, CCR7-Flag was immunoprecipitated from whole cell lysates, followed by immunoblotting with anti-GFP. Figure 6 (lane 6) shows that Vpu co-immunoprecipitated with CCR7. Surprisingly, both VpuRD and VpuA14F, mutants that failed to downregulate CCR7, also co-immunoprecipitated with CCR7 (Lanes 8 and 10). Interestingly, VpuS52,56N, which also co-immunoprecipitated with CCR7 (Lane 12), did not show the upper two bands, which we interpret to be the phosphorylated forms of Vpu at serine residues 52 and/or 56.
Figure 6. CCR7 interacts with Vpu.
A) HEK293T cells were transfected with GFP, Vpu-GFP, VpuA14F–GFP, VpuRD-GFP and VpuS52,56N–GFP either with an empty vector or in combination with CCR7-Flag. Twenty-four hours post transfection, cells were lysed and immunoprecipitated using anti-Flag antibody. Lysates were analyzed by western blot and probed for β-actin (42 kDa), GFP (37 kDa) and Flag (43 kDa- unglycosylated form of CCR7 (U-CCR7)) by loading 10 µg of lysate per sample. Antibodies probed against GFP and Flag were used to analyze immunoprecipitates by western blot. IgG Heavy Chain: 53 kDa. Results are representative of two different experiments.
The above results indicate that the interaction between CCR7 and Vpu, while necessary, is not sufficient for downmodulation of CCR7 surface levels. Further studies are needed in order to identify other potential requirements, beyond CCR7-Vpu binding, that may exist. One possibility is that interaction(s) of Vpu with additional cellular proteins may be required for the downmodulation of CCR7.
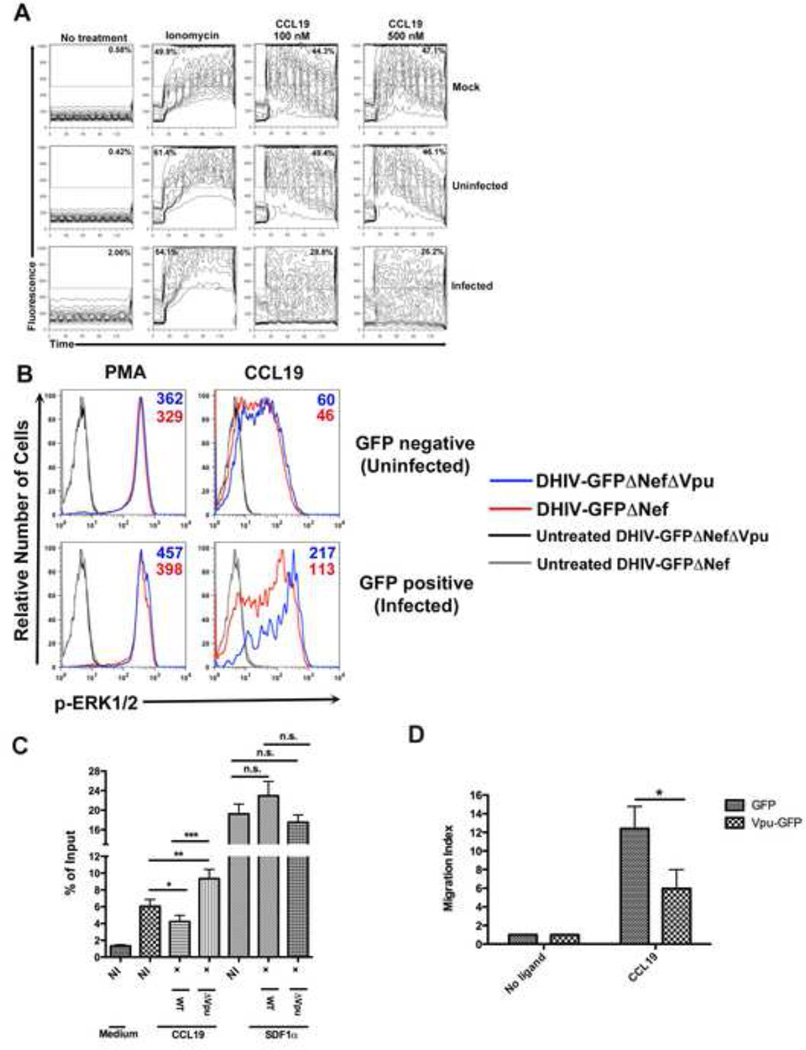
CCL19-mediated mobilization of intracellular calcium and Erk 1/2 phosphorylation are impaired in HIV-infected CD4+ T cells
Binding of either CCL19 or CCL21 to CCR7 initiates a signaling cascade that leads to the release of calcium from intracellular stores (Wu et al., 2000) and activates extracellular signal-regulated kinase-1/2 (Erk-1/2) (Tilton et al., 2000). In our preliminary tests, primary CD4+ T cells migrated in response to CCL19 more efficiently than to CCL21 in vitro (Figure S5). Therefore, to further examine potential effects of Vpu on CCR7 function, we decided to use CCL19 for the next set of experiments. We first asked whether decreased surface expression of CCR7 in HIV-infected cells impaired signaling by CCL19. To that end, we compared the efficiency of calcium mobilization in infected and uninfected cells after stimulation with CCL19. Cultured TCM were infected with a recombinant HIV-1NL4-3 construct encoding the murine heat stable antigen (HSA/CD24) in place of Vpr (Jamieson and Zack, 1998). As shown in Figure 7A, mock-infected and HIV-HSAneg (uninfected) cells responded in a similar fashion to CCL19, with 44.3% and 49.4% of the cells, respectively, increasing the [Ca2+]i at the lower dose utilized. In contrast, only 28.8% of HIV-HSApos (infected) cells upregulated [Ca2+]i in response to the same stimulation.
Figure 7. CCL19 mediated chemotaxis and chemotactic signaling responses are impaired within HIV-1 infected primary CD4+ T cells.
A) Primary CD4+ T cells obtained as described were either mock-infected or infected with DHIV-HSA. Two days post infection cells were loaded with Fluo3-AM followed by surface staining for HSA. Cells were left untreated (no treatment), stimulated with Ionomycin (20 ng/ml) or with either 100 nM or 500 nM CCL19. Changes in fluorescence were recorded over time by flow cytometry. Figure is representative of two independent experiments in two different donors.
B) Primary CD4+ T cells were either mock-infected or infected with the viruses DHIV-GFPΔNef or DHIV-GFPΔNefΔVpu. Forty-eight hours later, cells were stimulated with either 5 ng/ml PMA or 50 ng/ml CCL19 for 5 minutes and immediately stained to detect ERK1/2 phosphorylation. Histograms depicted are split into GFP negative (uninfected) and GFP positive (infected) rows. Blue line depicts cells infected with Vpu- virus whereas red line depicts cells infected with Vpu+ virus. At least 5 × 104 viable GFP positive and negative cells were collected
via flow cytometry. Figure is representative of two independent experiments performed in two different donors.
C) HIV-1NL4-3 or HIV-1NL4-3ΔVpu was used to infect primary CD4+ T cells at an MOI=.1. Five days post infection, cells were placed in the upper chamber of a transwell and either medium alone or medium containing CCL19 or SDF1α in the lower chamber. After 1 hr, the percentages of T cells within the lower chamber were calculated. Data is depicted as the percent of cells migrating in response to medium or ligand relative to total cells stained (input). Figure is representative of four independent experiments performed in duplicate and within different donors. (*, P < 0.05; **, P < 0.01; ***, P < 0.001)
D) CEM-CCRF cells transfected with GFP or Vpu-GFP plasmids were placed in the upper chamber of a transwell and either medium or CCL19 in the lower chamber. After 3 hrs, the numbers of GFP expressing T cells attracted towards the medium was calculated and is depicted as a migration index (MI). Data is represented as mean +/− SEM of three independent experiments performed in duplicate. (*, P < 0.05)
It is noteworthy that the basal levels (“No treatment”) of [Ca2+]i were higher in infected cells (2.06%) than in uninfected ones (0.42%). The reason for such a difference is unknown. It is possible that the binding of the staining antibody against muHSA could trigger a modest increase in the [Ca2+]i . Response to ionomycin was comparable in each sample analyzed, indicating that cells similarly incorporated the fluorescent dye and that calcium mobilization in response to other stimuli was preserved and not affected by infection.
Phosphorylation of Erk-1/2 is another event triggered by CCL19 binding to CCR7. We predicted that levels of phosphorylated ERK1/2 would be compromised in HIV-infected cells after stimulation with CCL19. To address this, activated CD4+ T cells were infected with viruses that expressed Vpu (DHIV-GFPΔNef) or did not (DHIV-GFPΔNefΔVpu). As expected, unstimulated cells infected with either DHIV-GFPΔNef or DHIV-GFPΔNefΔVpu showed no induction of p-ERK1/2 (Figure 7B, gray and black lines). PMA treatment (positive control) led to p-ERK1/2 levels that were comparable between GFPpos and GFPneg cells infected with either virus. When cells were stimulated with CCL19, the GFPneg population had fairly similar levels of p-ERK1/2 whether cultures were infected with DHIV-GFPΔNef (red line; MFI= 46) or DHIV-GFPΔNefΔVpu (blue line; MFI= 60). In contrast, GFPpos cells infected with DHIV-GFPΔNef had reduced levels of p-ERK1/2 (red line; MFI= 113) when compared with those infected with DHIV-GFPΔNefΔVpu (blue line; MFI= 217).
Interestingly, the levels of both PMA and p-ERK1/2 within GFPpos cells (whether the virus was DHIV-GFPΔNef or DHIV-GFPΔNefΔVpu) were generally higher than those within GFPneg cells. This may be a reflection that HIV infection (in particular, the viral protein Tat) may induce cellular stress, leading to the phosphorylation of Erk1/2 (Herbein and Khan, 2008). Therefore, it is likely that phosphorylation of ERK1/2 during HIV-1 infection occurs in response to multiple signals. Taken together, the above results suggest that Vpu-mediated downregulation of CCR7 upon viral infection results in an impaired ability for infected primary CD4+ T cells to respond to CCL19.
Vpu decreases the migratory capacity of CD4+ T cells towards CCL19
Based on the above results, we predicted that Vpu downregulation of CCR7 would result in decreased cellular migration in a CCL19 chemokine gradient. To test our hypothesis, primary CD4+ T cells were infected with either HIV-1NL4-3 or HIV-1NL4-3ΔVpu. Five days post infection, cells were placed in the upper chamber of a transwell plate and allowed to migrate towards either medium alone or chemokine ligands specific for CCR7 (CCL19) or CXCR4 (SDF1α). The cells in the lower chambers of the transwell plates were then fixed and permeabilized, stained for p24Gag and enumerated. As shown in Figure 7C, HIV-1NL4-3 infected cells showed a decreased ability to migrate towards CCL19 relative to non-infected (NI) cells, and also when compared with cells infected with HIV-1NL4-3ΔVpu. Migration defects were not observed in response to an SDF1α gradient. Interestingly, cells infected with HIV-1NL4-3ΔVpu showed a slightly enhanced ability to migrate relative to NI cells towards CCL19 (but not toward SDF1α). Taken together, these results indicate that Vpu negatively modulates the chemotactic potential of primary CD4+ T cells to migrate specifically towards CCL19.
Finally, to also determine whether Vpu was sufficient to induce an impaired chemotactic response, CEM-CCRF cells were nucleofected with expression plasmids encoding GFP or Vpu-GFP. Twenty-four hours later, the cells were subjected to in vitro transmigration assays. As shown in Figure 7D, Vpu-GFP expressing cells showed a reduced capacity to migrate towards CCL19 relative to GFP expressing cells (Migration Index (MI) of 6 versus 12), indicating that Vpu alone is still able to cause a CCR7 specific defect in cellular migration.
Discussion
Naïve and central memory CD4+ T cells are characterized by the ability to continuously transition through secondary lymphoid organs, where cognate antigen is expressed by professional antigen presenting cells. In order for T-cells to correctly home to peripheral lymphoid sites, cellular migration is orchestrated by chemokines and chemokine receptors. The chemokine receptor CCR7 and its two known ligands, CCL19 and CCL21, are crucial factors in this process. Previous reports showed that HIV-1 infection interferes with T-cell recirculation, mostly by accelerating T-cell differentiation and promoting a CCR7low phenotype (Pantaleo and Harari, 2006; Younes et al., 2003). Perez-Patrigeon and colleagues found that chemotaxis triggered by CCL19 was impaired in naïve, central memory and effector memory T cells from HIV-infected patients although they did not find differences in CCR7 surface expression levels between T-cells of HIV-1 infected patients and those of healthy subjects (Perez-Patrigeon et al., 2009). Thus, the findings by Perez-Patrigeon and colleagues could be in apparent contradiction with our observations in this study. However, one notable difference between both studies is that Perez-Patrigeon et al. did not analyze CCR7 surface levels in a manner that discriminated between infected and uninfected cells.. It is also worth noting that a recent report disputes the involvement CCR7 in the trafficking of memory CD4+ T cells from blood into the lymph nodes (Vander Lugt et al., 2013).
Surprisingly, immunoprecipitation of CCR7 from cells expressing wild-type Vpu, VpuRD or VpuA14F (the later two being unable to downregulate CCR7) showed a physical interaction in all cases. We surmised that the interaction between the two proteins, although necessary, was not sufficient toward Vpu-mediated modulation of CCR7. According to the solidstate nuclear magnetic resonance (NMR) structure of the HIV-1NL4-3 Vpu TMD in lipid membranes (Marassi et al., 1999; Park et al., 2003; Skasko et al., 2012), residues A14, A18 and W22 form a diagonal line on the TM alpha helix. Although previous studies implicated these residues as potential points of contact between Vpu and BST-2 (Kobayashi et al., 2011; Rong et al., 2009; Skasko et al., 2012; Vigan and Neil, 2010), McNatt and colleagues recently showed that such residues are responsible for maintaining the overall structure of Vpu TMD rather than constituting points of interaction (McNatt et al., 2013). Our experiments confirm that residues A14, W22 and, to a lesser extent, A18 in Vpu are important for CCR7 downmodulation.
Alteration of immune cell functionality is a hallmark of many viral infections. Specifically, CCR7 expression levels on DCs have been found to be downregulated by cytomegalovirus infection (Moutaftsi et al., 2004) and by HHV-8 (Cirone et al., 2012), with a consequent decrease in the ability of cells to migrate to peripheral lymphoid organs and coordinate the immune response. Data presented here suggest that, in a similar fashion, HIV-1 may inhibit migration of CD4+ infected T cells to peripheral lymphoid tissues, possibly hindering the initiation of effective immune responses.
Experimental procedures
Cells and Plasmids
For details on in vitro cultured TCM see the Supplemental Procedures. The T-lymphoblastoid CCRF-CEM cell line was maintained in RPMI complete (supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin-L glutamine). HEK293T and HeLa cells were cultured in DMEM complete. For a detailed description of the plasmids used throughout the manuscript, see Supplemental Procedures.
Transfections, co-immunoprecipitation and immunoblots
For overexpression of Vpu, CEM-CCRF cells were nucleofected with pAcGFP or pAcGFP-Vpu using the Amaxa Nucleofector Kit C (Lonza). For co-immunoprecipitation, HEK293T cells were transfected via calcium-phosphate with indicated plasmids and processed as explained in Supplemental Procedures.
Flow cytometry
Detection of both surface antigens and intracellular p24Gag was previously described (Ward et al., 2009). Total levels of CCR7 or CD4 and p24Gag within cells were detected by simultaneous staining with anti-APC-CCR7 or anti-APC-CD4 antibodies along with mouse-FITC-anti-p24. Surface levels of BST-2 were analyzed by staining cells with anti-BST2 (NIH AIDS Reagent Program; Dr. Klaus Strebel) and then staining cells with a goat anti-rabbit secondary antibody coupled to Alexa 647 (Molecular Probes, Invitrogen).
To measure relative levels of p-ERK1/2, cells were stimulated with CCL19 for 5 minutes at 37°C. Cells were immediately fixed in 2% formaldehyde (Polysciences) and permeabilized in 90% ice-cold methanol, prior to labeling with anti-p-ERK1/2 (Thr202/Tyr204), followed by staining with a goat anti-rabbit secondary antibody coupled to Alexa 647.
Calcium mobilization assay
Intracellular calcium mobilization was measured in primary CD4+ T cells infected with DHIV-HSA virus, encoding the heat-stable antigen (HSA/CD24) in place of Vpr, according to the procedure reported in the Supplemental Procedures.
Immunofluorescence Microscopy
HeLa cells were transfected and stained as described in the Supplemental Procedures. Images were acquired on an Olympus FV-1000 using a 60x oil lens and quantification was performed using the Velocity 3D image analysis software (Perkin-Elmer).
Supplementary Material
Highlights.
Vpu downregulates CCR7 from the surface of HIV-infected CD4+ T cells.
CCR7 and Vpu co-localize within the trans-Golgi network.
CCR7 downregulation requires the transmembrane region of Vpu.
Chemotactic responses to CCL19 are compromised in CD4+ T cells infected with HIV-1.
Acknowledgments
We would like to thank the excellent technical assistance of James Marvin and Chris Leukel at the Univeristy of Utah Flow Cytometry Core. We would also like to thank Drs. Diane Ward and Dustin Bagley, University of Utah, for providing antibodies for confocal microscopy. M.F. conducted this study as partial fulfillment of her Ph.D. degree of the International Ph.D. School of Molecular Medicine, Università Vita-Salute San Raffaele, Milan, Italy. This work was supported by NIAID grants R01 AI087508 to V.P and R01 AI065361 to E.B. P.R. was supported by Predoctoral Award T32 AI055434 from NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
P.W.R. and M.F. designed and performed the experiments, analyzed data and wrote the manuscript. B.S. and E.B. provided materials. A.B.S. designed and performed experiments. C.R. analyzed data and A.B and V.P designed experiments, analyzed data and wrote the manuscript.
References
- Barlow AL, Macleod A, Noppen S, Sanderson J, Guerin CJ. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of pearson’s correlation coefficient Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society. Microscopical Society of Canada. 2010;16:710–724. doi: 10.1017/S143192761009389X. [DOI] [PubMed] [Google Scholar]
- Binette J, Dube M, Mercier J, Halawani D, Latterich M, Cohen EA. Requirements for the selective degradation of CD4 receptor molecules by the human immunodeficiency virus type 1 Vpu protein in the endoplasmic reticulum. Retrovirology. 2007;4:75. doi: 10.1186/1742-4690-4-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butticaz C, Michielin O, Wyniger J, Telenti A, Rothenberger S. Silencing of both beta-TrCP1 and HOS (beta-TrCP2) is required to suppress human immunodeficiency virus type 1 Vpu-mediated CD4 down-modulation. Journal of virology. 2007;81:1502–1505. doi: 10.1128/JVI.01711-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirone M, Conte V, Farina A, Valia S, Trivedi P, Granato M, Santarelli R, Frati L, Faggioni A. HHV-8 reduces dendritic cell migration through down-regulation of cell-surface CCR6 and CCR7 and cytoskeleton reorganization. Virology journal. 2012;9:92. doi: 10.1186/1743-422X-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, Hay JB, Butcher EC. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a betaTrCP-dependent mechanism. Journal of virology. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M, Roy BB, Guiot-Guillain P, Binette J, Mercier J, Chiasson A, Cohen EA. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS pathogens. 2010;6:e1000856. doi: 10.1371/journal.ppat.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M, Roy BB, Guiot-Guillain P, Mercier J, Binette J, Leung G, Cohen EA. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. Journal of virology. 2009;83:4574–4590. doi: 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard-Todeschi N, Gharbi-Benarous J, Bertho G, Coadou G, Megy S, Benarous R, Girault JP. NMR studies for identifying phosphopeptide ligands of the HIV-1 protein Vpu binding to the F-box protein beta-TrCP. Peptides. 2006;27:194–210. doi: 10.1016/j.peptides.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Hauser H, Lopez LA, Yang SJ, Oldenburg JE, Exline CM, Guatelli JC, Cannon PM. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7:51. doi: 10.1186/1742-4690-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Khan KA. Is HIV infection a TNF receptor signalling-driven disease? Trends in immunology. 2008;29:61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Hopken UE, Winter S, Achtman AH, Kruger K, Lipp M. CCR7 regulates lymphocyte egress and recirculation through body cavities. J Leukoc Biol. 2010;87:671–682. doi: 10.1189/jlb.0709505. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Swigut T, Schindler M, Kirchhoff F, Skowronski J. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. Journal of virology. 2005;79:10650–10659. doi: 10.1128/JVI.79.16.10650-10659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabu Y, Fujita H, Kinomoto M, Kaneko K, Ishizaka Y, Tanaka Y, Sata T, Tokunaga K. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. The Journal of biological chemistry. 2009;284:35060–35072. doi: 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson BD, Zack JA. In vivo pathogenesis of a human immunodeficiency virus type 1 reporter virus. J Virol. 1998;72:6520–6526. doi: 10.1128/jvi.72.8.6520-6526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkau T, Bacik I, Bennink JR, Yewdell JW, Hunig T, Schimpl A, Schubert U. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell host & microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ode H, Yoshida T, Sato K, Gee P, Yamamoto SP, Ebina H, Strebel K, Sato H, Koyanagi Y. Identification of amino acids in the human tetherin transmembrane domain responsible for HIV-1 Vpu interaction and susceptibility. Journal of virology. 2011;85:932–945. doi: 10.1128/JVI.01668-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97:12694–12699. doi: 10.1073/pnas.97.23.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadan JG, Bonifacino JS. Transmembrane domain determinants of CD4 Downregulation by HIV-1 Vpu. Journal of virology. 2012;86:757–772. doi: 10.1128/JVI.05933-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadan JG, Perez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog. 2010;6:e1000869. doi: 10.1371/journal.ppat.1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Chen MY, Willey RL, Strebel K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. Journal of virology. 1993;67:5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS pathogens. 2009;5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marassi FM, Ma C, Gratkowski H, Straus SK, Strebel K, Oblatt-Montal M, Montal M, Opella SJ. Correlation of the structural and functional domains in the membrane protein Vpu from HIV-1. Proc Natl Acad Sci U S A. 1999;96:14336–14341. doi: 10.1073/pnas.96.25.14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- McNatt MW, Zang T, Bieniasz PD. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS pathogens. 2013;9:e1003299. doi: 10.1371/journal.ppat.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5:e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood. 2010;116:1876–1884. doi: 10.1182/blood-2009-09-243667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutaftsi M, Brennan P, Spector SA, Tabi Z. Impaired lymphoid chemokine-mediated migration due to a block on the chemokine receptor switch in human cytomegalovirus-infected dendritic cells. Journal of virology. 2004;78:3046–3054. doi: 10.1128/JVI.78.6.3046-3054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomaguchi M, Fujita M, Adachi A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes and infection / Institut Pasteur. 2008;10:960–967. doi: 10.1016/j.micinf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Otero C, Groettrup M, Legler DF. Opposite fate of endocytosed CCR7 and its ligands: recycling versus degradation. Journal of immunology. 2006;177:2314–2323. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nature reviews Immunology. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- Park SH, Mrse AA, Nevzorov AA, Mesleh MF, Oblatt-Montal M, Montal M, Opella SJ. Three-dimensional structure of the channel-forming trans-membrane domain of virus protein “u” (Vpu) from HIV-1. J Mol Biol. 2003;333:409–424. doi: 10.1016/j.jmb.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Perez-Patrigeon S, Vingert B, Lambotte O, Viard JP, Delfraissy JF, Theze J, Chakrabarti LA. HIV infection impairs CCR7-dependent T-cell chemotaxis independent of CCR7 expression. AIDS. 2009;23:1197–1207. doi: 10.1097/QAD.0b013e32832c4b0a. [DOI] [PubMed] [Google Scholar]
- Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- Rong L, Zhang J, Lu J, Pan Q, Lorgeoux RP, Aloysius C, Guo F, Liu SL, Wainberg MA, Liang C. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. Journal of virology. 2009;83:7536–7546. doi: 10.1128/JVI.00620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sandberg JK, Andersson SK, Bachle SM, Nixon DF, Moll M. HIV-1 Vpu interference with innate cell-mediated immune mechanisms. Curr HIV Res. 2012;10:327–333. doi: 10.2174/157016212800792513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Bacik I, Cox JH, Bour S, Bennink JR, Orlowski M, Strebel K, Yewdell JW. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. Journal of virology. 1998;72:2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U, Ferrer-Montiel AV, Oblatt-Montal M, Henklein P, Strebel K, Montal M. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett. 1996;398:12–18. doi: 10.1016/s0014-5793(96)01146-5. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Felber BK, Benko DM, Fenyo EM, Pavlakis GN. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. Journal of virology. 1990;64:2519–2529. doi: 10.1128/jvi.64.6.2519-2529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AH, Sowrirajan B, Davis ZB, Ward JP, Campbell EM, Planelles V, Barker E. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skasko M, Wang Y, Tian Y, Tokarev A, Munguia J, Ruiz A, Stephens EB, Opella SJ, Guatelli J. HIV-1 Vpu protein antagonizes innate restriction factor BST-2 via lipid-embedded helix-helix interactions. The Journal of biological chemistry. 2012;287:58–67. doi: 10.1074/jbc.M111.296772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K. HIV accessory genes Vif and Vpu. Advances in pharmacology. 2007;55:199–232. doi: 10.1016/S1054-3589(07)55006-4. [DOI] [PubMed] [Google Scholar]
- Strebel K, Klimkait T, Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Tiganos E, Friborg J, Allain B, Daniel NG, Yao XJ, Cohen EA. Structural and functional analysis of the membrane-spanning domain of the human immunodeficiency virus type 1 Vpu protein. Virology. 1998;251:96–107. doi: 10.1006/viro.1998.9368. [DOI] [PubMed] [Google Scholar]
- Tilton B, Ho L, Oberlin E, Loetscher P, Baleux F, Clark-Lewis I, Thelen M. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. The Journal of experimental medicine. 2000;192:313–324. doi: 10.1084/jem.192.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Lugt B, Tubo NJ, Nizza ST, Boes M, Malissen B, Fuhlbrigge RC, Kupper TS, Campbell JJ. CCR7 plays no appreciable role in trafficking of central memory CD4 T cells to lymph nodes. Journal of immunology. 2013;191:3119–3127. doi: 10.4049/jimmunol.1200938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzke S, Michel N, Allespach I, Fackler OT, Keppler OT. Expression of Nef downregulates CXCR4, the major coreceptor of human immunodeficiency virus, from the surfaces of target cells and thereby enhances resistance to superinfection. Journal of virology. 2006;80:11141–11152. doi: 10.1128/JVI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigan R, Neil SJ. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. Journal of virology. 2010;84:12958–12970. doi: 10.1128/JVI.01699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigan R, Neil SJ. Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin. Journal of virology. 2011;85:9737–9748. doi: 10.1128/JVI.00479-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS pathogens. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey RL, Maldarelli F, Martin MA, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. Journal of virology. 1992;66:7193–7200. doi: 10.1128/jvi.66.12.7193-7200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willimann K, Legler DF, Loetscher M, Roos RS, Delgado MB, Clark-Lewis I, Baggiolini M, Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur J Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wray V, Federau T, Henklein P, Klabunde S, Kunert O, Schomburg D, Schubert U. Solution structure of the hydrophilic region of HIV-1 encoded virus protein U (Vpu) by CD and 1H NMR spectroscopy. International journal of peptide and protein research. 1995;45:35–43. doi: 10.1111/j.1399-3011.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang CK, Jiang H. Roles of phospholipid signaling in chemoattractant-induced responses. Journal of cell science. 2000;113(Pt 17):2935–2940. doi: 10.1242/jcs.113.17.2935. [DOI] [PubMed] [Google Scholar]
- Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Nagira M, Kitaura M, Imagawa N, Imai T, Yoshie O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J Biol Chem. 1998;273:7118–7122. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. The Journal of experimental medicine. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.