Abstract
Rationale
Cardiovascular health depends on proper development and integrity of blood vessels. Etv2 (Ets variant 2), a member of the E26 transforming specific (ETS) family of transcription factors, is essential to initiate a transcriptional program leading to vascular morphogenesis in early mouse embryos. However, endothelial expression of the Etv2 gene ceases at mid-gestation; therefore, vascular development past this stage must continue independent of Etv2.
Objective
To identify molecular mechanisms underlying transcriptional regulation of vascular morphogenesis and homeostasis in the absence of Etv2.
Methods and Results
Utilizing loss- and gain-of-function strategies and a series of molecular techniques, we identify Fli1 (Friend leukemia integration 1), another ETS family transcription factor, as a downstream target of Etv2. We demonstrate that Etv2 binds to conserved Ets-binding sites (EBSs) within the promoter region of the Fli1 gene and governs Fli1 expression. Importantly, in the absence of Etv2 at mid-gestation, binding of Etv2 at EBSs in the Fli1 promoter is replaced by Fli1 protein itself, sustaining expression of Fli1 as well as selective Etv2-regulated endothelial genes to promote endothelial cell survival and vascular integrity. Consistent with this, we report that Fli1 binds to the conserved EBSs within promoter and enhancer regions of other Etv2-regulated endothelial genes, including Tie2, to control their expression at and beyond mid-gestation.
Conclusions
We have identified a novel positive feed-forward regulatory loop in which Etv2 activates expression of genes involved in vasculogenesis, including Fli1. Once the program is activated in early embryos, Fli1 then takes over to sustain the process in the absence of Etv2.
Keywords: Transcription factors, autoregulation, vasculogenesis, gene expression, embryogenesis, apoptosis, developmental biology, embryonic development
INTRODUCTION
Endothelial dysfunction is a cardinal feature of several human cardiovascular diseases1. During embryogenesis, growth and survival of the vertebrate embryo relies critically on proper morphogenesis and homeostasis of the organs, tissues, and cell types that comprise the circulatory and cardiovascular systems2. Endothelial and blood progenitor cells of the circulatory system derive from a common ancestor known as the hemangioblast3, 4. Progenitor cell survival, specification, and differentiation require precisely orchestrated interacting action of numerous transcription factors. However, details of the transcriptional network that governs endothelial cell (EC) function and homeostasis during embryogenesis are still being defined. Elegant work using gene disruption and mutation strategies have demonstrated that diverse transcription factors are involved in vascular development5. Among them, the importance of the E26 transforming specific (ETS) family of transcription factors has been highlighted5.
The ETS family of transcription factors is conserved in metazoans and first identified in avian erythroblastosis virus, E266, 7. They are highly conserved in their DNA-binding ETS domain and govern a plethora of biological processes including, but not limited to, development, cellular growth, differentiation, cell-cell contact and viability5, 8. To date, 29 different Ets genes have been identified in mammals. Each binds to a core “GGAA/T” DNA-binding element6, 7, suggesting that aspects of their functions overlap. Global inactivation or mutation of numerous Ets genes either in mouse or zebrafish indicates that despite high conservation of the DNA-binding motif and recognition of a similar cis-regulatory element, certain ETS factors play an essential role in vascular morphogenesis and homeostasis which cannot be compensated by other family members5.
Recently, we and others have reported that the ETS family transcription factor Etv2 (also called Etsrp71/ER71) is essential for the genesis of hemato-endothelial progenitor cells in mice9–11. Mice lacking Etv2 die in utero around embryonic (E) day 9.5 with complete loss of vasculature9, 11. Similarly, knockdown of the Etv2 ortholog Ets-related protein (Etsrp) in zebrafish provokes a profound impairment of vasculogenesis12. Furthermore, recent studies demonstrate that loss of the Etv2/Etsrp in mice and zebrafish redirects endothelial progenitor cells to a myogenic fate13, 14, highlighting a central role for this transcription factor in endothelial specification and vascular development in the early embryo. Intriguingly, endothelial expression of Etv2 in wild type (WT) mice is undetectable at mid-gestation9–11, thus raising two major questions: 1) how are vascular morphogenesis and integrity maintained through the remainder of gestation? and 2) what controls the continued expression of known Etv2 target genes, such as Tie2 (also known as Tek)9, in the absence of Etv2?
Genome-wide sequence analyses identified Ets-binding sites (EBSs) in the promoter and enhancer regions of many endothelial-expressed genes, including ones encoding other ETS proteins5, 15. Therefore, we postulated that once expression of an Ets gene is activated by Etv2 early in development, an Etv2-targeted Ets gene may function to maintain its own expression as well as that of additional endothelial genes involved in EC survival, vascular morphogenesis and homeostasis. Recently published studies demonstrate that compared with WT embryos and embryonic stem cells (ESCs), transcript levels of several transcription factors involved in hematopoietic and vascular development during early embryogenesis are attenuated in Etv2 knockout embryos and ESCs11, 16, 17. However, the identity of a specific Etv2 target and its role in vascular morphogenesis in the absence of Etv2 remain elusive.
The primary purpose of this study was to identify a specific Etv2 target which can activate a positive autoregulatory feedback mechanism that persists beyond mid-gestation (and in the absence of Etv2) to govern critical elements of embryonic vasculogenesis. We demonstrate Fli1 (Friend leukemia integration 1) has such properties, being dependent on Etv2 to initiate embryonic expression early in development, and then acting to regulate its own expression, as well as that of additional Etv2-target endothelial genes involved in EC survival, vascular morphogenesis and homeostasis, at and beyond mid-gestation.
METHODS
Lentivirus production, purification, and infection
HA-tagged mouse Fli1 cDNA was subcloned into a lentiviral expression vector (Clontech) and co-transfected with pCD and VSVG constructs into HEK293T cells according to the manufacturer’s instructions (Clontech). Lentivirus was harvested from the culture supernatant and concentrated using ultracentrifugation for 2 hours at 22k rpm using SW28 rotor. Expanded protocols for viral infection, gene expression and histological and immunohistochemical (IHC) analyses utilizing isolated mouse embryos are provided in the Supplementary Data section.
RESULTS
Excessive endothelial cell death leads to hemorrhage in Fli1-null embryos at mid-gestation
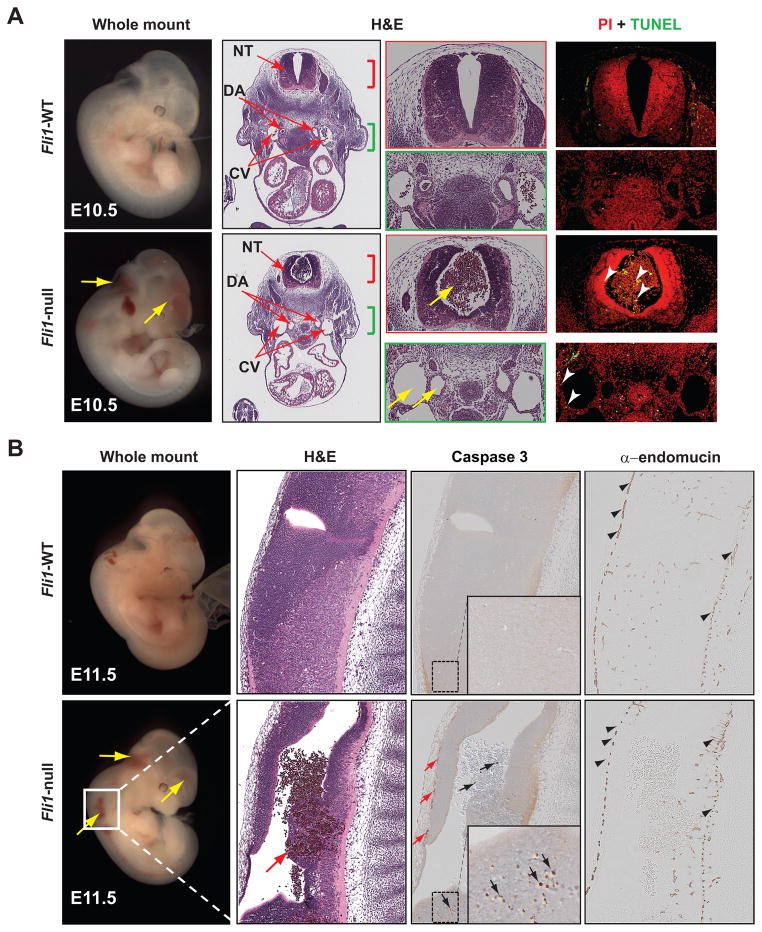
We and others have reported that mice lacking Fli1 die between E12.0–E12.5 due to widespread hemorrhage at mid-gestation18, 19. However, the underlying cause of this perturbation is unknown. To gain insights into mechanisms underlying vascular leakage in Fli1-null embryos, we mated Fli1 heterozygous mice19 and isolated WT and Fli1-null embryos from timed pregnant females at distinct developmental stages. Consistent with previously reported studies18, 19, Fli1-null embryos isolated at E10.5 (Figure 1A) and E11.5 (Figure 1B) manifested hemorrhage within the embryo proper as well as in the extra-embryonic (e.g. yolk sac) vasculature (data not shown) culminating ultimately in lethality by E12.0 (Supplemental Fig. IA). This was further supported by the absence of erythrocytes within the vasculature (e.g. the dorsal aorta and cardinal vein) and hemorrhage in the canal of the neural tube of Fli1-null, but not WT, embryos (Figure 1A). In addition, Fli1-null embryos also revealed diminished endocardial cushion formation (Supplemental Fig. IB), supporting a role of Fli1 in endocardial endothelium.
Figure 1. Vascular leakage in Fli1-null embryos at mid-gestation is due to excessive ECs death.
(A) Gross (whole-mount) and hematoxylin and eosin (H&E) sections of E10.5 WT and Fli1-null littermates are shown. Blood in the canal of neural tube (NT) but absent in the vasculature, dorsal aorta (DA) and cardinal vein (CV), due to hemorrhage (yellow arrows) is evident in null mice. Histology corresponding to NT (red bracket) and vasculatures (green bracket) are shown to demonstrate increased apoptotic cell death (TUNEL) of ECs, blood and neuropil (arrowheads) in null mice. (B) Gross and histologic (H&E) sections of E11.5 WT and Fli1-null littermates are shown. Higher magnification H&E images of NT illustrate hemorrhage in Fli1-null mice (red arrow). IHC analyses for activated caspase 3 and endomucin revealed increased apoptotic EC death (small red arrows), blood (black arrows) and cells of the neuropil (arrows in inset).
By contrast, Fli1-null embryos isolated at E8.5 (data not shown) and E9.5 (Supplemental Fig. IIA) are indistinguishable from WT littermates in terms of overall morphology, growth and cardiovascular structures. The presence of erythrocytes within the dorsal aorta of Fli1-null embryos and IHC analyses for the endothelial/endocardial marker α-endomucin9 supported the notion that embryonic vascular structures were normal in early (between E8.5–E9.5) WT and Fli1-null littermates (Supplemental Figs. IIA and IIB). This conclusion was further supported by similar transcript levels of two endothelial genes in both genotypes at E9.5 (Supplemental Fig. IIC). Collectively, these data support an essential role for Fli1 in vascular morphogenesis and homeostasis at mid-gestation.
Next, we analyzed whether vascular leakage in Fli1-null mice is associated with perturbation of endothelial proliferation and/or viability. IHC analyses for Ki67 demonstrated normal cellular proliferation in Fli1-null and WT littermates at E10.5 (Supplemental Fig. IB), suggesting that disruption of vascular integrity was not due to lack of endothelial proliferation. Utilizing terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and IHC analyses for activated caspase-3 and endomucin, we tested for evidence of apoptotic cell death in WT and Fli1-null embryos. Compared with WT embryos, Fli1-null embryos isolated at mid-gestation manifested excessive EC death (Figures 1A and 1B). It is worth noting that elevated levels of cell death within the blood and cells of the neuropil were also evident in Fli1-null embryos (Figure 1 and Supplemental Fig. IB). Taken together, we conclude that Fli1 is essential for EC viability and homeostasis at and beyond mid-gestation.
Etv2 is an essential upstream regulator of the Fli1 gene
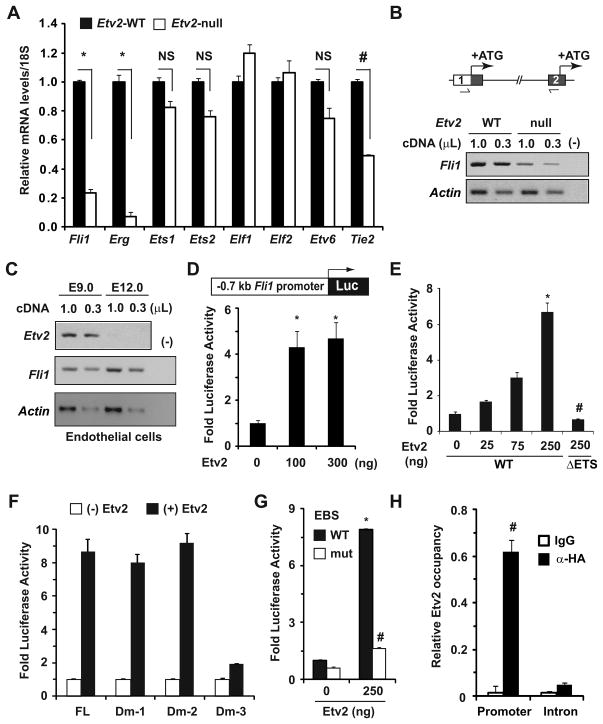
Having identified an essential role for Fli1 in ECs at and beyond mid-gestation, we sought to determine molecular mechanisms governing embryonic expression of the Fli1 gene. Embryonic expression of Fli1 occurring after Etv2 expression16, coupled with significant attenuation of Fli1 transcript levels in Etv2-null ESCs16, 17 and ECs13, together imply that Etv2 governs Fli1 expression in the early embryo. To test this concept, we purified RNA from E8.0 WT and Etv2-null littermates9 and analyzed transcript levels of numerous Ets genes. Quantitative RT-PCR (qRT-PCR) analyses showed that compared with WT, transcript levels of Fli1 and a closely-related Fli1 family member Erg5, were significantly attenuated in Etv2-null embryos (Figure 2A). By contrast, the transcript levels of several other Ets genes, including Ets1, Ets2, Elf1, Elf2, and Etv6, were unaffected (Figure 2A). Transcript levels of the Tie2 gene, an established downstream target of Etv2 in early embryo9, were also significantly decreased in mutant embryos (Figure 2A), which suggests that attenuation of Fli1 expression in the Etv2-null embryos was specific to the absence of Etv2. Based on these data, we hypothesized that Fli1 is a downstream target of Etv2 in the early mouse embryo.
Figure 2. Etv2 governs Fli1 gene expression in vitro and in vivo.
(A) qRT-PCR analyses for transcript levels of the indicated genes in E8.0 WT and Etv2-null embryos (n=3–4). Relative gene expression in WT embryos was normalized to 1. Note the significant attenuation of Fli1, Erg (*p<0.001 vs. WT) and Tie2 (#p<0.0001 vs. WT) gene expression in Etv2-null mice. NS: not significant. (B) Semi-quantitative RT-PCR (sqRT-PCR) analyses of Fli1 transcripts spanning exon 1 UTR and exon 2 (boxes) of the Fli1 gene (schematized top) in E8.0 WT and Etv2-null embryos. Translation initiation sites (ATG) in exon 1 and exon 2 are indicated. PCR loading (α-actin) and negative (−) controls are indicated. (C) sqRT-PCR analyses of Etv2 and Fli1 transcripts in GFP+ ECs isolated at the indicated developmental stages. Note the transient co-expression of the Etv2 and Fli1 genes in ECs of early embryos, while expression of the Fli1 gene persisted beyond mid-gestation. (D) Schematic of the Fli1 promoter region (−0.7kb) fused to a luciferase (Luc) reporter. Co-transfection of reporter and indicated amounts of Etv2 expression plasmids in HAECs resulted in significant induction of luciferase activity (*p<0.008 vs. control). (E) Transcriptional assays in COS1 cells elicited dose-dependent activation of luciferase activity by WT, but not mutant (lack of DNA-binding domain, ΔETS) Etv2. (F and G) Fold-activation of luciferase activity in the absence (−) and presence (+) of Etv2 (250ng) from FL and indicated Dm reporter plasmids are shown in panel F. Note the EBSs located between 200–250bp upstream of ATG were essential for efficient activation of luciferase activity, and that activity was significantly abrogated when they were mutated, panel G (*p<0.001 vs. control; # p<0.001 vs. WT). Luciferase activity without Etv2 was normalized to 1. (H) Quantitative analyses of Etv2 occupancy of the Fli1 promoter. Note the specific and significant induction of Etv2 occupancy of the Fli1 promoter (# p<0.001 vs. control).
It has been demonstrated that synthesis of two major isoforms of the Fli1 protein initiates from two distinct translation initiation sites (ATG) present within exon 1 and exon 2, respectively (Figure 2B)20. To confirm that Etv2-dependent Fli1 gene transcription initiates from exon 1, we utilized semi-quantitative RT-PCR (sqRT-PCR) techniques to analyze Fli1 transcripts using primers spanning the exon 1 untranslated region (UTR) and exon 2 (Figure 2B). We noted that Fli1 transcripts detected in WT embryos were significantly attenuated in Etv2-null embryos (Figure 2B). These data suggest that marked reduction of Fli1 transcripts in the Etv2-null embryos was associated with Etv2-dependent regulation of Fli1 gene transcription from exon 1.
We, and others, have reported that endothelial expression of the Etv2 gene stops at mid-gestation (E10.5–E11.5)9–11. This observation raised the possibility that expression of the Fli1 gene in whole embryo and/or in ECs will also be undetectable or decline at mid-gestation. Using sq- and qRT-PCR, we analyzed Etv2 and Fli1 gene expression in whole embryo and in green fluorescence protein (GFP)-positive ECs isolated from Tie2-GFP mice at distinct developmental stages. Consistent with our previously published study9, the Etv2 gene was expressed in the early embryos (E8.5–E9.5) (Supplemental Fig. IIIA) and ECs (Figure 2C), but its expression was undetectable at mid-gestation. In contrast, Fli1 transcripts were detected at all developmental stages and increased with age in whole embryos (Supplemental Fig. IIIA) and ECs (Figure 2C). Taken together, we conclude that Etv2 is required for endothelial Fli1 expression in the early embryo, while Fli1 expression at and beyond mid-gestation becomes Etv2-independent.
To complement our in vivo Etv2 loss-of-function data, we undertook in vitro approaches to validate Fli1 as a downstream target gene of Etv2. We fused a 0.7-kb Fli1 promoter region upstream of exon 1 harboring numerous conserved EBSs (Supplemental Fig. IIIB)21, 22 to a luciferase reporter cassette and conducted transcriptional analyses. Co-transfection of the reporter plasmid with increasing amounts of hemagglutinin (HA)-tagged Etv2 expression plasmid9 in primary human aortic endothelial cells (HAECs) resulted in significant activation of luciferase activity (Figure 2D). Since expression of numerous endothelial genes can be regulated by co-operative action of several ETS family transcription factors, including Etv29–11, 15, we repeated transcriptional assays in non-endothelial cells. Co-transfection of the reporter plasmid with Etv2 expression plasmid in C2C12 (Supplemental Fig. IIIC) or COS1 (Figure 2E) cells resulted in significant and dose-dependent activation of luciferase activity. However, co-transfection of COS1 cells with a mutant Etv2 that lacks the DNA-binding ETS domain failed to activate luciferase activity (Figure 2E), suggesting that DNA-binding by Etv2 is essential for the transcriptional activation of the Fli1 gene.
To test for the presence of EBSs in the Fli1 promoter critical for Etv2 activity, we generated a series of deletion constructs of the 0.7-kb promoter fragment (Supplemental Fig. IVA). In transcriptional assays utilizing each reporter plasmid in the presence or absence of Etv2, we identified three conserved EBSs, residing between 200 to 250-bp upstream of the ATG were essential and sufficient for Etv2-dependent activation of luciferase activity (Figure 2F and Supplemental Fig. IVA). Mutation of these EBSs disrupted Etv2-dependent activation of luciferase activity (Figure 2G and Supplemental Fig. IIIB). Utilizing quantitative analyses of chromatin immunoprecipitation (ChIP-qPCR) assays in C2C12 cells expressing HA-tagged Etv2, we confirmed specific occupancy of Etv2 at this Fli1 promoter region, and that Etv2 occupancy was not detected of an intronic region of the Fli1 gene which does not harbor a conserved EBS (Figure 2H and Supplemental Fig. IVB). These data suggest that the binding of Etv2 to these conserved EBSs is essential for activation of Fli1 expression. Indeed, ectopic Etv2 expression in C2C12 cells induced endogenous Fli1 transcript levels in a dose-dependent manner (Supplemental Fig. IVC). Collectively, these data suggest that Etv2 is an important upstream regulator of the Fli1 gene, and that ectopic Etv2 expression will activate Fli1 expression in a cell autonomous manner.
Fli1 promotes its own expression in the absence of Etv2
Having established Fli1 as an essential downstream target of Etv2 in early embryos, we set out to unveil molecular mechanisms underlying Etv2-independent transcriptional regulation of Fli1 expression at and beyond mid-gestation. Normal transcript levels of selected Ets genes in Etv2-null embryos (Figure 2A) along with the absence of vascular abnormalities at mid-gestation in mice lacking these factors5 suggest that they are not required for Fli1 expression at mid-gestation. Moreover, significant attenuation of transcript levels of Etv2 target genes, Erg and Gata2, in Fli1-null embryos at E10.5 (Supplemental Fig. VA) suggests that they are also not involved in Fli1 expression at mid-gestation. Based on these data, we hypothesized that Fli1 regulates its own promoter activity in the absence of Etv2.
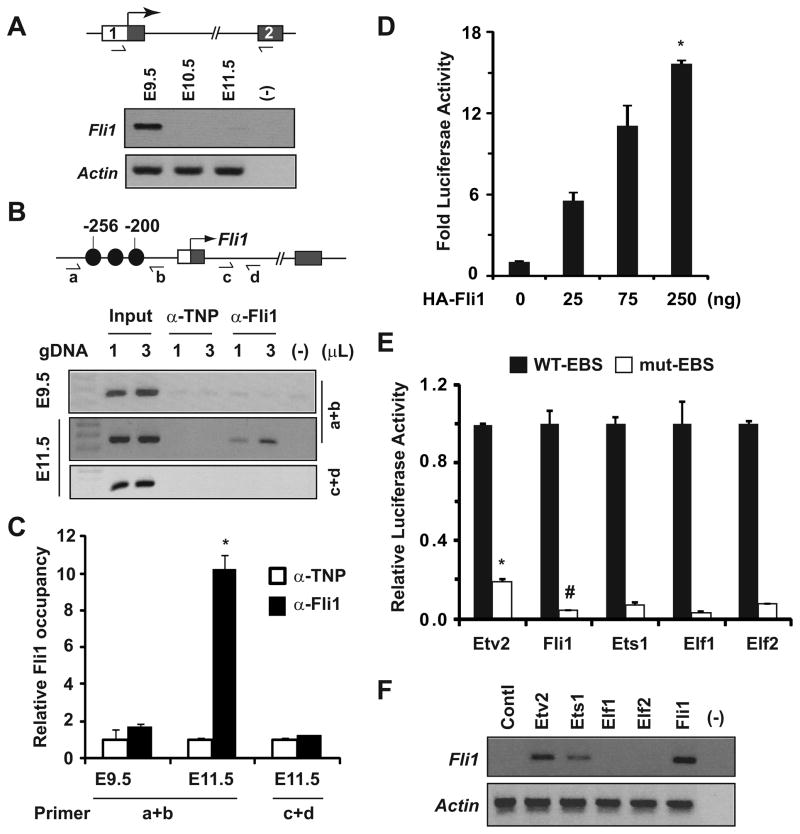
To test this hypothesis, we analyzed the N-terminal transcript levels of Fli1 in Fli1-null embryos which express very little Fli1 protein that lacks the C-terminal activation domain encoded by the exon 919. We detected Fli1 transcripts spanning the exon 1 UTR and exon 2 in E9.5, but not in E10.5 and E11.5, Fli1-null embryos (Figure 3A), while Fli1 transcripts at these developmental stages were readily detected in WT embryos (Supplemental Fig. IIIA). These data suggest that Fli1 is dispensable for Fli1 expression in early embryos but required for sustained expression at mid-gestation. To complement this observation, we performed ChIP assays using WT embryos isolated at distinct developmental stages and confirmed increased Fli1 occupancy of its own promoter in embryos isolated at E11.5 (Figures 3B and 3C). Surprisingly, such occupancy was not detected in embryos isolated at E9.5 or in the Fli1 intronic region in embryos isolated at E11.5 (Figures 3B and 3C). Collectively, these data lend strong support to our hypothesis that Fli1 occupancy of its own promoter region was associated with sustained endothelial Fli1 expression at mid-gestation in the absence of Etv2 (Figure 2C).
Figure 3. Fli1 governs Fli1 gene expression via positive autoregulation.
(A) Semi-quantitative RT-PCR analyses of Fli1 expression in Fli1-null embryos isolated at the indicated stages. Note the Fli1 transcripts detected at E9.5 were significantly attenuated at mid-gestation. (B and C) ChIP assays (B) and quantitative analyses (C) for Fli1 occupancy of the Fli1 promoter (schematized top) in embryos isolated at the indicated stages. Fli1-DNA complexes were immunoprecipitated with anti-Fli1 and anti-TNP (control) sera. Note that promoter-specific Fli1 binding was enriched at E11.5, but not at E9.5, embryos (*p<0.0001 vs. control). (D) Transcriptional assays using Dm-2 reporter and Fli1 expression plasmids in COS1 cells elicited robust and dose-dependent activation of luciferase activity (*p<0.0001 vs. control). (E) Transcriptional assays in the presence of indicated ETS factors (250ng) and Dm-2 reporter plasmids harboring WT and mutated EBSs are shown. Note that mutation of EBSs significantly attenuated luciferase activity. Luciferase activity for each ETS factor from reporter plasmid harboring WT EBS was normalized to 1. (F) RT-PCR analyses of the endogenous Fli1 transcripts following expression of the indicated ETS factors in C2C12 cells. Note that Etv2 and Fli1, but not Elf1 or Efl2, induced endogenous Fli1 transcript levels.
To complement these in vivo data, we performed transcriptional assays using a Fli1 reporter construct. In COS1 cells, we detected robust and dose-dependent activation of luciferase activity by Fli1 from a Fli1 reporter plasmid harboring WT (Figure 3D), but not mutated (Figure 3E), EBSs. Utilizing ChIP-qPCR, Western blot and RT-PCR analyses using primers designed to amplify only endogenous Fli1 transcripts, we further demonstrated that Fli1 occupancy of its own promoter region in C2C12 cells can induce endogenous Fli1, but not Etv2, transcript levels (Supplemental Figs. VB-D). These data further support our hypothesis that Fli1 acts downstream of Etv2 and can activate its own promoter activity. Interestingly, activation of Fli1 reporter activity by Etv2 or Fli1 was indistinguishable from that of other ETS factors, including Elf1 and Elf2 (Figure 3E). However, we found that unlike Etv2 and Fli1, ectopic expression of Elf1 and Elf2 in C2C12 cells did not induce endogenous Fli1 expression, and that expression of Ets1 showed only a modest effect (Figure 3F). Together, these data suggest that the failure of Elf1/Elf2 to induce endogenous Fli1 gene expression was secondary to an inability to access the EBSs sites in native chromatin but not due to an inability to recognize the EBSs in the Fli1 reporter plasmid. Based on our in vitro and in vivo data, we conclude that in the absence of Etv2 at mid-gestation, Fli1 binds to conserved EBSs within the Fli1 promoter to induce its own expression via positive autoregulation.
Fli1 is required for endothelial Fli1 expression for the remainder of gestation
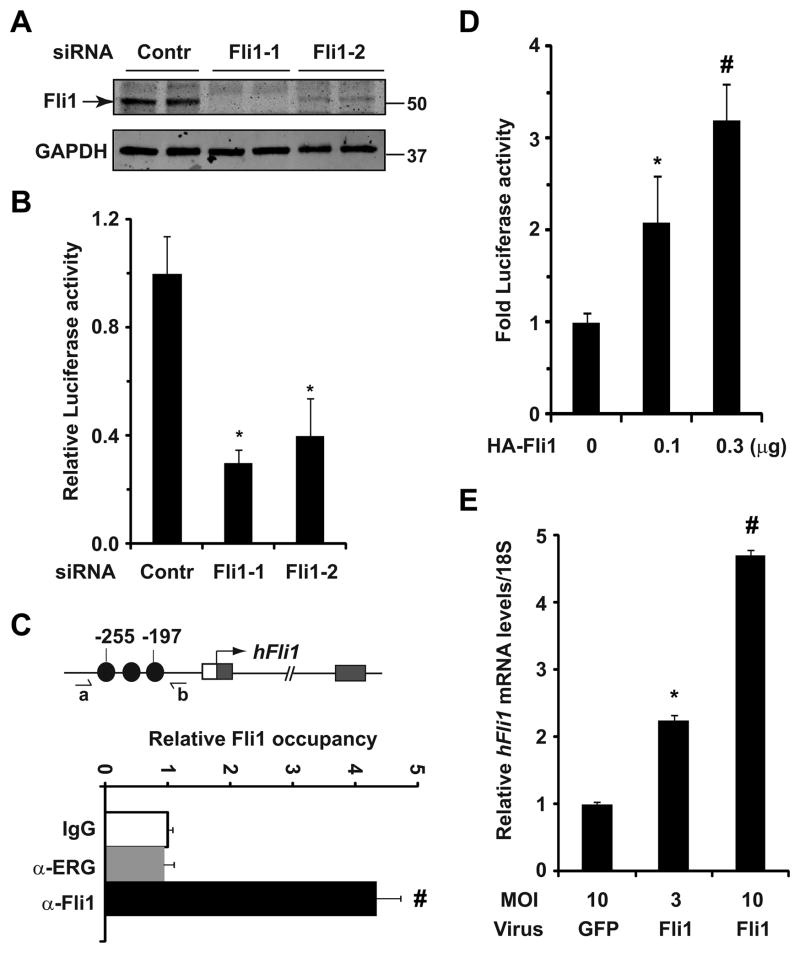
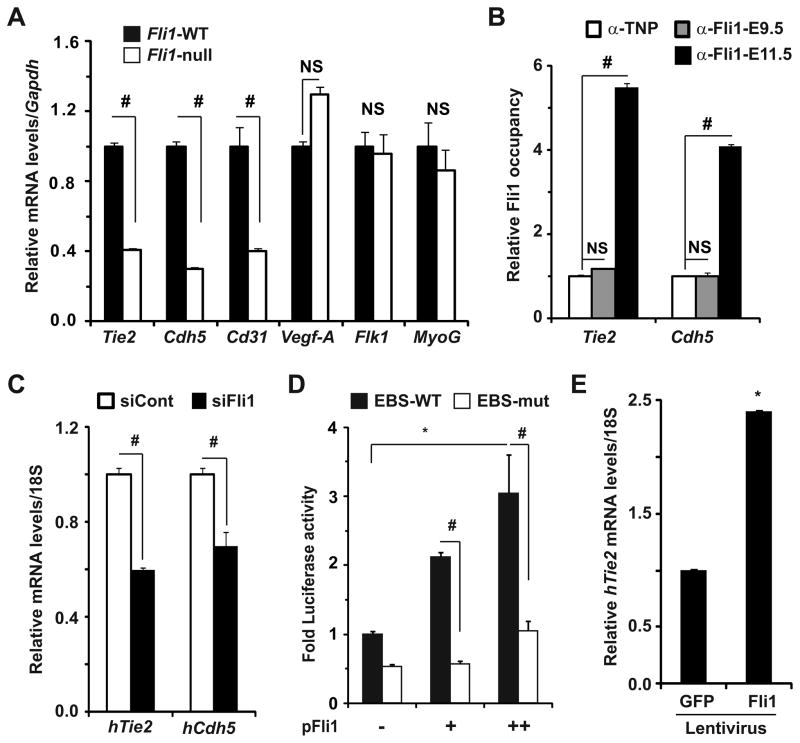
To determine whether feed-forward autoregulation of Fli1 is essential for sustained Fli1 expression during the remainder of gestation, we undertook Fli1 loss- and gain-of-function strategies in adult primary HAECs. Consistent with our recent findings of Fli1 expression in adult mouse ECs23, we detected marked reduction of Fli1 transcript (Supplemental Fig. VE) and protein (Figure 4A) levels in HAECs using two independent Fli1-specific siRNAs. We also noted that compared with COS1 cells, baseline Fli1 reporter activity was significantly higher in HAECs (data not shown), suggesting that endogenous Fli1 might activate its own reporter activity. To test this concept, we analyzed Fli1 reporter activity in HAECs and found that knockdown of Fli1 significantly attenuated Fli1 reporter activity (Figure 4B). Utilizing ChIP-qPCR, we detected Fli1, but not Erg, occupancy of the Fli1 promoter region in HAECs (Figure 4C). Taken together, we conclude that Fli1 is required to maintain Fli1 expression in adult ECs.
Figure 4. Fli1 is required for sustained Fli1 expression for the remainder of gestation.
(A) Western blot analyses of Fli1 in HAECs transfected with control (Contr) and Fli1-specific siRNAs. Note the marked reduction of Fli1 protein levels in siFli1-treated cells. GAPDH was used as a loading control. Numbers indicates protein molecular markers (kD). (B) Baseline Fli1 reporter activity in HAECs transfected with the indicated siRNA (p<0.05 vs. control). (C) ChIP-qPCR analyses of Fli1 occupancy of the Fli1 promoter. Note the Fli1, but not Erg, occupancy of Fli1 promoter (# p<0.003 vs. control). (D) Transcriptional assays reveal significant and dose-dependent induction of luciferase activity by Fli1 from Fli1 reporter plasmid in HAECs (*p<0.001 vs. control). (E) qRT-PCR analyses for the endogenous Fli1 transcripts in HAECs infected with control (GFP) and Fli1-encoding lentivirus (*p<0.003 vs. control, #p<0.0001 vs. control).
To further test our model, we undertook Fli1 gain-of-function approaches finding that co-transfection of a Fli1 reporter construct and HA-tagged Fli1 expression plasmids in HAECs resulted in significant and dose-dependent induction of luciferase activity (Figure 4D). Furthermore, lentivirus-mediated overexpression of mouse Fli1 in HAECs resulted in marked induction of endogenous Fli1 transcript levels (Figure 4E). Collectively, these data lend additional support to our hypothesis that Fli1 is essential for sustained Fli1 expression at and beyond mid-gestation.
Fli1 is an upstream regulator of selected Etv2-regulated endothelial genes in developing and adult endothelium
Finally, we set out to investigate whether Fli1 is essential for sustained expression of selected Etv2 target endothelial genes involved in EC viability and vascular homeostasis. For example, mice lacking Tie2, an endothelial receptor tyrosine kinase24, 25 and vascular endothelial cadherin 5 (VE-Cdh5)26, 27 manifest excessive EC death, abnormal vascular remodeling, hemorrhage and embryonic lethality. Consistent with previously reported studies18, 23, transcript levels of Tie2, Cdh5 and Cd31, but not Vegf-A and Flk1/Vegfr2, genes were significantly attenuated in Fli1-null embryos at E10.5 (Figure 5A). Furthermore, similar transcript levels of the myogenic transcription factor myogenin in both genotypes at E10.5 suggest that attenuation of transcript levels of endothelial genes, including Tie2 was specific to the loss of Fli1. IHC analyses for Tie2 and Fli1 also supported the notion that significant attenuation of Tie2 protein levels in vascular ECs of Fli1-null littermates was associated with EC-specific loss of Fli1 (Supplementary Fig. VIA). Given that Tie2 transcripts in Fli1-null embryos declined to a similar extent in Etv2-null embryos (Figure 2A), we reasoned that the regulation of endothelial genes abundance in mid-gestational endothelium occurred at the level of gene transcription.
Figure 5. Fli1 governs selective Etv2-regulated endothelial gene expression at and beyond mid-gestation.
(A) qRT-PCR analyses for transcript levels of the indicated genes in E10.5 WT and Fli1-null embryos (n=3). Relative gene expression in WT mice was normalized to 1 (*p<0.001 vs. WT). NS: not significant. (B) ChIP-qPCR analyses demonstrating Fli1 occupancy of the promoter of indicated endothelial genes in E11.5, but not E9.5, embryos (# p<0.001 vs. control). (C) qRT-PCR analyses revealing significant attenuation of Tie2 and Cdh5 transcript levels in siFli1-treated HAECs (#p<0.01 vs. control). (D) Transcriptional assays in COS1 cells reveal significant and dose-dependent induction of luciferase activity by Fli1 from Tie2-UPF reporter plasmid harboring WT (black), but not mutated (white), EBSs (*p<0.001 vs. control; # p<0.002 vs. WT EBS). (E) qRT-PCR analyses for Tie2 transcript levels in HAECs infected with control (GFP) and Fli1-encoding lentivirus (MOI 10) (*p<0.001 vs. control).
It has been reported that an upstream promoter fragment (UPF) and a distal first intronic enhancer fragment (IEF) are essential for sustained endothelial Tie2 expression at mid- and late gestation28. Moreover, both the UPF and IEF have been reported to harbor several conserved EBSs9. Therefore, we examined whether immunoprecipitation of Fli1-DNA complexes from embryos (Figure 3B) harvested regulatory elements of Tie2 and a known Fli1 downstream target Cdh523. Consistent with our previous report23, we detected Fli1 occupancy of the Cdh5 promoter in vivo (Figure 5B). In fact, we also noted Fli1 occupancy of conserved EBSs within the UPF (Figure 5B) and IEF (Supplemental Fig. VIB) of the Tie2 gene in embryos isolated at E11.5, but not at E9.5. These data suggest that Fli1 binds to the conserved EBSs within the promoter of selected Etv2 target endothelial genes, including Tie2, to govern their expression at and beyond mid-gestation.
To complement our in vivo data, we undertook Fli1 loss- and gain-of-function strategies and analyzed transcript levels of Tie2 and Cdh5 in vitro. We found that siRNA-mediated knockdown of Fli1 in HAECs resulted in marked reduction of transcripts levels of Tie2 and Cdh5 (Figure 5C). Furthermore, co-transfection of the Tie2 reporter with increasing amounts of Fli1 expression plasmid in COS1 cells revealed marked and dose-dependent induction of luciferase activity, while mutation of the EBSs in a 2.1-kb upstream promoter fragment of the Tie2 gene9 significantly attenuated the induction of reporter activity (Figure 5D). Importantly, ectopic Fli1 expression in C2C12 cells (Supplementary Fig. VIC) and HAECs (Figure 5E) resulted in significant induction of endogenous Tie2 expression. Thus, we conclude that in the absence of Etv2 at mid-gestation, Fli1 governs EC survival and vascular integrity by regulating expression of selected Etv2 target transcription (Supplementary Fig. VA) and signaling (e.g. Tie2) factors at and beyond mid-gestation.
DISCUSSION
The overall goal of this study was to decipher the Etv2-mediated transcriptional network that integrates vascular morphogenesis from early to late gestation in the developing mouse embryo. Our study reports three important findings. First, using a combination of molecular and Etv2 loss- and gain-of-function experiments, we have identified Fli1 as a downstream target of Etv2 in the early embryo and defined the underlying regulatory mechanisms. Second, we have uncovered a previously unrecognized positive autoregulatory mechanism controlling Fli1 gene expression at and beyond mid-gestation, thereby providing insight into mechanisms governing vascular morphogenesis and homeostasis in the absence of Etv2. We demonstrate that when Etv2 expression is extinguished, Fli1 acts to regulate its own expression, as well as that of selected other Etv2 target genes, at and beyond mid-gestation. In so doing, it governs EC viability and vascular integrity, which accounts for the vascular leakage and ultimate embryonic lethality reported in Fli1 mutant mice18, 19. Third, we have identified Fli1 as an upstream regulator of Tie2 and demonstrated that Fli1 binds to the conserved EBS within the UPF and IEF of the Tie2 gene to regulate endothelial Tie2 expression at and beyond mid-gestation. Collectively, our findings identify Etv2 and Fli1 as a classic example of a feed-forward autoregulatory feedback loop that initiates and maintains vascular morphogenesis, homeostasis and subsequent fetal growth and survival during development.
Since the discovery of Etv2 as an essential regulator of endothelial fate of progenitor cells, and its temporal and spatial expression pattern in the EC of early mouse embryo9–11, the general consensus has been that Etv2 activates a downstream transcriptional network in early embryo, which in turn regulates EC homeostasis and vascular morphogenesis in the absence of Etv2. Indeed, compared with WT ESCs16, 17 and ECs13, 16, transcript levels of several transcription factors, including Fli1, were significantly attenuated in Etv2-null cells. Reciprocally, ectopic expression of Etv2 in zebrafish embryo29 and differentiating ESCs30 induces endogenous Fli1 transcript levels. Although these data are consistent with our hypothesis that Etv2 can induce Fli1 expression in a cell autonomous manner, the specificity of this response and the underlying mechanisms of Fli1 gene expression regulation by Etv2 were incompletely understood. A recently published study describes modest activation of the Fli1 gene by Etv2 through binding to a conserved EBS located 11.4-kb downstream of the Fli1 translation initiation site in exon 1, but mutation of that site did not significantly attenuate Fli1 reporter activity16, highlighting the physiological significance of cis-regulatory motifs within the Fli1 promoter region for transcriptional regulation of Fli1 expression by Etv2.
Although all ETS factors recognize a similar cis-regulatory element6, 7, attenuation of Fli1 expression in Etv2-null mice was not complemented by several other ETS factors, which are known to be expressed in endothelial cells21, 22. However, all of them, including Elf1 and Elf2, induced Fli1 reporter activity in vitro. These data suggest that the binding specificity of ETS factors to a cis-regulatory element is uniquely regulated in native chromatin, which is often indistinguishable in naked DNA. It is also plausible that the transcriptional regulation of Fli1 and Tie2 genes by other ETS factors in endothelial and non-endothelial cells is context-dependent or dependent on their cooperative action with other family members. Indeed, cooperative actions among numerous ETS factors, including Etv2, Fli1 and Erg, and other transcription factors, are known to play essential roles in endothelial gene expression during development10, 31. Consistent with these studies, a cooperative action between Fli1 and Erg is required for hematopoiesis in mice32 and mice lacking Fli1 and endothelial isoforms of Erg (isoforms 5–7)33 manifest vascular as well as cardiac malformations at mid-gestation. Therefore, it is conceivable that a cooperative action between Fli1 and other ETS factors governs endothelial genes expression and vascular morphogenesis in the absence of Etv2.
Autoregulation of gene expression is a common theme among transcription factors that govern expression of a large number of genes during embryogenesis34. Although autoregulatory mechanisms can be positive or negative, the significance of autoregulation relates to maintenance of cell fate and cellular homeostasis. Positive autoregulation has been described for numerous developmental transcription factors including, but not limited to, paired box protein 6 (Pax6)35, homeobox (Hox) proteins and several myogenic factors, MyoD and myocyte enhancer factor 2 (Mef2)34, 36,37, which are essential for cardiac and skeletal myogenesis. For example, the basic helix-loop-helix transcription factor Twist, which governs mesodermal specification and myogenesis36 and Mef2 proteins are co-expressed during early phases of mesodermal development. However, Twist expression is extinguished before muscle cell differentiation, while Mef2 expression persists throughout mesodermal development and subsequent muscle differentiation34. Of interest is that positive autoregulatory mechanisms related to Twist and Mef2 in skeletal myogenesis parallel those reported here for Etv2 and Fli1 in vascular morphogenesis.
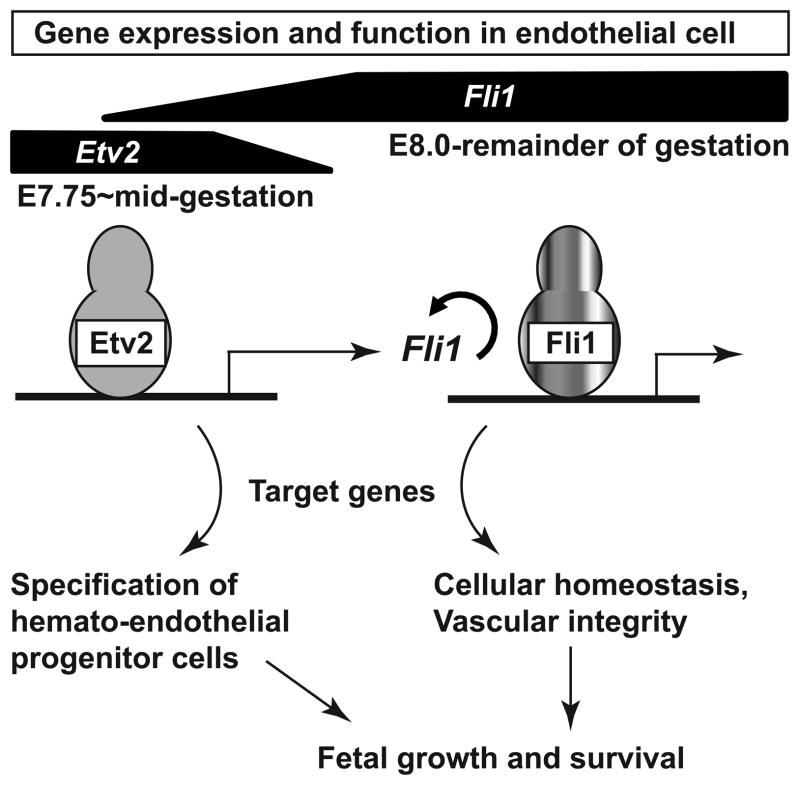
Our model suggests that in the early embryo, Etv2 activates a transcriptional network16, 17 involving Fli1 and Tie29 to govern the hemato-endothelial fate of progenitor cells and subsequent fetal growth and survival (Figure 6). At mid-gestation, when Etv2 expression turns off, Fli1 autoregulates its own expression as well as that of selected Etv2 target genes (such as Erg, Cdh5 and Tie2) for the remainder of gestation to govern EC survival and vascular integrity (Figure 6). Consistent with our model, endothelial expression of a lacZ reporter driven by the native Fli1 promoter is significantly diminished at mid-gestation and undetectable at E14.5 in Fli1-null embryos, while reporter expression in ECs persists in Fli1-heterozygous embryos during these developmental stages18, 19. By contrast, endothelial expression of a lacZ reporter driven by the native Erg promoter is not reduced in Erg-null embryo at mid-gestation33, suggesting that Fli1, but not Erg, is required to govern its own promoter activity at mid-gestation. Although embryonic lethality of Etv2-null mice is not associated with excessive EC death9, our data along with previously published studies24–27 support a model in which attenuation of endothelial Tie2 and Cdh5 expression is associated with excessive EC death in Fli1-null embryos. However, we cannot rule out the possibility that additional factors might be involved in this process. Previously, we demonstrated that Etv2 governs Tie2 expression in early embryonic ECs9, yet the molecular mechanisms underlying transcriptional regulation of Tie2 expression at and beyond mid-gestation were unknown. Our study is the first to report that Fli1 is an important regulator of endothelial Tie2 expression at and beyond mid-gestation.
Figure 6.
Working model for positive autoregulation between Etv2 and Fli1 regulates vascular morphogenesis and integrity at early and mid-gestation of mouse embryogenesis. Embryonic expression of Etv2 and Fli1 genes at distinct developmental stages and their role in EC are indicated. See text for details.
In conclusion, we have uncovered a unique and previously unrecognized positive autoregulatory transcriptional circuit whereby coordinated transcriptional activity of Etv2 and Fli1 regulates vascular morphogenesis and homeostasis at distinct stages of embryogenesis. Given the essential role of Fli1 in cardiovascular morphogenesis and vascular inflammation6, 7, it is conceivable that Fli1 may be intimately involved in vascular and cardiovascular diseases. Moreover, Fli1 is associated with thymus development and cancer pathogenesis38–40. Looking to the future, development of small molecules that positively or negatively modulate Fli1 activity will provide molecular insights into a novel mechanism with potential clinical relevance.
Supplementary Material
Novelty and Significance.
What Is Known?
The E26 transforming specific (ETS) family transcription factor Etv2 governs endothelial fate of the progenitor cells and vascular morphogenesis in early development.
In early mouse embryo, the Etv2 activates numerous endothelial genes, including the ETS family transcription factor Fli1 gene.
Endothelial expression of the Etv2 gene stops at mid-gestation; hence, molecular mechanisms underlying transcriptional regulation of vascular morphogenesis and homeostasis beyond this stage remain incompletely understood.
What New Information Does This Article Contribute?
We have identified the mechanism underlying Etv2-dependent regulation of the Fli1 gene expression in early mouse embryo.
At mid-gestation, when Etv2 expression turns off, Fli1 regulates its own expression as well as that of selected Etv2 target endothelial genes at and beyond mid-gestation.
This positive autoregulatory mechanism of Fli1 gene expression is critical for endothelial cell (EC) survival and vascular integrity.
Mice lacking Etv2 die in utero around embryonic day 9.5 with complete loss of vasculature. Etv2 is an essential upstream regulator of numerous endothelial genes and governs endothelial fate of the progenitor cells. Intriguingly, in mice endothelial expression of the Etv2 gene ceases at mid-gestation. This study was designed to identify a specific Etv2 target that controls its own expression as well as that of additional Etv2-regulated genes and maintains vascular morphogenesis and integrity through the remainder of gestation. We demonstrate that Fli1 has such properties, being dependent on Etv2 to initiate embryonic expression early in development, and then acting to regulate its own expression as well as that of selected Etv2 target endothelial genes. In so doing, Fli1 governs EC viability and vascular integrity for the remainder of gestation critical for fetal growth and survival. Our study identifies the Etv2 and Fli1 axis as a classic example of a feed-forward autoregulatory feedback loop in the endothelium that initiates and maintains cardiovascular health at distinct developmental stages.
Acknowledgments
We thank Drs. Tamara K. Nowling of Medical University of South Carolina, Peter Oettgen of Harvard Medical School, Eric N. Olson, Philip Shaul, Hiromi Yanagisawa and Ondine Cleaver of University of Texas Southwestern Medical Center for providing reagents. We also thank Drs. Joseph A. Hill, Beverly A. Rothermel, James A. Richardson, and Thomas G. Gillette for critical reading of the manuscript, and Dr. Ken Chambliss of Shaul lab and members of Hill, Rothermel and Ferdous labs for technical supports.
SOURCES OF FUNDING
Funding for these studies was provided by the National Institutes of Health (P30-HL101254-01) and the March of Dimes (5-FY09-21).
Nonstandard Abbreviations and Acronyms
- ETS
E26 transforming specific
- E
Embryonic
- EC
Endothelial cell
- ChIP
Chromatin immunoprecipitation
- UPF
Upstream promoter fragment
- IEF
Intronic enhancer fragment
- EBS
Ets-binding site
- sqRT-PCR
semi-quantitative RT-PCR
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
DISCLOSURES
None
References
- 1.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson EN, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. doi: 10.1126/science.272.5262.671. [DOI] [PubMed] [Google Scholar]
- 3.Choi K. The hemangioblast: A common progenitor of hematopoietic and endothelial cells. J Hematother Stem Cell Res. 2002;11:91–101. doi: 10.1089/152581602753448568. [DOI] [PubMed] [Google Scholar]
- 4.Chung YS, Zhang WJ, Arentson E, Kingsley PD, Palis J, Choi K. Lineage analysis of the hemangioblast as defined by Flk1 and Scl expression. Development. 2002;129:5511–5520. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- 5.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oettgen P. Regulation of vascular inflammation and remodeling by ETS factors. Circ Res. 2006;99:1159–1166. doi: 10.1161/01.RES.0000251056.85990.db. [DOI] [PubMed] [Google Scholar]
- 7.Laudet V, Hanni C, Stehelin D, Duterque-Coquillaud M. Molecular phylogeny of the Ets gene family. Oncogene. 1999;18:1351–1359. doi: 10.1038/sj.onc.1202444. [DOI] [PubMed] [Google Scholar]
- 8.Sharrocks AD. The Ets-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 9.Ferdous A, Caprioli A, Iacovino M, Martin CM, Morris J, Richardson JA, Latif S, Hammer RE, Harvey RP, Olson EN, Kyba M, Garry DJ. Nkx2-5 transactivates the Ets-related protein 71 gene and specifies an endothelial/endocardial fate in the developing embryo. Proc Natl Acad Sci U S A. 2009;106:814–819. doi: 10.1073/pnas.0807583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL. Combinatorial regulation of endothelial gene expression by Ets and Forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasmussen TL, Kweon J, Diekmann MA, Belema-Bedada F, Song Q, Bowlin K, Shi X, Ferdous A, Li T, Kyba M, Metzger JM, Koyano-Nakagawa N, Garry DJ. ER71 directs mesodermal fate decisions during embryogenesis. Development. 2011;138:4801–4812. doi: 10.1242/dev.070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palencia-Desai S, Kohli V, Kang J, Chi NC, Black BL, Sumanas S. Vascular endothelial and endocardial progenitors differentiate as cardiomyocytes in the absence of Etsrp/Etv2 function. Development. 2011;138:4721–4732. doi: 10.1242/dev.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sementchenko VI, Watson DK. Ets target genes: Past, present and future. Oncogene. 2000;19:6533–6548. doi: 10.1038/sj.onc.1204034. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRα+ primitive mesoderm. Blood. 2012;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 17.Wareing S, Mazan A, Pearson S, Gottgens B, Lacaud G, Kouskoff V. The Flk1-cre-mediated deletion of Etv2 defines its narrow temporal requirement during embryonic hematopoietic development. Stem Cells. 2012;30:1521–1531. doi: 10.1002/stem.1115. [DOI] [PubMed] [Google Scholar]
- 18.Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177. doi: 10.1016/s1074-7613(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 19.Spyropoulos DD, Pharr PN, Lavenburg KR, Jackers P, Papas TS, Ogawa M, Watson DK. Hemorrhage, impaired hematopoiesis, and lethality in mouse embryos carrying a targeted disruption of the Fli1 transcription factor. Mol Cell Biol. 2000;20:5643–5652. doi: 10.1128/mcb.20.15.5643-5652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarrazin S, Starck J, Gonnet C, Doubeikovski A, Melet F, Morle F. Negative and translation termination-dependent positive control of Fli-1 protein synthesis by conserved overlapping 5′ upstream open reading frames in Fli-1 mRNA. Mol Cell Biol. 2000;20:2959–2969. doi: 10.1128/mcb.20.9.2959-2969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lelievre E, Lionneton F, Mattot V, Spruyt N, Soncin F. Ets-1 regulates Fli-1 expression in endothelial cells. Identification of ETS binding sites in the Fli-1 gene promoter. J Biol Chem. 2002;277:25143–25151. doi: 10.1074/jbc.M201628200. [DOI] [PubMed] [Google Scholar]
- 22.Svenson JL, Chike-Harris K, Amria MY, Nowling TK. The mouse and human Fli1 genes are similarly regulated by Ets factors in T cells. Genes Immun. 2010;11:161–172. doi: 10.1038/gene.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asano Y, Stawski L, Hant F, Highland K, Silver R, Szalai G, Watson DK, Trojanowska M. Endothelial Fli1 deficiency impairs vascular homeostasis: A role in scleroderma vasculopathy. Am J Pathol. 2010;176:1983–1998. doi: 10.2353/ajpath.2010.090593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niu Q, Perruzzi C, Voskas D, Lawler J, Dumont DJ, Benjamin LE. Inhibition of Tie-2 signaling induces endothelial cell apoptosis, decreases Akt signaling, and induces endothelial cell expression of the endogenous anti-angiogenic molecule, thrombospondin-1. Cancer Biol Ther. 2004;3:402–405. doi: 10.4161/cbt.3.4.735. [DOI] [PubMed] [Google Scholar]
- 25.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, Tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 27.Birdsey GM, Dryden NH, Amsellem V, Gebhardt F, Sahnan K, Haskard DO, Dejana E, Mason JC, Randi AM. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood. 2008;111:3498–3506. doi: 10.1182/blood-2007-08-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 30.Koyano-Nakagawa N, Kweon J, Iacovino M, Shi X, Rasmussen TL, Borges L, Zirbes KM, Li T, Perlingeiro RC, Kyba M, Garry DJ. Etv2 is expressed in the yolk sac hematopoietic and endothelial progenitors and regulates lmo2 gene expression. Stem Cells. 2012;30:1611–1623. doi: 10.1002/stem.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R, Pei H, Watson DK. Regulation of ETS function by protein - protein interactions. Oncogene. 2000;19:6514–6523. doi: 10.1038/sj.onc.1204035. [DOI] [PubMed] [Google Scholar]
- 32.Kruse EA, Loughran SJ, Baldwin TM, Josefsson EC, Ellis S, Watson DK, Nurden P, Metcalf D, Hilton DJ, Alexander WS, Kile BT. Dual requirement for the ETS transcription factors Fli-1 and Erg in hematopoietic stem cells and the megakaryocyte lineage. Proc Natl Acad Sci U S A. 2009;106:13814–13819. doi: 10.1073/pnas.0906556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayaraj P, Le Bras A, Mitchell N, Kondo M, Juliao S, Wasserman M, Beeler D, Spokes K, Aird WC, Baldwin HS, Oettgen P. Erg is a crucial regulator of endocardial-mesenchymal transformation during cardiac valve morphogenesis. Development. 2012;139:3973–3985. doi: 10.1242/dev.081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crews ST, Pearson JC. Transcriptional autoregulation in development. Curr Biol. 2009;19:R241–246. doi: 10.1016/j.cub.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol. 2003;257:1–13. doi: 10.1016/s0012-1606(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 36.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 37.Dekel I, Magal Y, Pearson-White S, Emerson CP, Shani M. Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol. 1992;4:217–224. [PubMed] [Google Scholar]
- 38.Melet F, Motro B, Rossi DJ, Zhang L, Bernstein A. Generation of a novel Fli-1 protein by gene targeting leads to a defect in thymus development and a delay in friend virus-induced erythroleukemia. Mol Cell Biol. 1996;16:2708–2718. doi: 10.1128/mcb.16.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oikawa T. ETS transcription factors: Possible targets for cancer therapy. Cancer Sci. 2004;95:626–633. doi: 10.1111/j.1349-7006.2004.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seth A, Watson DK. Ets transcription factors and their emerging roles in human cancer. Eur J Cancer. 2005;41:2462–2478. doi: 10.1016/j.ejca.2005.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.