Abstract
Drug self-administration procedures in laboratory settings allow us to closely model drug-taking behavior in real-world settings. This review provides an overview of many of the common self-administration methods used in human laboratory research. Typically, self-administration studies provide a quantifiable measure of the reinforcing effect of a drug, which is believed to be predictive of its potential for abuse. Several adaptations of the self-administration paradigm exist, the simplest of which allows participants free access to the drug under investigation. Free-access procedures allow investigators to observe patterns of drug self-administration and drug effects in a controlled setting. Allowing participants to choose between two simultaneously available reinforcers (choice procedures) is another well-established method of assessing the reinforcing effects of a drug. Offering a choice between two reinforcers (e.g. two different doses of the same drug, two different drugs, or drug and nondrug reinforcers) provides researchers with a point of comparison (e.g. between a drug of known abuse potential and a novel drug). When combined with other endpoints, such as subjective effects ratings, physiological responses, and cognitive performance, human self-administration paradigms have contributed significantly to our understanding of the factors that contribute to, maintain, and alter drug-taking behavior including: craving, positive subjective effects, toxicity, drug interactions and abstinence. This area of research has also begun to incorporate other techniques such as imaging and genetics to further understand the multifaceted nature of substance abuse. The present paper summarizes the different self-administration techniques that are commonly used today and the application of other procedures that may complement interpretation of the drug
Keywords: abuse potential, choice procedures, drug abuse, free-access, reinforcer, self-administration
Why model drug abuse?
Drug abuse is characterized by compulsive drug seeking and use (http://www.who.int/topics/substance_abuse/en/). Substance use disorders worsen other psychiatric conditions, contribute to crime and the spread of HIV and hepatitis C, and cause the deaths of millions of people each year [United Nations Office on Drugs and Crime (UNODC), 2012]. A number of experimental paradigms have been developed to study the various aspects of the addictive process from prenatal exposure to relapse. Other procedures attempt to quantify the abuse potential of drugs and ways to manipulate them to deter abuse. Evaluation of the abuse liability of new compounds is also an important component of the drug development process. Self-administration testing provides valuable data so that rational decisions can be made about the abuse potential of new medications in relation to their clinical efficacy. As such, self-administration data are required by many government regulatory authorities before approval. Subjective-effects batteries that assess self-reported drug effects such as ‘liking’ and ‘high,’ can provide critical information concerning the positive and negative subjective effects of a drug, the balance of which may determine whether the drug will be abused (Foltin and Fischman, 1991).
Laboratory models of self-administration synthesize the physiological, behavioral, and cognitive aspects of a drug’s effects, and translate them into a quantifiable indicator of abuse liability, drug-taking behavior itself. In general, drugs of abuse are thought of as reinforcers, because they increase the likelihood/occurrence of the behavior(s) that lead to their delivery (Catania, 1971; Brady and Lucas, 1984). Within the context of self-administration studies, the reinforcing effects of a drug have typically been quantified in terms of: the amount of drug participants ingest, the rate of responding to receive the drug, the amount of effort they are willing to devote to receive the drug, and/or the percentage of times drug is chosen over placebo or a nondrug alternative reinforcer.
Because drug taking is an essential feature of drug abuse, the face validity of the self-administration procedure is clear. Furthermore, the strong correlation between self-administration of a drug in the laboratory and its abuse in the natural environment indicates that this technique also has good predictive validity (Brady et al., 1987; Balster, 1991; for reviews see: Comer et al., 2008a; Haney and Spealman, 2008). Accordingly, self-administration in a controlled laboratory environment is commonly used to assess the abuse potential of novel compounds, drug combinations, and abuse-deterrent formulations. Self-administration can also be used to predict which medications will reduce the abuse potential of a drug by decreasing the motivation to use the drug and/or drug-seeking behavior. Within the context of the self-administration model, this is reflected as reduced drug taking in the laboratory.
Researchers have been systematically studying drug self-administration in human research volunteers for decades (Wikler, 1952; Mello and Mendelson, 1965). Clinical laboratory procedures were adapted from preclinical models and have been modified to investigate the abuse liability of all of the major classes of commonly abused drugs (Comer et al., 2010a). Many different types of self-administration procedures have been used in humans. Methodologies vary on the basis of not only differences in the various classes of drugs being tested, but also according to the goals of the study. This review attempts to summarize many of the most common self-administration techniques used by clinical investigators (Table 1).
Table 1.
Commonly used drug self-administration procedures
| Procedure | Advantages | Disadvantages |
|---|---|---|
| Free access Participants request drug (most often verbally) whenever theywant it Dependent measures: number of doses chosen, interval between dose requests |
Simple Somewhat naturalistic for some drugs, such as coffee or cigarettes |
Can be insensitive to dose conditions Most drugs of abuse are not available in unlimited supply, and are not safe to deliver in this manner |
| Choice (discrete trial choice) Participants perform a verbal or nonverbal operant to select between two potential reinforcers (drug vs. drug, drug vs. placebo, drug vs. money) Dependent measures: number or percentage of drug chosen in comparison with alternative reinforcers |
Simple Experimenter controls the interval at which doses are available Enhanced safety It is possible to test the relative reinforcing effects of two or more different drugs |
Requires many sessions to examine dose– response relationships The appropriate alternative money amount may vary for different populations |
| Multiple-choice procedure Participants are asked to make a series of choices between the drug under investigation and other reinforcers of increasing monetary value or monetary values that escalate Dependent measure: the monetary value at which participants switch from choosing drug to choosing money (crossover point) |
Does not require multiple-drug administrations Sensitive to dose manipulations |
Not all choices are reinforced There is often considerable delay between the time the choice is made and when the reinforcer is given Generally not considered a direct measure of a drug’s reinforcing effects |
| Fixed-ratio operant procedure Participants are given the opportunity to ‘work’ for the drug under investigation by responding on a manipulandum a fixed number of times Dependent measures: amount of drug consumed or rate of responding |
This procedure is similar to that used in a large number of preclinical studies, which allows for indirect comparisons across species | Depending on the dose, drug, and ratio requirement, it is sometimes difficult to differentiate the reinforcing effects of different doses Results can vary depending on the fixed-ratio value chosen, so an ‘appropriate’ fixed-ratio value can be difficult to determine |
| Progressive-ratio operant procedure Participants are given the opportunity to ‘work’ for a drug by responding on a manipulandum; the ratio requirement increases after each drug delivery Dependent measures: amount of drug consumed, rate of responding, maximum ratio value completed (break point) |
Provides a dependent measure that allows for direct comparison across different drugs Sensitive to dose manipulations Amenable to a behavioral economic analysis, which generates a rich array of dependent measures |
If shorter intertrial intervals are used, carryover effects from one dose to another can interfere with the ability to perform the operant If longer intertrial intervals are used to minimize carryover effects, the study duration can become prohibitively long |
Free-access procedures
One of the most straightforward self-administration methods provides participants with unconditional or free access to the drug under investigation (Griffiths et al., 1986). Free-access procedures are typically used when investigating drugs with minimal acute aversive effects and risk of overdose, such as nicotine and caffeine (Liguori et al., 1997; Mooney et al., 2006; Lee et al., 2011). For example, sex-related differences in the reinforcing effects of nicotine were assessed by allowing research participants unlimited use of nicotine gum (2, 4 mg) and comparing the number of pieces of gum consumed by men and women (Hatsukami et al., 1995). Utilizing a free-access procedure, the investigators were able to observe patterns of drug self-administration and drug effects throughout the day in a controlled setting. As a result, they uncovered differences in gum chewing that provided data indicating that the response to nicotine withdrawal varied between sexes.
Choice procedures
Free-access procedures sometimes use an additional choice alternative to the drug under investigation. These comparators are often another drug of known reinforcing effect or a placebo/inactive control. Choice procedures allow participants access to two or more concurrently available drugs. Hale et al. (1995), for example, investigated caffeine self-administration in adolescents by allowing participants unrestricted access to caffeinated (33.3 mg/240 ml) and decaffeinated sodas. Participants were provided with sufficient bottles of each type of soda to use at home during the period of testing, and instructed to drink the two sodas in any manner they wished. Participants were allowed concurrent access to each type of beverage. The conclusion that caffeine was a robust reinforcer was determined from the increased rate at which participants self-administered caffeinated as opposed to decaffeinated soda.
Another free-access study by Griffiths et al. (1986), allowed heavy coffee drinkers free access to coffee. The researchers manipulated the presence or absence of caffeine and observed the participants’ pattern of consumption of caffeinated (100 mg/180 ml) and decaffeinated coffee, which were available over the course of 10 alternating days. During these periods of observation, participants verbally requested coffee from research staff whenever they desired it. In this inpatient study, verbal selection was the operant (a behavior that is modifiable by its consequences) that led to caffeine administration. Researchers recorded the daily number of cups of caffeinated versus decaffeinated coffee consumed throughout the inpatient testing period. Participants’ preference for the active drug (caffeinated beverage) over the control (decaffeinated beverage) demonstrated the reinforcing effects of caffeine.
Multiple-choice procedures
To mimic real world situations, many choice designs require participants to make two or more discrete choices between potential reinforcers. Griffiths et al. (1993) first introduced the multiple-choice procedure (MCP) as a quick and efficient method of estimating the reinforcing effects of a drug. In this study, the participants made a series of choices on a questionnaire. For each choice, they were required to select one of two potential reinforcers (e.g. drug vs. drug, drug vs. placebo, or drug vs. money). Using this procedure, dose-related choice of pentobarbital over money and choice of a higher dose of pentobarbital (400 mg/70 kg) over a lower dose (200 mg/70 kg) and placebo were demonstrated. Following the choice trials, only one of the selected choices was randomly provided to the participant (i.e. delivery of the reinforcer via a lottery).
A version of the MCP has also been developed that asks participants to make choices between hypothetical rewards (Chutuape et al., 1998). The use of hypothetical outcomes may circumvent problems involving delivery of the drug choice discussed later in this review. However, there are concerns that the choices an individual makes for hypothetical outcomes may not be synonymous with real world choices (Kirby, 1997). As such, the reinforcing value of a hypothetical outcome may be altered in these scenarios. Although some self-administration studies use an MCP, many opt to deliver the chosen reinforcer consistently in a manner similar to traditional self-administration designs, as opposed to having hypothetical outcomes or lottery-like delivery of the reinforcer. Other disadvantages of the MCP are listed in Table 1.
Designing a choice procedure
Though less commonly used in preclinical studies (Banks and Negus, 2012), choice procedures are the most common clinical self-administration designs currently in use. Choice designs can be used to assess reinforcement as a function of which reinforcer is chosen and/or how much of the reinforcer is chosen. Comparison of these dependent measures to those of a drug whose abuse potential is known, or another robust reinforcer such as money, can provide investigators with an estimate of a drug’s abuse potential. Certain choice procedures generate a large amount of responding, but provide only minimal reinforcement of behavior and are popular because of their efficiency (Griffiths et al., 1996; Comer et al., 2008a). Choice procedures also have utility in evaluating new medications to treat drug abuse. Within the context of this design, an efficacious medication would significantly reduce the number of drug choices. There are some disadvantages to using choice designs, however. In many choice designs, for example, there is only a single delivery of drug or money, at the end of the choice session. As such, there is often a considerable delay between the operant behavior and delivery of the reinforcer. The temporal relation between response and reinforcer is important because increasing the delay proportionally decreases the value of the reinforcer (Catania, 1979). Despite these limitations, choice procedures have been used in abuse liability assessments of: prescription opioids (tramadol; Babalonis et al., 2013; oxycodone, Jones et al., 2011), marijuana (Cooper et al., 2012), alcohol (Barrett et al., 2006), D-amphetamine (Vansickel et al., 2010), and cocaine (Stoops et al., 2010).
Choice of participants and route of drug delivery
Most self-administration studies use samples of volunteers who have experience with the drug under investigation, yet are unambiguous in their intent to continue drug use. This is done partly in response to the ethical considerations involved in the administration of drugs of abuse, but also to maintain the face and predictive validity of the procedure. Human participants who have used the drug (or a similar drug) nonmedically have demonstrated their susceptibility to the reinforcing effects of the drug. As such, the researcher is engaging an exact sample of the population of interest, or a sample of those who are most at risk of abusing a drug with similar effects.
Using this logic researchers typically choose to administer the drug through a route that has been shown to be abused, or whose pharmacodynamic and pharmacokinetic profiles suggest that they have the greatest potential to be abused. Failure to correctly model the route of administration can significantly impact the predictive validity of the assessment of the drug’s abuse potential. For example, initial clinical assessments of the abuse liability of the partial µ-opioid agonist and κ antagonist buprenorphine concluded that the drug had a ‘low’ potential for abuse (Jasinski et al., 1978). These conclusions were made by administering the drug subcutaneously. Later research found that when the drug is administered intravenously, intramuscularly, or intranasally, it can produce robust increases in positive subjective effects, and in some cases acts as a reinforcer in clinical self-administration studies (Stoller et al., 2001; Comer et al., 2005, 2010b; Duke et al., 2010). Field research has proven the recent findings to be more accurate: since its introduction to the market, parenteral abuse of buprenorphine has been a significant problem in several countries (Obadia et al., 2001; Jenkinson et al., 2005; Carrieri et al., 2006; Chua and Lee, 2006; Hakansson et al., 2007; Nielsen et al., 2007; Vicknasingam et al., 2010).
Restricting drug access
As mentioned above, choice procedures can be used to assess reinforcing efficacy as a function of how much of the drug is self-administered. However, concerns about drug toxicity often necessitate that researchers place temporal or dose limitations on the amount of drug that participants can self-administer. Determination of these variables exemplifies the decision-making process that must occur when designing studies of the reinforcing effects of a drug: a dose of drug must be chosen that is likely to be abused but at the same time is safe to administer. The dose, dose range, or interdose interval to be used in a self-administration study is typically determined by previous experimental research and/or clinical experience with the drug in question. These decisions are exemplified in a study that sought to confirm or reject anecdotal evidence that the hypnotic anesthetic propofol had abuse potential. In this design, participants choose to self-administer either propofol or vehicle placebo (Zacny et al., 1993a). The researchers selected a dose of propofol (0.6 mg/kg) that had previously been shown to have robust subjective effects, but was also subanesthetic, to avoid significant sedation (Zacny et al., 1993b). Restrictions on drug availability are also commonly utilized because they tend to stabilize the pattern of drug self-administration, allowing researchers additional sensitivity to determine the influence of experimental manipulations (Woods and Winger, 1971).
Choosing a comparator
As demonstrated in the study by Zacny et al. (1993a), comparing drug preference to an agent with little or minimal reinforcing efficacy (drug vs. placebo) has been valuable in quantifying the abuse liability of novel drugs or those for whom abuse has not been extensively observed in the natural environment. A similar method was also used to assess the abuse potential of the inhaled anesthetic nitrous oxide. As in many choice procedures, participants were blind (unaware of certain study procedures such as the independent variable) to the drug options [nitrous oxide (20, 30, and 40%) or O2 (100%) for 30 min; Dohrn et al., 1993a, 1993b)]. Alternatively, researchers often compare preference to a known drug of abuse (drug vs. drug). The use of a positive control condition is often used when comparing a drug to an abuse-deterrent formulation of that same drug. For example, several studies have sought to examine the abuse potential of the buprenorphine/naloxone combination, the design of which is intended to dissuade parenteral abuse of buprenorphine. These studies often use the intravenous administration of buprenorphine itself as a standard of comparison for the abuse-deterrent formulation (Mendelson et al., 1999; Comer et al., 2010b).
In addition to deterring agents, such as naloxone, other abuse deterrents include physical barriers (e.g. hardened tablets). The development of these tamper-resistant drug formulations is intended to prevent abuse by creating obstacles to crushing or dissolving tablets and capsules (Hamed and Moe, 2010). Standards for abuse liability assessment of tamper-resistant formulations have not been developed. The ability of these formulations to alter abuse potential is often difficult to study using traditional self-administration procedures. Many formulations congeal and become viscous when mixed with water or heated, or fail to crush into particle sizes that can be readily insufflated (Vosburg et al., 2012, 2013). Self-administration studies where these formulations could be injected or snorted may impose significant health risks to study participants.
As an alternative to drug versus drug or placebo comparisons, some researchers make use of a monetary alternative reinforcer (drug vs. money). Comer et al. (1997) evaluated the effects of a monetary alternative (US$10, US$20, US$40) on intranasal heroin (0, 12.5, 25, 50, 100 mg) self-administration. Participants chose to receive either heroin or money, with each dollar amount being tested against each heroin dose. The data revealed that the reinforcing effects of heroin could be modified by the availability of a monetary alternative reinforcer. When using a drug versus money procedure, discovering the point where choice behavior switches from drug to money can also provide a monetary measure of the reinforcing value of the drug.
Also using a drug versus money design, another group of investigators tested the ability of two κ-opioid agonists to alter choice preference for intravenous cocaine (40 mg) versus money (Walsh et al., 2001). Neither compound was found to alter cocaine choice behavior. If either drug had decreased the monetary value of cocaine, this would have indicated that the medication might serve as a pharmacotherapy to deter cocaine abuse.
There are two well-established disadvantages of the drug versus money procedure. First, monetary alternatives may not accurately reflect the current street value of the drug under investigation, which can fluctuate significantly over time and from location to location. Second, variation in the participants’ socioeconomic background and current financial circumstances may provide data on the reinforcing nature of a drug that is only generalizable to drug users in similar circumstances. For example, data from poor participants may not be generalizable to drug users with more disposable incomes because the reinforcing value of the monetary alternative is reduced.
An advantage of the drug versus money procedure is that its external validity is readily apparent. In the vast majority of circumstances drug users must pay for drugs. Each time they purchase drugs they are making a choice between the drug and money. As such, drug versus money choice procedures are commonly used in clinical abuse liability assessments. The drug versus money design has also been likened to the contingency management treatment approach, which asserts that providing an alternative reinforcer can reduce or stop drug use: drug treatment patients are therefore provided monetary or other tangible reinforcement for abstinence and/or activities incompatible with drug use (Higgins et al., 1994). In both contingency management and drug versus money choice designs, patients and research participants are choosing between the use of a drug and money. In the laboratory, participants must still work for both reinforcers. This feature is only comparable to forms of contingency management where patients are given employment as an incentive for abstinence (Silverman et al., 2001; DeFulio and Silverman, 2011).
Familiarizing participants with their choices
Whether choosing between two drugs, or between a drug and money, choice procedures are often preceded by a session in which participants are administered/receive each of the potential reinforcers. These sample sessions allow participants the chance to experience the physiological and subjective effects of each drug before having to choose between them. Sample sessions may also be used to screen out nonresponders and ensure that participants can safely tolerate the drug(s) to be tested later. In these studies, participants are often specifically instructed to attend to the drug effects along with cues associated with that drug choice, such as the color, size, and shape of the pill. This allows participants to better associate and retain information about the drug’s effects for a later choice session. Sample sessions are often referred to as ‘safety’ sessions because they can also be used to minimize the chances of adverse drug effects. In their safety session, Matuskey et al. (2012) administered a bolus dose of intravenous cocaine to each participant to establish their ability to tolerate the drug before examining self-administration. In their attempt to quantify the reinforcing properties of intravenous nicotine, Sofuoglu et al. (2008) performed sample and choice sessions on the same day. In some cases, the duration of drug effects may necessitate that sample and choice sessions occur on separate days, as was done by Kirkpatrick et al. (2012) to compare the reinforcing effects of intranasal methamphetamine to D-amphetamine. However, other studies have suggested that potential carryover effects from sample to choice sessions may not significantly alter the reinforcing effects of a drug (Comer et al., 2001; Comer and Collins, 2002), although carryover effects are likely to be a factor when very high doses of drug are administered (Comer et al., 1999). Another methodological consideration involves allowing participants to sample full doses of the drug and later self-administer portions of the full amount, or sample and self-administer the same dose. To the authors’ knowledge there are no data arguing in favor of one design over the other. However, this methodological decision is usually driven by practical considerations. If the drug being studied has a rapid pharmacology (e.g. intravenous or smoked cocaine), then it may be preferable to have participants sample a full dose and earn that same dose repeatedly in the choice session. If the drug has a slower onset and offset (e.g. an orally administered drug or longacting opioid), it would be more feasible and safe (i.e. avoiding carryover effects) to have participants first sample the full dose of the drug, then work for portions of that drug. The division of the dose in this manner allows for multiple opportunities to self-administer a drug in a manner comparable to what can be done using a drug with a rapid onset/offset.
Forced-choice versus free-choice designs
Another methodological consideration in designing a choice procedure involves forced-choice versus free-choice preparations. Free-choice designs (also known as single choice) allow participants to choose between an active drug condition or nothing. In forced-choice designs, the participant must choose between active drug and placebo, or between several drug options. Although direct methodological comparisons are rare, some studies have reported that forced-choice and free-choice procedures produce comparable results (Oliveto et al., 1992; Roehrs et al., 1997). However, forced-choice options are considerably more commonplace in clinical studies. It is believed that the choice between competing reinforcers most accurately reflects real-world decisions concerning whether or not to use drugs.
How participants ‘choose’ drug
In the natural environment, drug users often make the choice between competing reinforcers. When modeling this behavior in the laboratory, simple operant behaviors, such as a ‘yes’ or ‘no’ verbal response, are often used in cases where drug intoxication could significantly impair participants’ ability to perform more complex or effortful behavior. Another commonly used simple operant behavior allows for selection between alternatives using finger clicks on a computer mouse or movement of a joystick (Bozarth, 1987). The use of these nonverbal operants in humans (Mendelson and Mello, 1966) began as extensions of preclinical models (Thompson and Schuster, 1964; Deneau et al., 1969).
How and when drug is delivered
Although simple operant behaviors have their utility, it has been noted that choice procedures with simpler operant requirements can lead to placebo being chosen with high frequency, making it difficult to demonstrate the reinforcing nature of the active compound in comparison (i.e. a ceiling effect; Roehrs et al., 1997). To address this issue, many self-administration studies use an operant behavior with a more significant work contingency (e.g. a large number of responses on a computer mouse or joystick).
A reinforcement schedule specifies the relationship between the operant behavior and its consequences. Within the context of drug self-administration, the reinforcement schedule defines the number and pattern of responses required for participants to receive the reinforcer(s) under investigation. Interval schedules require that a minimum amount of time passes between successive reinforced responses (e.g. 15 min). In contrast to an interval schedule, in which the reinforcer is delivered when a response is made after a certain amount of time has elapsed, a ratio schedule is based on the reinforcer being delivered after a certain number of operant responses is emitted. One commonly used ratio schedule of reinforcement is the fixed-ratio (FR) schedule. When responding on an FR schedule, a fixed number of responses are required to receive drug (e.g. Griffiths et al., 1976). For example, with every 100 finger presses on a computer mouse, participants receive a dose of drug (FR 100). The required number of responses is also known as the ‘response cost.’ A ‘timeout’ or ‘refractory period,’ during which drug is unavailable, usually follows each drug delivery to minimize the occurrence of adverse drug effects, and limit the number of doses that can be self-administered. The speed of responding, pattern of responding, number of drug deliveries, and amount of drug received are often used as primary dependent measures. Increases in the rate of operant responding or amount of drug received are thought to reflect increases in the reinforcing effects of the drug.
A strength of an FR schedule of reinforcement lies in its ability to assess changes in the amount of drug self-administered under a fixed set of conditions, because the response cost of receiving the drug is constant. However, behavioral incapacitation (inability to respond) may occur if the drug being self-administered affects motor behavior or if the participant is satiated and/or feels overly intoxicated. These effects may confound the speed and pattern of responding as measures of reinforcing potential and limit the reinforcing efficacy observed in these studies. In fact, inverted U-shaped dose–response curves are often seen in preclinical self-administration studies where higher doses produce lower rates of responding (e.g. Skoldager et al., 1991; Winger et al., 2002), although these are less often seen in clinical self-administration studies (e.g. Comer et al., 1999). As noted above, concerns about drug toxicity can restrict the range of doses tested, and limit the number of drug deliveries to human participants. In one such study, Foltin and Fischman (1992) compared the self-administration of smoked (0, 25, and 50mg) versus intravenous (0, 16, and 32mg) cocaine. Their FR schedule required participants to complete a fixed response requirement of 200 button clicks on a joystick to receive cocaine. To address concerns about drug toxicity, before drug delivery they first confirmed that participants’ vital signs (heart rate, blood pressure) were within safe parameters for drug administration.
Progressive ratio (PR) schedules are another type of reinforcement schedule that is commonly used in clinical studies of drug self-administration. PR schedules differ from fixed schedules in that the behavioral requirement for reinforcement varies with each delivery of the reinforcer. Under a PR schedule, the operant response required to receive each drug delivery progressively increases (e.g. logarithmically or arithmetically) so that more ‘work’ is required to receive the same amount of drug. The primary dependent variable in studies using PR schedules is known as the ‘break point,’ which is the point at which responding for the reinforcer stops. Drugs that maintain larger break point values (i.e. drugs for which participants are willing to perform more work to receive) are considered to be more efficacious reinforcers and to have greater abuse liability (Katz, 1990; Stafford et al., 1998).
As with FR schedules, direct drug effects can confound the rate of responding on a PR schedule if drug is delivered after completion of each ratio requirement. However, unlike FR schedules, PR schedules can be used to examine reinforcer effectiveness without relying directly on response rates, if drug is delivered after all of the responding has been completed. As such, PR schedules can provide a less confounded measure of the reinforcing effects of a drug (Hodos, 1961; Hodos and Kalman, 1963).
Another utility of a PR schedule is its ability to provide a standard way to compare the reinforcing efficacies of several different doses and/or drugs. This was demonstrated in a study by Comer et al. (2008b), where the relative reinforcing effects of various doses of intravenously administered opioids were tested [fentanyl (0, 0.0625, 0.125, 0.187, and 0.250 mg/70 kg), oxycodone (0, 6.25, 12.5, 25, and 50 mg/70 kg), morphine (0, 6.25, 12.5, 25, and 50 mg/70 kg), buprenorphine (0, 0.125, 0.5, 2, and 8 mg/70 kg), and heroin (0, 3.125, 6.25, 12.5, and 25 mg/70 kg]. Self-administration of each drug was assessed in a 10-trial procedure where participants were able to choose between tenths of US$20 or each drug dose that they had previously sampled (e.g. US$2 or 2.5 mg of heroin). As illustrated in Fig. 1, the response requirement for each option increased progressively (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800) each time the option was selected. So if the participant chose to receive 7U of drug, the break point for that drug/dose was 1600 operant responses. Conversely, this would mean that they made three choices for money, which would equate to a break point of 200 and the participant receiving US$6. Importantly, in this procedure, the drug reinforcer was not delivered to the participants until after all of the responses were completed so that the potential motor-impairing effects of the drugs did not affect the participants’ ability to respond. The procedure provided a direct measure of comparison (break point) of the reinforcing effects of each drug and dose.
Fig. 1.
In this illustration of a 10-trial drug versus money self-administration session, upon completion of responding, the participant has made seven choices for drug and three choices for money. As such, the participants would receive a combined dose of 7U of the drug and US$6. The respective break point values for drug and money would be 1600 and 200 responses.
In designs where the operant response requirement for the two choices escalates, responding for one reinforcer may not alter the response requirement for the alternative reinforcer (independent PR schedule), or alternatively, responding for either reinforcer may decrease or increase the ratio requirement of the alternative (non-independent PR schedule). The upper limit of the operant response requirement can increase to 12 000 or more (Shahan et al., 2001; Greenwald et al., 2002). Depending on the number of trials, participants may make tens of thousands of responses in total throughout each self-administration session.
Unlike FR and PR designs, second-order schedules are comprised of two reinforcement schedule components. On a second-order schedule, a participant responds according to one type of schedule for a brief presentation of a stimulus such as a light or tone. Responding on this schedule is then reinforced with the drug according to a second type of reinforcement schedule (Goldberg, 1973; Goldberg and Gardner, 1981; Bergman et al., 1989; Spear and Katz, 1991). For example, Lamb et al. (1991) used a second-order schedule to examine the reinforcing effects of intramuscular morphine (0, 3.75, 7.5, 15, 30 mg). An illuminated green light indicated the start of the session, and each operant response (right lever press) resulted in a brief flash of a white light. Each 100th response turned off the green light and turned on a red stimulus light for 1 s (FR 100). The 30th time the FR 100 requirement was met the red light was turned on for 15 min and then the participant received morphine [FR 30 (FR 100): S].
Under second-order schedules, responding is maintained not only by the self-administered drug, but also by contingent presentation of the cue stimulus that has become a secondary reinforcer (i.e. a cue that has obtained value as a reinforcer through its association with the primary reinforcer, in this case the drug). Using second-order schedules, responding is intermittently reinforced and the operant behavior can be maintained with relatively few drug deliveries per session. Second-order schedules allow investigators to separate responding for the drug (i.e. drug-seeking behavior maintained by the reinforcing efficacy of the drug that is eventually administered), from those affected by the drug itself (i.e. subsequent responses that may be affected by the drug in ways that alters rate or pattern of responding and are not necessarily related to its reinforcing effects, Everitt and Robbins, 2000).
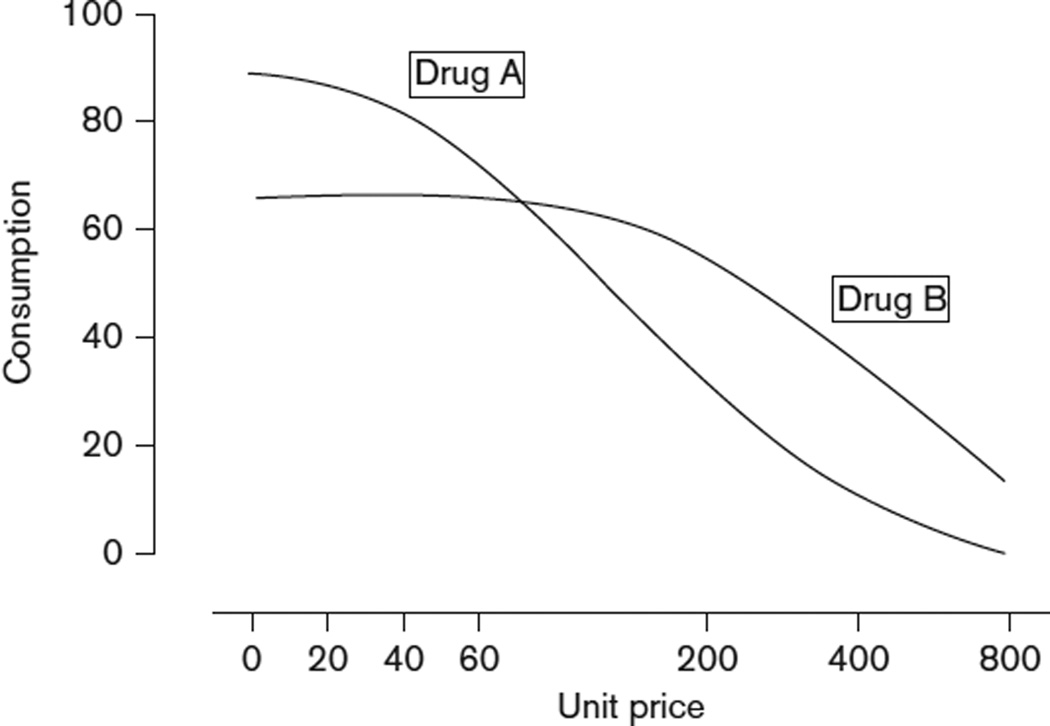
Data from studies involving permutations in the schedule of reinforcement can subsequently be used to examine the relationship between drug self-administration and drug price (i.e. operant response requirement), graphically depicted as a demand curve (Fig. 2). Demand curves can be generated from incrementally increasing PR schedules across trials, or through randomly altering the FR schedules across trials. In either case, the elasticity of demand (degree to which drug responding varies as a function of ‘price’) for the reinforcer being investigated can be used as a proxy for reinforcing efficacy and abuse liability.
Fig. 2.
Mean consumption of two hypothetical drugs as a function of unit price.
Study of the behavioral economics of drug abuse provides us with an understanding of how altering the ‘price’ of the drug affects self-administration behavior (i.e. drug reinforcement within a system of limited or constrained resources; Bickel et al., 1995; DeGrandpre and Bickel, 1996). One such behavioral economic analysis compared the reinforcing efficacy of nicotine-containing (0.6 mg) and denicotinized (0.07 mg) cigarettes (Shahan et al., 2001). Demand curves for each type of cigarette [with and without money (US$0.25 or US$0.50) as an alternative reinforcer] were developed by increasing the response requirement for a standardized cigarette puff on a PR of: 2, 100, 300, 1000, 3000, 6000, and 12 000. Comparison of the curves revealed that the two cigarette types did not differ in their sensitivity to price or alternative nondrug reinforcement. This type of finding confirms the importance of nonpharmacological variables in the maintenance of drug-taking behavior.
Designs to assess participant variables and environmental factors
The abuse potential of a drug and its ability to maintain self-administration is not exclusively dependent on its pharmacodynamics. As such, a number of self-administration models have been developed to examine the effects of participant variables and environmental circumstances on drug reinforcement. For example, the effects of drug abstinence or withdrawal have been investigated where drug is available for self-administration following a period of abstinence (Haney et al., 2008; Lee et al., 2011). A relapse model developed by Walsh et al. (2001) differs from other self-administration procedures in that a sample dose is not administered, such that participants’ choice behavior begins in a state of abstinence (> 48 h).
The reinforcing efficacy of a drug can also vary once self-administration has begun. For some drugs such as cocaine, shifting self-administration away from drug to alternate reinforcers has proven to be particularly difficult after drug use has been initiated (Foltin and Fischman, 1994; Walsh et al., 2001). Novel models have been developed that attempt to examine ways to alter cocaine self-administration under conditions in which cocaine use has already begun. One such human laboratory model developed a game of chance for monetary gain as an alternative reinforcer to cocaine use under binge conditions (Vosburg et al., 2010). Cocaine users completed 5 consecutive cocaine self-administration days with two sessions per day (a binge model), and following 9 days without cocaine, 2 additional cocaine self-administration laboratory session days (a second binge model). A game of chance was used as an alternative reinforcer to cocaine. In this game, participants blindly chose from a container that held three pieces of paper, each bearing a single number (2, 4, or 6), which represented the number of balls the participant could draw from a bingo wheel. The bingo wheel contained 20 balls, each labeled with a monetary amount ranging from US$0 to US$20. After drawing a number from the container, participants then chose whether to draw that number of balls from the bingo wheel (i.e. 2, 4, or 6 balls) or to smoke a dose of cocaine (25 mg). The game led to a decrease in choice to self-administer cocaine, therefore showing promise as a nondrug alternative reinforcer.
Single and multiday paradigms of ‘self-regulated’ (i.e. free access) cocaine administration have been developed specifically to allow participants flexibility and control of the frequency of drug intake (Sughondhabirom et al., 2005; Lynch et al., 2006; Matuskey et al., 2012). Similarly, Donny et al. (2003) developed a cessation model to evaluate cocaine self-administration behavior once cocaine taking had already been initiated. They used a standard choice procedure, but increased the monetary alternative with each trial from US$1 to US$16. The researchers also varied the time between each of the six drug choice trials (15, 30 min, and an interval selected by the volunteer). Each dose of cocaine (12.5, 25, or 50 mg/70 kg) was assessed under each interchoice interval. This model modified these two criteria to provide a further incentive to shift choice behavior from drug to the alternative reinforcer; however, their results only confirmed the persistence of cocaine self-administration once cocaine taking had been initiated.
Other behavioral manipulations have been developed to study how participant factors alter drug self-administration in the laboratory and the abuse potential of drugs. One such clinical technique investigates how reactivity to stress or drug-associated cues affect drug craving, drug seeking, and ultimately drug self-administration. Numerous studies have shown that drug craving can be induced in the laboratory by exposing participants to stress and cues associated with drug use (Ehrman et al., 1992; Childress et al., 1993; Margolin et al., 1994; Berger et al., 1996; Sinha et al., 1999; Wolfling et al., 2008).
Several investigations have exposed participants to drugrelated paraphernalia within the context of self-administration designs to observe the extent to which these conditioned reinforcers influence drug taking. In one such study with eight cocaine-dependent participants, Foltin and Haney (2000) paired different sets of neutral cues with smoked placebo cocaine and active cocaine (25 mg) over the course of 18 drug plus cue association trials. After the cue association trials, researchers observed a preference for cues paired with active cocaine (vs. smoked cocaine). However, presentation of either cue did not alter whether participants chose to purchase six doses of cocaine or placebo; no participant purchased a dose of placebo under any condition.
Similarly, McKee et al. (2011) developed a novel human laboratory model to examine the effects of stress on tobacco self-administration. Thirty-seven nicotine-deprived daily smokers received stress or neutral imagery induction on 2 separate days. Imagery induction was followed by the options to self-administer tobacco immediately, or to delay tobacco use for up to 50 min in exchange for three levels of monetary reinforcement (US$0.50, US$1.00, or US$1.50/5 min delay). Following stress induction, participants were less able to resist smoking, smoked more, and reported greater positive subjective effects from smoking.
What is next in drug self-administration?
The procedural evolution of self-administration research appears to involve the incorporation of novel technologies into this well-established paradigm. Genetic techniques have provided strong evidence for genetic involvement in drug use disorders (Goldman et al., 2005). A more recent hypothesis-driven approach uses select genetic markers with relevant biological function to investigate how these variants may impact many aspects of drug abuse. The majority of pharmacogenetic research focuses on how specific genes or gene variants alter drug effects, the effectiveness of pharmacotherapy, and self-administration behavior (Kalayasiri et al., 2007; Levran et al., 2008; Kim et al., 2009; Stapleton et al., 2011). These types of investigations use traditional self-administration techniques but use the presence or absence of one or a combination of genes/genetic variants as an independent variable across which to compare effects. One such study explored associations of gene variants in the dopamine (DRD2/ANKK1, SLC6A3), opioid (OPRM1), and serotonin (SLC6A3/4) pathways with smoking reinforcement (Perkins et al., 2008). Smokers were allowed to smoke nicotine (0.6 mg) and denicotinized (0.05 mg) cigarettes under varying mood states. After sampling each type of cigarette, participants were allowed to smoke additional cigarettes at will. This study provided strong evidence for genetic associations with the increase in nicotine reinforcement (latency to first puff and total puffs) during negative mood states.
Similar to pharmacogenetic studies, brain imaging techniques such as PET and MRI have been added to self-administration studies to further elucidate factors affecting the reinforcing effects of drugs. One such study explored the association between D1 receptor availability and cocaine-taking behavior (Martinez et al., 2009). D1 receptor availability in the limbic, striatum, and cortical brain regions was measured by PET imaging using the radiotracer [11C]NNC-112, whereas cocaine-dependent participants completed a drug (cocaine: 0, 6, and 12 mg) versus money (voucher worth US$5) self-administration procedure. Low D1 receptor availability in the ventral striatum was associated with the choice to self-administer smoked cocaine (Fig. 3: see also Martinez et al., 2007; MacKillop et al., 2012). The integration of genetics and imaging techniques into traditional self-administration procedures may help researchers account for the significant individual variation in a drug’s abuse liability, thereby identifying more vulnerable populations and novel treatment approaches.
Fig. 3.
Correlation between [11C]NNC-112 BPND in the ventral striatum and the choice to self-administer cocaine (6mg) over a monetary alternative (US$5 voucher). Reprinted from Martinez et al. (2009), originally published in Neuropsychopharmacology.
Summary and conclusion
In conclusion, examining drug self-administration in the clinical laboratory is a scientifically meaningful way to study the many variables that control excessive consumption of drugs. Quantification of abuse liability using clinical self-administration can be a valid predictor of whether a drug will be abused in the real world. Additionally, observation of how medications or environment-based strategies alter a drug’s reinforcing potential in the clinical laboratory has served as an important proxy for assessing how these interventions may alter their abuse in the real world. Typically, strategies that shift preference away from the drug to alternative sources of reinforcement have significant potential as treatments. Although there are many variations of this experimental paradigm, they all contribute to our understanding of the factors that maintain drug use. Better understanding of these factors should improve our methods of predicting the abuse liability of a novel drug, and the effectiveness of potential medications for treating dependence on commonly abused drugs.
Acknowledgements
The authors would like to thank all of the faculty and staff of the Division on Substance Abuse, the New York State Psychiatric Institute, and NIDA for its generous research support of our research over the years (K01-DA030446, DA09236, DA016759, DA031022).
S.D.C. and J.D.J. are responsible for the content and preparation of this manuscript. Both authors contributed significantly to this work and have approved this manuscript for publication.
Conflicts of interest
The authors declare that over the past 3 years S.D.C. and J.D.J. have received compensation (in the form of partial salary support) from investigator-initiated studies supported by Reckitt-Benckiser Pharmaceuticals, Schering-Plough Corporation, Johnson & Johnson Pharmaceutical Research & Development, Endo Pharmaceuticals, and MediciNova. In addition, S.D.C. has served as a consultant to the following companies: Analgesic Solutions, BioDelivery Sciences, Cephalon, Grunenthal, Inflexxion, King, Neuromed, Pfizer, Salix, and Shire.
References
- Babalonis S, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Abuse liability and reinforcing efficacy of oral tramadol in humans. Drug Alcohol Depend. 2013;129:116–124. doi: 10.1016/j.drugalcdep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL. Drug abuse potential evaluation in animals. Br J Addict. 1991;86:1549–1558. doi: 10.1111/j.1360-0443.1991.tb01747.x. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci. 2012 doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall S, Mickalian J, Reid MS. Haloperidol antagonism of cueelicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in non-human primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Bickel WK, Green L, Vuchinich RE. Behavioral economics. J Exp Anal Behav. 1995;64:257–262. doi: 10.1901/jeab.1995.64-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA. Methods of assessing the reinforcing properties of abused drugs. New York, NY: Springer-Verlag Publishing; 1987. [Google Scholar]
- Brady JV, Lucas SE. NIDA Research Monograph #52. Washington, DC: US Government Printing Office; 1984. Testing drugs for physical dependence potential and abuse liability. [PubMed] [Google Scholar]
- Brady JV, Griffiths RR, Hienz RD, Ator NA, Lukas SE, Lamb RJ. Assessing drugs for abuse liability and dependence potential in laboratory primates. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 45–86. [Google Scholar]
- Carrieri MP, Amass L, Lucas GM, Vlaho D, Wodak A, Woody GE. Buprenorphine use: the international experience. Clin Infect Dis. 2006;43:S197–S215. doi: 10.1086/508184. [DOI] [PubMed] [Google Scholar]
- Catania AC. Reinforcement schedules: the role of responses preceding the one that produces the reinforcer. J Exp Anal Behav. 1971;15:271–287. doi: 10.1901/jeab.1971.15-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania AC. Learning. Englewood Cliffs, NJ: Prentice Hall; 1979. [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Chua SM, Lee TS. Abuse and prescription buprenorphine, regulatory controls. Ann Acad Med Singapore. 2006;35:492–495. [PubMed] [Google Scholar]
- Chutuape MA, Silverman K, Stitzer ML. Survey assessment of methadone treatment services as reinforcers. Am J Drug Alcohol Abuse. 1998;24:1–16. doi: 10.3109/00952999809001695. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Choice between money and intranasal heroin in morphine-maintained humans. Behav Pharmacol. 1997;8:677–690. doi: 10.1097/00008877-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. Comparison of intranasal and intravenous heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 1999;143:327–338. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology (Berl) 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED. Self-administration of intravenous buprenorphine and the buprenorphine/naloxone combination by recently detoxified heroin abusers. J Pharmacol Exp Ther. 2002;303:695–703. doi: 10.1124/jpet.102.038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Walker EA. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. J Pharmacol Exp Ther. 2005;315:1320–1330. doi: 10.1124/jpet.105.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008a;96(1–2):1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008b;33:1179–1191. doi: 10.1038/sj.npp.1301479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Bickel WK, Yi R, de Wit H, Higgins ST, Wenger GR, et al. Human behavioral pharmacology, past, present, and future: symposium presented at the 50th Annual Meeting of the Behavioral Pharmacology Society. Behav Pharmacol. 2010a;21:251–277. doi: 10.1097/FBP.0b013e32833bb9f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Manubay J, Amass L, Cooper ZD, et al. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010b;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Hart CL, Vosburg SK, Comer SD, Haney M. A human laboratory study investigating the effects of quetiapine on marijuana withdrawal and relapse in daily marijuana smokers. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: post-intervention outcomes. Addiction. 2011;106:960–967. doi: 10.1111/j.1360-0443.2011.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK. Drug dependence as consumer demand. In: Green L, Kagel J, editors. Advances in behavioral economics. Vol. 3. Norwood, NJ: Ablex; 1996. pp. 1–36. [Google Scholar]
- Deneau GE, Yanagita T, Seevers M. Self-administration of psychoactive substances by the monkey: a measure of psychological dependence. Psychopharmacology (Berl) 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- Dohrn CS, Lichtor JL, Coalson DW, Flemming D, Zacny JP. Reinforcing effects of extended inhalation of a low nitrous oxide concentration in humans. Pharmacol Biochem Behav. 1993a;46:927–932. doi: 10.1016/0091-3057(93)90224-h. [DOI] [PubMed] [Google Scholar]
- Dohrn CS, Lichtor JL, Coalson DW, Uitvlugt A, de Wit H, Zacny JP. Reinforcing effects of extended inhalation of nitrous oxide in humans. Drug Alcohol Depend. 1993b;31:265–280. doi: 10.1016/0376-8716(93)90009-f. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Duke AN, Correia CJ, Walsh SL, Bigelow GE, Strain EC. Acute effects of intramuscular and sublingual buprenorphine and buprenorphine/naloxone in non-dependent opioid abusers. Psychopharmacology (Berl) 2010;211:303–312. doi: 10.1007/s00213-010-1898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berl) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Assessment of abuse liability of stimulant drugs in humans: a methodological survey. Drug Alcohol Depend. 1991;28:3–48. doi: 10.1016/0376-8716(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Self-administration of cocaine by humans: choice between smoked and intravenous cocaine. J Pharmacol Exp Ther. 1992;261:841–849. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of buprenorphine on the self-administration of cocaine by humans. Behav Pharmacol. 1994;5:79–89. doi: 10.1097/00008877-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or D-ampheta-mine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- Goldberg SR, Gardner ML. Second-order schedules: extended sequences of behavior controlled by brief environmental stimuli associated with drug self-administration. NIDA Res Monogr. 1981;37:241–270. [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Schuh KJ, Hopper JA, Schuster CR, Johanson CE. Effects of buprenorphine sublingual tablet maintenance on opioid drug-seeking behavior by humans. Psychopharmacology (Berl) 2002;160:344–352. doi: 10.1007/s00213-001-0975-0. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson IA. Human coffee drinking: reinforcing and physical dependence producing effects of caffeine. J Pharmacol Exp Ther. 1986;239:416–425. [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Behav Pharmacol. 1996;4:3–13. [PubMed] [Google Scholar]
- Hakansson A, Medvedeo A, Andersson M, Berglund M. Buprenorphine misuse among heroin and amphetamine users in Malmo, Sweden: purpose of misuse and route of administration. Eur Addict Res. 2007;13:207–215. doi: 10.1159/000104883. [DOI] [PubMed] [Google Scholar]
- Hale KL, Hughes JR, Oliveto AH, Higgins ST. Caffeine self-administration and subjective effects in adolescents. Exp Clin Psychopharmacol. 1995;3:364–370. [Google Scholar]
- Hamed E, Moe D. Development of tamper deterrent formulations: state of the pharmaceutical industry. Curr Drug Abuse Rev. 2010;3:139–146. doi: 10.2174/1874473711003030139. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology (Berl) 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Exp Clin Psychopharmacol. 1995;3:163–173. [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive-ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive-ratio performance. J Exp Anal Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- Jenkinson RA, Clark NC, Fry CL, Dobbin M. Buprenorphine diversion and injection in Melbourne, Australia: an emerging issue? Addiction. 2005;100:197–205. doi: 10.1111/j.1360-0443.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD. The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenor-phine/naloxone. Neuropsychopharmacology. 2011;36:411–422. doi: 10.1038/npp.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, et al. Dopamine beta-hydroxylase gene (DbetaH) −1021C→T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry. 2007;61:1310–1313. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Katz JL. Models of relative reinforcing efficacy of drugs and their predictive utility. Behav Pharmacol. 1990;1:283–301. doi: 10.1097/00008877-199000140-00003. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, et al. A mu opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- Kirby KN. Bidding on the future: evidence against normative discounting of delayed rewards. J Exp Psychol Gen. 1997;126:54–70. [Google Scholar]
- Kirkpatrick MG, Gunderson EW, Johanson CE, Levin FR, Foltin RW, Hart CL. Comparison of intranasal methamphetamine and D-amphetamine self-administration by humans. Addiction. 2012;107:783–791. doi: 10.1111/j.1360-0443.2011.03706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, et al. The reinforcing and subjective effects of morphine in post-addicts: a dose- response study. J Pharmacol Exp Ther. 1991;259:1165–1173. [PubMed] [Google Scholar]
- Lee DC, Perkins KA, Zimmerman E, Robbins G, Kelly TH. Effects of 24 h of tobacco withdrawal and subsequent tobacco smoking among low and high sensation seekers. Nicotine Tob Res. 2011;13:943–954. doi: 10.1093/ntr/ntr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, O’Hara K, Peles E, Li D, Barral S, Ray B. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17:2219–2227. doi: 10.1093/hmg/ddn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori A, Hughes JR, Oliveto AH. Caffeine self-administration in humans. 1. Efficacy of cola vehicle. Exp Clin Psychopharmacol. 1997;5:286–294. doi: 10.1037//1064-1297.5.3.286. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Sughondhabirom A, Pittman B, Gueorguieva R, Kalayasiri R, Joshua D, et al. A paradigm to investigate the regulation of cocaine self-administration in human cocaine users: a randomized trial. Psychopharmacology (Berl) 2006;185:306–314. doi: 10.1007/s00213-006-0323-5. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Wier LM, David SP, Ray LA, Bickel WK, Sweet LH. The neuroeconomics of nicotine dependence: a preliminary functional magnetic resonance imaging study of delay discounting of monetary and cigarette rewards in smokers. Psychiatry Res. 2012;202:20–29. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Kosten TR. Cue-elicited cocaine craving and autogenic relaxation: association with treatment outcome. J Subst Abuse Treat. 1994;11:549–552. doi: 10.1016/0740-5472(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, et al. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology. 2009;34:1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Chen JI, Wanyiri J, Nadim H, Jatlow P, et al. A single-day paradigm of self-regulated human cocaine administration. Pharmacol Biochem Behav. 2012;103:95–101. doi: 10.1016/j.pbb.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Operant analysis of drinking patterns of chronic alcoholics. Nature. 1965;206:43–46. doi: 10.1038/206043a0. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Experimental analysis of drinking behavior of chronic alcoholics. Ann N Y Acad Sci. 1966;133:828–845. doi: 10.1111/j.1749-6632.1966.tb50930.x. [DOI] [PubMed] [Google Scholar]
- Mendelson J, Jones RT, Welm S, Baggott M, Fernandez I, Melby AK, Nath RP. Buprenorphine and naloxone combinations: the effects of three dose ratios in morphine-stabilized, opiate-dependent volunteers. Psychopharmacology (Berl) 1999;141:37–46. doi: 10.1007/s002130050804. [DOI] [PubMed] [Google Scholar]
- Mooney M, Green C, Hatsukami D. Nicotine self-administration: cigarette versus nicotine gum diurnal topography. Hum Psychopharmacol. 2006;21:539–548. doi: 10.1002/hup.808. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, Taylor D. Concurrent buprenorphine and benzodiazepine use and self-reported opioid toxicity substitution treatment. Addiction. 2007;102:616–622. doi: 10.1111/j.1360-0443.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–272. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Hughes JR, Higgins ST, Bickel WK, Pepper SL, Shea PJ, Fenwick JW. Forced-choice versus free-choice procedures: caffeine self-administration in humans. Psychopharmacology (Berl) 1992;109(1–2):85–91. doi: 10.1007/BF02245484. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Grottenthaler A, Ciccocioppo MM, Milanak M, Conklin CA, et al. Dopamine and opioid gene variants are associated with increased smoking reward and reinforcement owing to negative mood. Behav Pharmacol. 2008;19(5–6):641–649. doi: 10.1097/FBP.0b013e32830c367c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrs T, Pedrosi B, Rosenthal L, Zorick F, Roth T. Hypnotic self-administration: forced-choice versus single-choice. Psychopharmacology (Berl) 1997;133:121–126. doi: 10.1007/s002130050381. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Bickel WK, Badger GJ, Giordano LA. Sensitivity of nicotine-containing and de-nicotinized cigarette consumption to alternative non-drug reinforcement: a behavioral economic analysis. Behav Pharmacol. 2001;12:277–284. doi: 10.1097/00008877-200107000-00006. [DOI] [PubMed] [Google Scholar]
- Silverman K, Svikis D, Robles E, Stizer ML, Bigelow GE. A reinforcement-based therapeutic workplace for the treatment of drug abuse: six-month abstinence outcomes. Exp Clin Psychopharmacol. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Skoldager P, Winger G, Woods JH. Analysis of fixed-ratio behavior by drug reinforcers. J Exp Anal Behav. 1991;56:331–343. doi: 10.1901/jeab.1991.56-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008;33:715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- Spear DJ, Katz JL. Cocaine and food as reinforcers: effects of reinforcer magnitude and response requirement under second- order fixed-ratio and progressive-ratio schedules. J Exp Anal Behav. 1991;56:261–275. doi: 10.1901/jeab.1991.56-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stapleton JA, Sutherland G, O’Gara C, Spirling LI, Ball D. Association between DRD2/ANKK1 Taq1A genotypes, depression and smoking cessation with nicotine replacement therapy. Pharmacogenet Genomics. 2011;21:447–453. doi: 10.1097/FPC.0b013e328347473a. [DOI] [PubMed] [Google Scholar]
- Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/ naloxone in opioid-dependent humans. Psychopharmacology (Berl) 2001;154:230–242. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol. 2010;644(1–3):101–105. doi: 10.1016/j.ejphar.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, et al. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology (Berl) 2005;180:436–446. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Thompson T, Schuster CR. Morphine self-administration, food-reinforced and avoidance behaviors in rhesus monkeys. Psychopharmacology (Berl) 1964;5:87–94. doi: 10.1007/BF00413045. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs, Crime (UNODC) World drug report. Sales No E.12.XI.1. Vienna, Austria: United Nations Publication; 2012. [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR. Human sex differences in D-amphetamine self-administration. Addiction. 2010;105:727–731. doi: 10.1111/j.1360-0443.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicknasingam B, Mazlan M, Schottenfeld RS, Chawarski MC. Injection of buprenorphine and buprenorphine/naloxone tablets in Malaysia. Drug Alcohol Depend. 2010;111:44–49. doi: 10.1016/j.drugalcdep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Vosburg SK, Haney M, Rubin E, Foltin RW. Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend. 2010;110(1–2):144–150. doi: 10.1016/j.drugalcdep.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Benedek IH, Comer SD. Assessment of a formulation designed to be crush-resistant in prescription opioid abusers. Drug Alcohol Depend. 2012;126(1–2):206–215. doi: 10.1016/j.drugalcdep.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Shapiro DY, Comer SD. A comparison among tapentadol tamper-resistant formulations (TRF) and OxyContins (non-TRF) in prescription opioid abusers. Addiction. 2013;108:1095–1106. doi: 10.1111/add.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Wikler A. A psychodynamic study of a patient during experimental self-regulated re-addiction to morphine. Psychiatr Q. 1952;26:270–293. doi: 10.1007/BF01568465. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eu J Neurosci. 2008;27:976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Woods J, Winger G. A critique of methods for inducing ethanol self-intoxication in animals. In: Mello N, Mendelson J, editors. Recent advances in studies of alcoholism. Washington, DC: US Government Printing Officepp; 1971. pp. 413–436. [Google Scholar]
- Zacny JP, Lichtor JL, Thompson W, Apfelbaum JL. Propofol at a subanesthetic dose may have abuse potential in healthy volunteers. Anesth Analg. 1993a;77:544–552. doi: 10.1213/00000539-199309000-00020. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Zaragoza JG. Assessing the behavioral effects and abuse potential of propofol bolus injections in healthy volunteers. Drug Alcohol Depend. 1993b;32:45–57. doi: 10.1016/0376-8716(93)90021-h. [DOI] [PubMed] [Google Scholar]