Abstract
Vibrio cholerae O1 biotype El Tor (ET), causing the seventh cholera pandemic, was recently replaced in Bangladesh by an altered ET possessing ctxB of the Classical (CL) biotype, which caused the first six cholera pandemics. In the present study, V. cholerae O1 strains associated with endemic cholera in Dhaka between 2006 and 2011 were analysed for major phenotypic and genetic characteristics. Of 54 representative V. cholerae isolates tested, all were phenotypically ET and showed uniform resistance to trimethoprim/sulfamethoxazole (SXT) and furazolidone (FR). Resistance to tetracycline (TE) and erythromycin (E) showed temporal fluctuation, varying from year to year, while all isolates were susceptible to gentamicin (CN) and ciprofloxacin (CIP). Year-wise data revealed erythromycin resistance to be 33.3 % in 2006 and 11 % in 2011, while tetracycline resistance accounted for 33, 78, 0, 100 and 27 % in 2006, 2007, 2008, 2009 and 2010, respectively; interestingly, all isolates tested were sensitive to TE in 2011, as observed in 2008. All V. cholerae isolates tested possessed genetic elements such as SXT, ctxAB, tcpAET, rstRET and rtxC; none had IntlI (Integron I). Double mismatch amplification mutation assay (DMAMA)-PCR followed by DNA sequencing and analysis of the ctxB gene revealed a point mutation at position 58 (C→A), which has resulted in an amino acid substitution from histidine (H) to asparagine (N) at position 20 (genotype 7) since 2008. Although the multi-resistant strains having tetracycline resistance showed minor genetic divergence, V. cholerae strains were clonal, as determined by a PFGE (NotI)-based dendrogram. This study shows 2008–2010 to be the time of transition from ctxB genotype 1 to genotype 7 in V. cholerae ET causing endemic cholera in Dhaka, Bangladesh.
Introduction
Cholera is a life-threatening form of dehydrating diarrhoeal disease caused by the toxigenic serogroup strains of Vibrio cholerae. Of more than 200 O serogroups of V. cholerae, epidemics of cholera were caused by V. cholerae serogroup O1 until 1992. V. cholerae O1 has two biological variants or designated biotypes, namely Classical (CL) and El Tor (ET), which differ from each other in both phenotypic and genetic characteristics, as well as by the type of cholera toxin (CT) that they harbour (Dziejman et al., 2002; Kaper et al., 1995; Olsvik et al., 1993). In addition, the two biotypes differ in terms of infection patterns of disease; for example, the CL biotype strains cause more severe disease, while ET strains are more efficient in host-to-host transmission than their CL counterparts. The CL biotype is believed to have caused the first six cholera pandemics, which began in the Indian subcontinent, and subsequently appeared in other areas of the world between 1817 and 1923 (Politzer, 1959). V. cholerae O1 biotype ET, which was first reported in 1905 (Politzer, 1959) initiated the seventh cholera pandemic in the early 1960s by displacing the CL biotype (Kaper et al., 1995). In 1992, a V. cholerae non-O1 serovar, designated V. cholerae O139 synonym Bengal, appeared as the cause of epidemic cholera in Bangladesh (Cholera Working Group, 1993) and India (Ramamurthy et al., 1993). V. cholerae O139 Bengal emerged by displacing V. cholerae O1 ET, an occurrence that was considered to be significant in the history of cholera. V. cholerae O1 ET is still the major pathogen of cholera, although O139 Bengal continues to co-exist by sharing a niche with O1 ET in the estuarine ecosystem of the Bay of Bengal (Alam et al., 2006).
In spite of significant advances in our understanding of diarrhoeal diseases, including V. cholerae pathogenesis, endemic cholera still kills many people in the Ganges delta of the Bay of Bengal. In Bangladesh, the pattern of cholera disease shows two seasonal peaks, the first during spring (March–May), and the second in autumn (September–November) (Alam et al., 2006; Sack et al., 2004). The treatment of cholera, which includes appropriate oral or intravenous rehydration therapy together with a 3-day course of effective antibiotics, can significantly shorten the duration of diarrhoea (Sack et al., 2004), disease severity and hospitalization (Lindenbaum et al., 1967). However, in recent years antibiotic therapy has faced difficulties, with the rapid emergence of multiple antibiotic-resistant strains of V. cholerae being reported in Africa, Asia and America (Jesudason & John, 1990; Maimone et al., 1986). During the past two decades, several cholera-endemic countries including India and Bangladesh have reported V. cholerae serogroup O1 resistance to tetracycline (TE), ampicillin (AMP), kanamycin (KN), sulfonamides, streptomycin (SM), trimethoprim/sulfamethoxazole (SXT), norfloxacin (NOR), gentamicin (CN), furazolidone (FR), ciprofloxacin (CIP) and erythromycin (E) (Faruque et al., 2007; Sack et al., 2004; Jain et al., 2011). The increasing trend of multi-drug resistance of V. cholerae associated with severe disease is becoming a serious public health concern globally (Jain et al., 2011; Kumar et al., 2010; Quilici et al., 2010).
In Asia and Africa, V. cholerae O1 biotype ET, which is the cause of the current seventh pandemic, has recently been replaced by an altered ET possessing the CT of the CL biotype. Over the past few years, ET causing Asiatic cholera has shown remarkable changes in its phenotypic and genetic characteristics (Nair et al., 2002). Recent molecular analysis of ET strains causing acute watery diarrhoea in Bangladesh shows them to be hybrids because they possess phenotypic and genotypic traits of the CL biotype against an ET background (Nair et al., 2002). Subsequent retrospective studies showed that all of the O1 ET strains isolated in Bangladesh since 2001 were hybrids of both CL and ET biotypes, while those isolated before 2001 contained all the ET attributes of the seventh pandemic V. cholerae O1 (Nair et al., 2006). V. cholerae hybrid ET continues to be routinely isolated from clinical cholera cases in Asia and Africa (Safa et al., 2008), and has been reported to be a new pandemic pathogen capable of causing more severe disease (Siddique et al., 2010), which is spreading globally (Chin et al., 2011). A recent study in India reported that a new CT variant of V. cholerae O1 ET with an amino acid substitution at position 20 caused a large cholera outbreak in Orissa, Eastern India (Kumar et al., 2009). Subsequently, the same CT variant was found to have been associated with cholera in Kolkata, India, since 2006 (Naha et al., 2012). Considering these recent changes, the current study was undertaken in order to understand the phenotypic and genetic traits of contemporary V. cholerae causing endemic cholera in Dhaka, Bangladesh.
Methods
Bacterial strains.
A set of 54 randomly selected strains isolated between 2006 and 2011, as part of the 2 % surveillance of cholera patients seeking treatment at the hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), were included in the study. Rectal swabs were collected from suspected cholera patients and transported to the laboratory within 3 h using Cary Blair transport media. The isolation of V. cholerae began by enriching the rectal swab in alkaline peptone water (APW) (pH 8.4) at 37 °C for 4–6 h, and the resulting solution was cultured on selective media as described previously (Alam et al., 2007). V. cholerae was identified and confirmed using standard cultural, biochemical and molecular methods (Alam et al., 2007).
Serogrouping.
The serogroups of the V. cholerae strains identified by biochemical and molecular methods were confirmed serologically by a slide agglutination test using specific polyvalent antisera for V. cholerae O1 and O139, followed by a monoclonal antibody that is serotype-specific (Alam et al., 2007).
Biotyping.
Biotyping involved a number of phenotypic tests: chicken erythrocyte agglutination (chicken cell agglutination; CCA), sensitivity to polymyxin B, and Mukerjee CL phage IV and Mukerjee ET phage V tests (Kaper et al., 1995). To complement the biotype characterization by phenotypic traits, PCR assays were carried out using previously described procedures that were targeted to detect the tcpA allele (CL and ET) (Alam et al., 2010), the type of the rstR gene encoding the phage transcriptional regulator (Kimsey et al., 1998), and the rtxC gene of RTX (repeat in toxin) (Chow et al., 2001).
Genomic DNA preparation.
Genomic DNA extraction was carried out following previously described methods (Alam et al., 2010).
Confirmation of serogrouping by PCR assay.
All strains that were preliminarily identified as V. cholerae were reconfirmed using a V. cholerae species-specific ompW PCR. The serogroups of these strains were reconfirmed using polyvalent O1 and monovalent Inaba and Ogawa antisera, and by multiplex PCR targeted to identify genes encoding O1- (wbe) and O139-(wbf) specific O biosynthetic genes, as well as the CT gene (ctxA) (Hoshino et al., 1998).
Antimicrobial susceptibility.
Bacterial susceptibility to antimicrobial agents was determined by standard disc diffusion methods (Bauer et al., 1966; CLSI, 2010) using commercially available discs (Oxoid International). A total of six antibiotics, E (15 µg), TE (30 µg), CN (10 µg), CIP (5 µg), SXT (30 µg) and FR (100 µg), were used.
PCR assay for the detection of SXT and class 1 integron.
Using PCR assays, all antibiotic-resistant V. cholerae O1 strains were examined for the presence of the SXT element and class 1 integron. The detection of SXT and intI1 was performed using primers and procedures described elsewhere (Thungapathra et al., 2002).
Double mismatch amplification mutation assay (DMAMA)-PCR for determination of ctxB genotype.
DMAMA-PCR was recently developed to discriminate the CL (ctxB genotype 1), ET (ctxB gneotype 3) and Haitian types (ctxB genotype 7) of ctxB alleles by focusing on nucleotide positions 58 and 203 of the ctxB gene (Naha et al., 2012). DMAMA-PCR was performed in this study to detect the ctxB genotype using the primers and conditions described elsewhere (Naha et al., 2012). V. cholerae O1 strains O395 (CL), N16961 (ET) and 2010EL-1786 (Haiti variant, genotype 7) were used as control strains for DMAMA-PCR analysis.
Nucleotide sequence analysis of the ctxB gene.
To complement the results of DMAMA-PCR, the ctxB gene of representative V. cholerae O1 strains was sequenced using primers and conditions as described elsewhere (Olsvik et al., 1993). PCR amplification of the ctxB gene was performed in a 25 µl reaction mixture in an automated Peltier thermal cycler (PTC-200, MJ Research). Subsequently, PCR products were purified with a Microcon centrifugal filter device (Millipore Corporation) and sequenced using an ABI PRISM BigDye Terminator Cycle Sequencing Reaction kit (Applied Biosystems) on an ABI PRISM 310 automated sequencer (Applied Biosystems). The sequences of the ctxB gene for other V. cholerae O1 ET and CL strains listed in Fig. 1 were retrieved from GenBank (accession nos NC_002505, U25679, EU496278). The deduced amino acid sequences of the ctxB gene from all strains were aligned using clustal w.
Fig. 1.
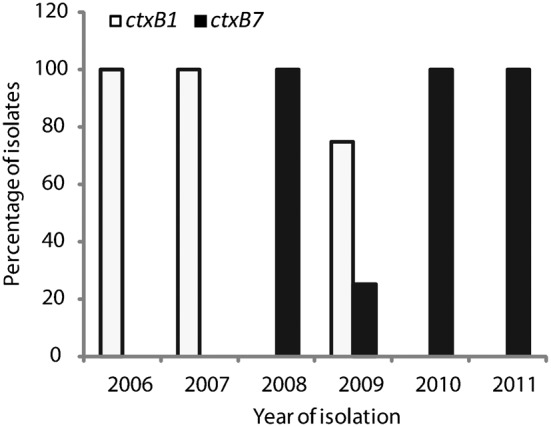
Results of DMAMA-PCR performed to determine the ctxB genotype of tested V. cholerae O1 strains (n = 54) from Dhaka, Bangladesh. All representative strains from 2006 and 2007 possessed ctxB genotype 1 only (ctxB1), while ctxB genotype 7 (ctxB7) was predominant during 2008, 2010 and 2011. Although ctxB1 and ctxB7 genotypes co-existed during 2009, none of the strains that were isolated during 2010 and thereafter possessed ctxB1, suggesting that the transition from genotype 1 to genotype 7 took place during 2008–2010.
PFGE.
The whole agarose-embedded genomic DNA for V. cholerae was prepared. PFGE was carried out with a contour-clamped homogeneous electrical field (CHEF-DR II) apparatus (Bio-Rad), according to procedures described elsewhere (Cooper et al., 2006). The conditions used for separation were as follows: 2–10 s for 13 h, followed by 20–25 s for 6 h. An electrical field of 6 V cm−1 was applied at an included field angle of 120°. Genomic DNAs of the test strains were digested by the NotI restriction enzyme (Gibco-BRL), and Salmonella enterica serovar Braenderup was digested by XbaI, with the fragments being used as molecular size markers. The restriction fragments were separated in 1 % pulsed-field-certified agarose in 0.5× TBE (Tris/borate-EDTA) buffer. In the post-electrophoresis gel treatment step, the gel was stained and de-stained. The DNA was visualized using a UV transilluminator, and images were digitized by a 1D gel documentation system (Bio-Rad).
Image analysis.
The fingerprint pattern in the gel was analysed using the software package Bionumeric (Applied Maths). After background subtraction and gel normalization, the fingerprint patterns were subjected to typing on the basis of banding similarity and dissimilarity by the Dice similarity coefficient and unweighted-pair group method (UPGMA), using average linkage clustering methods, as recommended by the manufacturer; these were graphically represented as dendrograms.
Results
Microbiological and serological tests
All tested strains (n = 54) produced characteristic colonies typical of V. cholerae when they were grown on the selective medium, taurocholate tellurite gelatin agar (TTGA). The characteristic colonies gave biochemical reactions typical of V. cholerae, and all strains reacted to polyvalent antibody specific for V. cholerae serogroup O1 followed by positive agglutination with monovalent Ogawa antisera, suggesting that all belonged to V. cholerae O1 of Ogawa serotype (Table 1).
Table 1. Phenotypic and genotypic traits of V. cholerae O1 ET causing endemic cholera in Dhaka, 2006–2011.
Abbreviations: Clin, clinical; Poly B, polymyxin B; R, resistant; S, sensitive.
| Year of isolation | No. of strains or accession no. | Source | Serotype | wbeO1 | ctxA | Phenotypic trait | Genotypic trait | Antibiotic resistance profile | sxt | intI1 | ||||||
| Poly B (50 U) | CCA | Phage IV | Phage V | tcpA type | rstR type | RTX | ctxB genotype* | |||||||||
| 2006 | 6 | Clin† | Ogawa | + | + | R | + | R | S | ET | ET | ET | B1 | SXT, FR | + | − |
| 3 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B1 | SXT, FR, TE, E | + | − | |
| 2007 | 2 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B1 | SXT, FR | + | − |
| 7 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B1 | SXT, FR, TE | + | − | |
| 2008 | 8 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR | + | − |
| 2009 | 6 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B1 | SXT, FR, TE | + | − |
| 2 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR, TE | + | − | |
| 2010 | 8 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR | + | − |
| 3 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR, TE | + | − | |
| 2011 | 8 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR | + | − |
| 1 | Clin | Ogawa | + | + | R | + | R | S | ET | ET | ET | B7 | SXT, FR, E | + | − | |
| 1971 | N16961 | Clin | Inaba | + | + | R | + | R | S | ET | ET | ET | B3 | All sensitive | − | − |
| 1965 | O395 | Clin | Ogawa | + | + | S | − | S | R | CL | CL | CL | B1 | All sensitive | − | − |
Determined by DMAMA-PCR (Naha et al., 2012).
Amplification of primers specific for V. cholerae serogroup O1 and ctxA by PCR assay
All 54 strains amplified the primers for the V. cholerae species-specific gene ompW, and O-antigen biosynthetic gene wbeO1, but failed to amplify the primers specific for the wbfO139 gene. In addition, all of the strains serologically identified to be O1 amplified the primers for the CT gene ctxA, confirming that all tested V. cholerae O1 strains harboured the toxigenic CTX prophage in their genome (Table 1).
Phenotypic and related genetic characteristics
The phenotypic and related genetic characteristics of the V. cholerae serogroup O1 strains are presented in Table 1. All of the V. cholerae O1 strains were primarily identified as ET biotype based on specific phenotypic characteristics such as positive CCA, sensitivity to ET-specific phage V, and resistance to both polymyxin B (50 U) and CL-specific phage IV. All phenotypically confirmed ET strains amplified the primers for the rtxC gene, which is unique to the ET biotype, thereby confirming their ET traits. In addition, all ET strains amplified the primers for the tcpAET and rstRET genes, but not for the tcpACL and rstRCL genes, further confirming that the strains to belong to the ET biotype.
Antibiotic susceptibility testing
The response of V. cholerae O1 strains towards six different antibiotics revealed that multidrug resistant (MDR) strains having resistance to 3-4 antibiotics were circulating between 2006 and 2011 (Table 1). The drugs to which all of the 54 tested V. cholerae O1 strains showed resistance throughout the study period were SXT and FR, while resistance against TE and E was highly unstable, varying between years. All of the tested strains were uniformly susceptible to CN and CIP. Three of the nine V. cholerae O1 strains (33.3 %) showing resistance to four drugs, namely SXT, FR, TE and E, were found in 2006 only. Year-wise data revealed that none of the tested strains showed resistance to E during 2007–2010, although one of nine (11 %) V. cholerae strains was resistant to E in 2011. The year-wise data also revealed that TE resistance in MDR V. cholerae occurred in 33.3, 77.8, 0, 100 and 27.3 % of the strains in 2006, 2007, 2008, 2009 and 2010, respectively, whereas all tested strains were sensitive to TE in 2011. The overall data suggest that continuous monitoring of the drug susceptibility of V. cholerae is important, considering that the response can change temporarily, making an effective drug ineffective.
PCR assay for detecting SXT and class 1 integron
All drug-resistant V. cholerae strains (n = 54) supported the amplification of the primers for the SXT gene, a mobile genetic element carrying multidrug resistance gene cassettes. None of the strains in the present study except the positive control amplified the primers targeted to the genetic element intI1, confirming the absence of the class 1 integron among the tested V. cholerae O1 strains.
ctxB typing by DMAMA-PCR
All V. cholerae O1 strains (n = 54), including the O395 (CL), N16961 (ET) and 2010EL-1786 (Haiti variant, ctxB genotype 7), were analysed by the DMAMA-PCR technique to determine the CTX-B genotype. As shown in Table 1, 24 V. cholerae O1 ET strains isolated between 2006 and 2009 amplified only the CL biotype-specific ctxB allele (ctxB genotype 1), confirming them to be the altered ET that replaced the prototype seventh pandemic ET (ctxB genotype 3) in Dhaka from 2001 onwards. The remaining ET strains isolated between 2008 and 2011 amplified the primers specific for ctxB genotype 7, which was reported recently from V. cholerae O1 strains in India and Haiti. Fig. 1 shows the time of transition from ctxB genotype 1 to genotype 7 that took place in Dhaka, Bangladesh. Although genotype 1 and genotype 7 coexisted in 2009, all V. cholerae O1 strains tested in 2010 and thereafter were of ctxB genotype 7 alone.
Sequencing of the ctxB gene
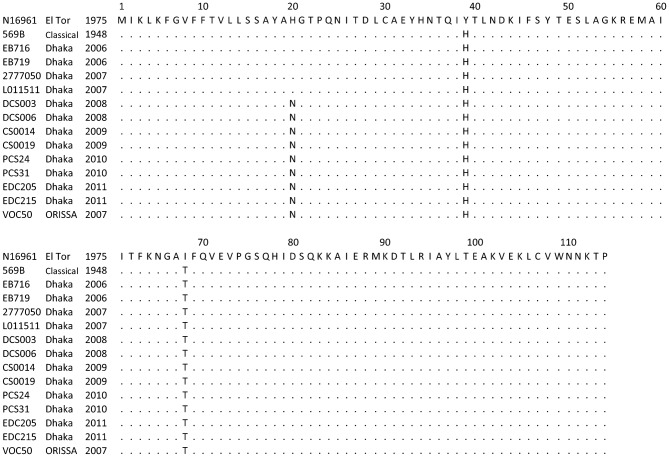
PCR-amplified ctxB genes (460 bp) of representative V. cholerae O1 strains (n = 12) from each year between 2006 and 2011 were sequenced, and the amino acid sequences were determined by employing bioinformatics tools. As shown in Fig. 2, V. cholerae O1 isolated before 2008 showed an amino acid sequence identical to that of the CL biotype CT (ctxB genotype 1), with histidine and threonine at positions 39 and 68, respectively. However, an additional sequence variation was observed at position 20, where the histidine found in CL and ET biotype CT was replaced by asparagine (H→N) in strains isolated during 2008 and thereafter (Fig. 2). The DNA sequence and the deduced amino acids matched the ctxB genotype 7 that was reported recently from India and Haiti. Our ctxB sequencing data were consistent, complementing the results of DMAMA-PCR.
Fig. 2.
Amino acid sequence alignment of the CTXB subunit of V. cholerae O1 ET strains isolated in Bangladesh (2006–2011) with ET reference (N16961), CL reference (569B) and altered ET (Orissa variant) possessing ctxB genotype 7. Identical amino acid residues are indicated by dots. The amino acid sequences of the CTXB reference and Orissa variant used in the alignment were taken from GenBank.
PFGE analysis
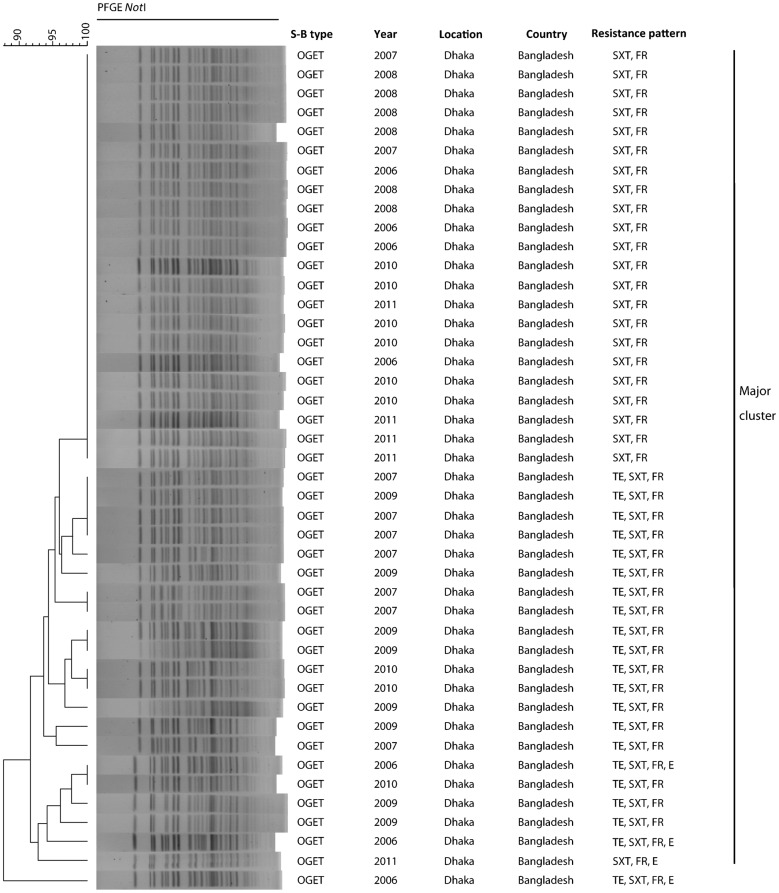
A total of 54 representative V. cholerae O1 strains isolated between 2006 and 2011 were analysed by PFGE to reveal the genetic relatedness of the MDR strains that were circulating in Dhaka. The NotI restriction enzyme digested the genomic DNA into variable fragments, and the fragment sizes ranged from 20 to 350 kb (Fig. 3). All of the tested V. cholerae strains had very closely related PFGE banding patterns, suggesting that they belonged to the same clonal lineage. Thirty-two strains with resistance to FR and SXT had identical banding patterns, whereas the MDR strains showing resistance to FR, SXT and TE and/or E were slightly divergent in their PFGE banding patterns (Fig. 3). Cluster analysis by dendrogram using the PFGE images revealed a close clonal relationship, as most of the V. cholerae strains of different temporal origins formed a major cluster, although MDR strains showing resistance to TE, in addition to FRR and SXTR, belonged to separate but closely related clusters.
Fig. 3.
Genomic fingerprinting of drug-resistant V. cholerae O1 ET strains isolated in Dhaka between 2006 and 2011. The dendrogram was constructed by Dice similarity coefficient and UPGMA clustering using PFGE images of NotI-digested genomic DNA. The scale bar at the top left indicates the correlation coefficient (range: 90–100 %). The banding patterns and the similarity coefficient, which is within 90 % for all V. cholerae strains included in the dendrogram, suggest that they all belong to the same clonal lineage. Cluster analysis shows that all V. cholerae strains resistant to SXT and FR, irrespective of their year of isolation, shared a major cluster with an identical banding pattern, while MDR strains having TE resistance showed genetic divergence in their patterns and formed several closely related subclusters.
Discussion
Clinical management of cholera and its prevention are often hindered because of rapid genetic changes and the emergence of multiple antibiotic resistance in V. cholerae (Glass et al., 1980; Sack et al., 2004; Saha et al., 2006). The altered V. cholerae ET, possessing CT of the CL biotype, emerged in 2001 by displacing ET of the seventh pandemic prototype (Nair et al., 2006). Recently, the altered V. cholerae ET has undergone another base substitution, resulting in a switch of the ctxB gene from genotype 1 (CL) to genotype 7 in eastern India in 2007 (Kumar et al., 2009). Here we present data on the antibiotic resistance and related phenotypic and genetic characteristics of V. cholerae O1 associated with epidemic and endemic cholera in Dhaka, between 2006 and 2011, showing 2008–2010 to be the time of a genetic transition from ctxB genotype 1 to genotype 7. During this period, V. cholerae exhibited temporal variation in drug resistance in Dhaka, rendering V. cholerae strains sensitive to TE, which was the drug of choice for treatment of cholera in the 1960s (Greenough et al., 1964).
The results of microbiological culture, biochemical and serological tests preliminarily confirmed that all the tested V. cholerae strains belonged to serogroup O1. These microbiological test results were complemented by simplex and multiplex PCR assays for V. cholerae species-specific gene ompW, the virulence-associated gene ctxA encoding the subunit A of the CT, and the wbe gene encoding a serogroup O1-specific marker (Hoshino et al., 1998). All of these assays further confirmed the V. cholerae strains to be toxigenic and as belonging to serogroup O1 (Alam et al., 2010). The presence of biotype-specific marker genes, such as rtxC, tcpAET, rstRET and ctxBCL, confirmed that the V. cholerae O1 strains causing endemic cholera in Dhaka between 2006 and 2011 were ET but contained the ctxB marker gene of the CL biotype, as reported previously (Nair et al., 2006; Alam et al., 2010).
Ever since effective antimicrobial agents were employed as successful therapeutic options in the treatment of cholera (Greenough et al., 1964; Lindenbaum et al., 1967), TE was the drug of choice worldwide, except for young children and pregnant women (Greenough et al., 1964; Sack et al., 2004). Other effective drugs included FR, E, SXT and chloramphenicol (C) (Greenough et al., 1964). Following a few years of successful use of these antimicrobial agents for the treatment of cholera (Lindenbaum et al., 1967), a rapid emergence of V. cholerae strains resistant to antimicrobial agents was reported in Africa (Mhalu et al., 1979). Likewise, in December 1979, V. cholerae O1 that was resistant to TE, AMP, KN, SM and SXT emerged as the causal agent of cholera in Bangladesh (Glass et al., 1980). Subsequent studies reported V. cholerae resistant to nalidixic acid (NA), and most recently to E and CIP. Higher numbers of MIC and clinical failures forced clinicians to stop using CIP, and to start administering azithromycin (AZM) as the only effective drug against cholera at the Dhaka Hospital of icddr,b. Recent studies in southwestern India have shown V. cholerae to be resistant to several antibiotics, including TE, FR, NOR and CIP (Jain et al., 2011). In the present study, V. cholerae strains isolated at the icddr,b Dhaka Hospital between 2006 and 2011 were found to be susceptible to CIP. Although all tested V. cholerae strains were resistant to SXT and FR, and the resistance to TE and E varied between 2006 and 2010, all of the tested strains were sensitive to TE in 2011, once again supporting its use as an effective drug for treating cholera, as reported many years ago (Greenough et al., 1964).
After MDR V. cholerae first emerged (Mhalu et al., 1979; Glass et al., 1980, 1983), the antibiotic susceptibility patterns of epidemic strains have changed frequently. Likewise, the emergence of V. cholerae O1 or O139 having resistance towards different drugs has been reported in Bangladesh (Faruque et al., 1998). The genetic basis for such fluctuation in drug resistance was shown to be due to lateral acquisition of the self-transmissible genetic element designated SXT, which carries multiple antibiotic resistance markers (Waldor, et al., 1996). In the present study, a significant proportion of the tested V. cholerae strains associated with the cholera epidemic in Dhaka between 2006 and 2011 were found to be MDR. Although four different antibiotic resistance profiles were found, all of the tested V. cholerae strains had the SXT element, presumably in their genome, a fact that appears to be in line with their consistent resistance towards SXT and FR. Temporal variation in TE resistance was observed, as none of the tested strains isolated in the years 2008 and 2011 showed resistance to TE. TE resistance in V. cholerae is known to be plasmid-mediated, although plasmids have been shown to be highly unstable in vibrios (Taneja et al., 2010). This could be a reason for the temporal fluctuation in TE resistance observed in the present study. Given that antibiotic susceptibility patterns of epidemic strains change frequently, continuous monitoring of V. cholerae drug resistance is crucial for choosing an effective drug for the treatment of cholera.
CT, encoded by the ctxAB genes, is the major virulence factor of V. cholerae that is responsible for causing severe cholera disease. Divergence within the ctxB gene and the corresponding amino acid sequence was first reported in the early 1990s. Based on amino acid substitutions at positions 39, 46 and 68, three different ctxB genotypes of V. cholerae O1 strains have been identified (Olsvik et al., 1993). Genotype 1 is associated with strains of the CL biotype worldwide and along the USA gulf coast, genotype 2 is found in the pre-seventh pandemic ET biotype strains from Australia, and genotype 3 is found in ET biotype strains from the seventh pandemic and the Latin American epidemic (Olsvik et al., 1993). However, subsequent investigation of the ctxB gene sequence revealed the presence of 11 distinct genotypes in different serogroups of V. cholerae (Marin & Vicente, 2012). Genotypes 1, 2, 3, 7, 10 and 11 were found in serogroup O1 strains, genotypes 3, 4, 5 and 6 were found in serogroup O139 strains, and genotypes 8 and 9 were found only in serogroups O27 and O37, respectively (Marin & Vicente, 2012). A significant and recent incident in the history of cholera has been the emergence of V. cholerae altered ET, which has the ctxB gene of V. cholerae CL biotype (Nair et al., 2006), and which has been recognized as a new pandemic pathogen with the capacity to spread globally (Chin et al., 2011; Nair et al., 2002, 2006), causing more severe disease (Siddique et al., 2010). According to a recent study, a new ctxB variant of V. cholerae with an amino acid substitution at position 20 [histidine (H)→asparagine (N)], designated genotype 7, was found to be associated with a large cholera outbreak in India (Kumar et al., 2009). Results from the present study confirm the emergence of genotype 7 in Dhaka, and 2008–2010 was the time of transition from ctxB genotype 1 to genotype 7. V. cholerae O1 ET possessing ctxB genotype 7 and showing reduced susceptibility to CIP, as reported in India (Kumar et al., 2009), has been reported recently in western Africa (Quilici et al., 2010). V. cholerae strains with ctxB genotype 7 in Dhaka were uniformly sensitive to CIP; however, switching from ctxB genotype 1 to genotype 7 may be yet another major turning point, especially if the altered ET with the same ctxB genotype 7 was responsible for the severe cholera epidemic that continues in Haiti (Chin et al., 2011).
PFGE has been a reliable molecular tool for typing enteric pathogens, including V. cholerae. Results from contemporary literature reviews on comparative genomic studies concur with PFGE-based conclusions that suggest circulation of very closely related clones of V. cholerae in the global wave of the seventh pandemic cholera worldwide (Chun et al., 2009; Chin et al., 2011; Mutreja et al., 2011). The overall PFGE results presented in this study, including the clustering of strains observed in the resulting dendrograms, demonstrated high genetic relatedness between V. cholerae O1 strains that have been associated with cholera in Dhaka between 2006 and 2011. This clustering of V. cholerae O1 strains correlated highly with the antibiotic resistance markers, not to the genetic change observed in the ctxB genotype, which is simply a base substitution and unlikely to be reflected at the PFGE level. Nonetheless, the data presented in this study showed the transmission of varyingly antibiotic resistant, but genetically very closely related, V. cholerae O1 ET in Dhaka city. The minor divergence observed in the strains showing resistance to SXT, FR and TE and/or E is presumed to be due to the acquisition of mobile genetic elements (Chun et al., 2009; Chin et al., 2011; Mutreja et al., 2011) carrying a variable number of antibiotic resistance gene cassettes (Thungapathra et al., 2002; Jesudason & John, 1990).
Cholera has been established as a climate-driven disease that continues to be a growing concern for Dhaka city, where population density is already very high and people living in slums do not have access to safe drinking water. While the changing climate and a sea-level rise will result in major cities like Dhaka becoming even more densely populated, severe infection caused by MDR-altered V. cholerae ET (Nair et al., 2006; Chin et al., 2011) could lead to higher case fatality rates, prolonged hospitalization, increased secondary infections, and increased healthcare costs for the growing population. In Bangladesh, the ET biotype of V. cholerae, the most prolific pandemic strain to date, has switched its CT gene from ET type to CL type (altered ET) since 2001 (Nair et al., 2006). Evidence provided here shows that the ET biotype has been undergoing yet another change in its ctxB gene in Dhaka since 2008. Although the change is subtle and the epidemiological significance of such a genetic change is not fully understood, it is presumed that this may be a factor in the increasing severity of disease caused by altered ET in recent outbreaks (Siddique et al., 2010; Goel & Jiang, 2010; Chin et al., 2011). Finally, the data presented in this study underscore the need for close monitoring of V. cholerae causing endemic cholera in Bangladesh. This is important not only in Bangladesh to ensure that the correct antibiotic is chosen according to resistance variations, but also around the world, considering the increasing global burden of cholera, and the emergence and spread of new variants that will significantly influence the clinical management of cholera and its prevention.
Acknowledgements
This research was supported by a grant-in-aid from the Ministry of Health, Labour and Welfare, the Government of Japan (H23-Shinkou-shitei-020), and National Institutes of Health grant no. RO1AI039129, under collaborative agreements between the Johns Hopkins Bloomberg School of Public Health, the University of Maryland, College Park, and the icddr,b. icddr,b acknowledges the following donors, which provide unrestricted support to the Centre’s research efforts: Australian Agency for International Development (AusAID), Government of the People’s Republic of Bangladesh, Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (SIDA), and the Department for International Development, UK (DFID).
Abbreviations:
- AMP
ampicillin
- CCA
chicken cell agglutination
- CIP
ciprofloxacin
- CL
Classical
- CN
gentamicin
- CT
cholera toxin
- E
erythromycin
- ET
El Tor
- FR
furazolidone
- icddr,b
International Centre for Diarrhoeal Disease Research, Bangladesh
- KN
kanamycin
- MDR
multidrug resistant
- NOR
norfloxacin
- RTX
repeat in toxin
- SM
streptomycin
- SXT
trimethoprim/sulfamethoxazole
- TE
tetracycline
- UPGMA
unweighted-pair group method
Reference
- Alam M., Hasan N. A., Sadique A., Bhuiyan N. A., Ahmed K. U., Nusrin S., Nair G. B., Siddique A. K., Sack R. B. & other authors (2006). Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl Environ Microbiol 72, 4096–4104 10.1128/AEM.00066-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Sultana M., Nair G. B., Siddique A. K., Hasan N. A., Sack R. B., Sack D. A., Ahmed K. U., Sadique A. & other authors (2007). Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci U S A 104, 17801–17806 10.1073/pnas.0705599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Nusrin S., Islam A., Bhuiyan N. A., Rahim N., Delgado G., Morales R., Mendez J. L., Navarro A. & other authors (2010). Cholera between 1991 and 1997 in Mexico was associated with infection by classical, El Tor, and El Tor variants of Vibrio cholerae. J Clin Microbiol 48, 3666–3674 10.1128/JCM.00866-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. (1966). Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45, 493–496 [PubMed] [Google Scholar]
- Chin C. S., Sorenson J., Harris J. B., Robins W. P., Charles R. C., Jean-Charles R. R., Bullard J., Webster D. R., Kasarskis A. & other authors (2011). The origin of the Haitian cholera outbreak strain. N Engl J Med 364, 33–42 10.1056/NEJMoa1012928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholera working group (1993). Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet 342, 387–390 10.1016/0140-6736(93)92811-7 [DOI] [PubMed] [Google Scholar]
- Chow K. H., Ng T. K., Yuen K. Y., Yam W. C. (2001). Detection of RTX toxin gene in Vibrio cholerae by PCR. J Clin Microbiol 39, 2594–2597 10.1128/JCM.39.7.2594-2597.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J., Grim C. J., Hasan N. A., Lee J. H., Choi S. Y., Haley B. J., Taviani E., Jeon Y. S., Kim D. W. & other authors (2009). Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci U S A 106, 15442–15447 10.1073/pnas.0907787106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2010. ). Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. Approved Guideline 2nd edn, document M45–A2 (ISBN 1–56238–732–4). Wayne, PA: Clinical and Laboratory Standards Institute [Google Scholar]
- Cooper K. L., Luey C. K., Bird M., Terajima J., Nair G. B., Kam K. M., Arakawa E., Safa A., Cheung D. T. & other authors (2006). Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog Dis 3, 51–58 10.1089/fpd.2006.3.51 [DOI] [PubMed] [Google Scholar]
- Dziejman M., Balon E., Boyd D., Fraser C. M., Heidelberg J. F., Mekalanos J. J. (2002). Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc Natl Acad Sci U S A 99, 1556–1561 10.1073/pnas.042667999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque A. S. G., Salam M. A., Faruque S. M., Fuchs G. J. (1998). Aetiological, clinical and epidemiological characteristics of a seasonal peak of diarrhoea in Dhaka, Bangladesh. Scand J Infect Dis 30, 393–396 10.1080/00365549850160701 [DOI] [PubMed] [Google Scholar]
- Faruque A. S. G., Alam K., Malek M. A., Khan M. G., Ahmed S., Saha D., Khan W. A., Nair G. B., Salam M. A. & other authors (2007). Emergence of multidrug-resistant strain of Vibrio cholerae O1 in Bangladesh and reversal of their susceptibility to tetracycline after two years. J Health Popul Nutr 25, 241–243 [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Huq I., Alim A. R. M. A., Yunus M. (1980). Emergence of multiply antibiotic-resistant Vibrio cholerae in Bangladesh. J Infect Dis 142, 939–942 10.1093/infdis/142.6.939 [DOI] [PubMed] [Google Scholar]
- Glass R. I., Huq M. I., Lee J. V., Threlfall E. J., Khan M. R., Alim A. R., Rowe B., Gross R. J. (1983). Plasmid-borne multiple drug resistance in Vibrio cholerae serogroup O1, biotype El Tor: evidence for a point-source outbreak in Bangladesh. J Infect Dis 147, 204–209 10.1093/infdis/147.2.204 [DOI] [PubMed] [Google Scholar]
- Goel A. K., Jiang S. C. (2010). Genetic determinants of virulence, antibiogram and altered biotype among the Vibrio cholerae O1 isolates from different cholera outbreaks in India. Infect Genet Evol 10, 815–819 10.1016/j.meegid.2009.06.022 [DOI] [PubMed] [Google Scholar]
- Greenough W. B., III, Gordon R. S., Jr, Rosenberg I. S., Davies B. I., Benenson A. S. (1964). Tetracycline in the treatment of cholera. Lancet 1, 355–357 10.1016/S0140-6736(64)92099-9 [DOI] [PubMed] [Google Scholar]
- Hoshino K., Yamasaki S., Mukhopadhyay A. K., Chakraborty S., Basu A., Bhattacharya S. K., Nair G. B., Shimada T., Takeda Y. (1998). Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol Med Microbiol 20, 201–207 10.1111/j.1574-695X.1998.tb01128.x [DOI] [PubMed] [Google Scholar]
- Jain M., Goel A. K., Bhattacharya P., Ghatole M., Kamboj D. V. (2011). Multidrug resistant Vibrio cholerae O1 El Tor carrying classical ctxB allele involved in a cholera outbreak in South Western India. Acta Trop 117, 152–156 10.1016/j.actatropica.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Jesudason M. V., John T. J. (1990). Transferable trimethoprim resistance of Vibrio cholerae O1 encountered in southern India. Trans R Soc Trop Med Hyg 84, 136–137 10.1016/0035-9203(90)90407-6 [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Morris J. G., Jr, Levine M. M. (1995). Cholera. Clin Microbiol Rev 8, 48–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimsey H. H., Nair G. B., Ghosh A., Waldor M. K. (1998). Diverse CTXϕs and evolution of new pathogenic Vibrio cholerae. Lancet 352, 457–458 10.1016/S0140-6736(05)79193-5 [DOI] [PubMed] [Google Scholar]
- Kumar P., Jain M., Goel A. K., Bhadauria S., Sharma S. K., Kamboj D. V., Singh L., Ramamurthy T., Nair G. B. (2009). A large cholera outbreak due to a new cholera toxin variant of the Vibrio cholerae O1 El Tor biotype in Orissa, Eastern India. J Med Microbiol 58, 234–238 10.1099/jmm.0.002089-0 [DOI] [PubMed] [Google Scholar]
- Kumar P., Wilson P. A., Bhai R., Thomas S. (2010). Characterization of an SXT variant Vibrio cholerae O1 Ogawa isolated from a patient in Trivandrum, India. FEMS Microbiol Lett 303, 132–136 10.1111/j.1574-6968.2009.01868.x [DOI] [PubMed] [Google Scholar]
- Lindenbaum J., Greenough W. B., Islam M. R. (1967). Antibiotic therapy of cholera. Bull World Health Organ 36, 871–883 [PMC free article] [PubMed] [Google Scholar]
- Maimone F., Coppo A., Pazzani C., Ismail S. O., Guerra R., Procacci P., Rotigliano G., Omar K. H. (1986). Clonal spread of multiply resistant strains of Vibrio cholerae O1 in Somalia. J Infect Dis 153, 802–803 10.1093/infdis/153.4.802 [DOI] [PubMed] [Google Scholar]
- Marin M. A., Vicente A. C. (2012). Variants of Vibrio cholerae O1 El Tor from Zambia showed new genotypes of ctxB. Epidemiol Infect 140, 1386–1387, author reply 1387–1388 [DOI] [PubMed] [Google Scholar]
- Mhalu F. S., Mmari P. W., Ijumba J. (1979). Rapid emergence of El Tor Vibrio cholerae resistant to antimicrobial agents during first six months of fourth cholera epidemic in Tanzania. Lancet 1, 345–347 10.1016/S0140-6736(79)92889-7 [DOI] [PubMed] [Google Scholar]
- Mutreja A., Kim D. W., Thomson N. R., Connor T. R., Lee J. H., Kariuki S., Croucher N. J., Choi S. Y., Harris S. R. & other authors (2011). Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477, 462–465 10.1038/nature10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naha A., Pazhani G. P., Ganguly M., Ghosh S., Ramamurthy T., Nandy R. K., Nair G. B., Takeda Y., Mukhopadhyay A. K. (2012). Development and evaluation of a PCR assay for tracking the emergence and dissemination of Haitian variant ctxB in Vibrio cholerae O1 strains isolated from Kolkata, India. J Clin Microbiol 50, 1733–1736 10.1128/JCM.00387-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G. B., Faruque S. M., Bhuiyan N. A., Kamruzzaman M., Siddique A. K., Sack D. A. (2002). New variants of Vibrio cholerae O1 biotype El Tor with attributes of the classical biotype from hospitalized patients with acute diarrhea in Bangladesh. J Clin Microbiol 40, 3296–3299 10.1128/JCM.40.9.3296-3299.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G. B., Qadri F., Holmgren J., Svennerholm A. M., Safa A., Bhuiyan N. A., Ahmad Q. S., Faruque S. M., Faruque A. S. & other authors (2006). Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol 44, 4211–4213 10.1128/JCM.01304-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsvik O., Wahlberg J., Petterson B., Uhlén M., Popovic T., Wachsmuth I. K., Fields P. I. (1993). Use of automated sequencing of polymerase chain reaction-generated amplicons to identify three types of cholera toxin subunit B in Vibrio cholerae O1 strains. J Clin Microbiol 31, 22–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politzer R. (1959. ). Cholera, pp. 11–50 Geneva, Switzerland: World Health Organization [Google Scholar]
- Quilici M. L., Massenet D., Gake B., Bwalki B., Olson D. M. (2010). Vibrio cholerae O1 variant with reduced susceptibility to ciprofloxacin, Western Africa. Emerg Infect Dis 16, 1804–1805 10.3201/eid1611.100568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy T., Garg S., Sharma R., Bhattacharya S. K., Nair G. B., Shimada T., Takeda T., Karasawa T., Kurazano H. & other authors (1993). Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341, 703–704 10.1016/0140-6736(93)90480-5 [DOI] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B., Nair G. B., Siddique A. K. (2004). Cholera. Lancet 363, 223–233 10.1016/S0140-6736(03)15328-7 [DOI] [PubMed] [Google Scholar]
- Safa A., Sultana J., Dac Cam P., Mwansa J. C., Kong R. Y. (2008). Vibrio cholerae O1 hybrid El Tor strains, Asia and Africa. Emerg Infect Dis 14, 987–988 10.3201/eid1406.080129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D., Karim M. M., Khan W. A., Ahmed S., Salam M. A., Bennish M. L. (2006). Single-dose azithromycin for the treatment of cholera in adults. N Engl J Med 354, 2452–2462 10.1056/NEJMoa054493 [DOI] [PubMed] [Google Scholar]
- Siddique A. K., Nair G. B., Alam M., Sack D. A., Huq A., Nizam A., Longini I. M., Jr, Qadri F., Faruque S. M. & other authors (2010). El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol Infect 138, 347–352 10.1017/S0950268809990550 [DOI] [PubMed] [Google Scholar]
- Taneja N., Samanta P., Mishra A., Sharma M. (2010). Emergence of tetracycline resistance in Vibrio cholerae O1 biotype El Tor serotype Ogawa from north India. Indian J Pathol Microbiol 53, 865–866 10.4103/0377-4929.72014 [DOI] [PubMed] [Google Scholar]
- Thungapathra M., Amita, Sinha K. K., Chaudhuri S. R., Garg P., Ramamurthy T., Nair G. B., Ghosh A. (2002). Occurrence of antibiotic resistance gene cassettes aac(6′)-Ib, dfrA5, dfrA12, and ereA2 in class I integrons in non-O1, non-O139 Vibrio cholerae strains in India. Antimicrob Agents Chemother 46, 2948–2955 10.1128/AAC.46.9.2948-2955.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Tschäpe H., Mekalanos J. J. (1996). A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J Bacteriol 178, 4157–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]