Abstract
Aims—(1) To study the frequency of putative malignancy associated point mutations and a 30 base pair (bp) deletion in exon 3 of the C-terminus of the Epstein-Barr virus (EBV) encoded latent membrane protein (LMP)-1 (BNLF-1) gene in wild type EBV strains. (2) To assess the influence of these mutations on the tumorigenicity of lymphoblastoid cell lines (LCL).
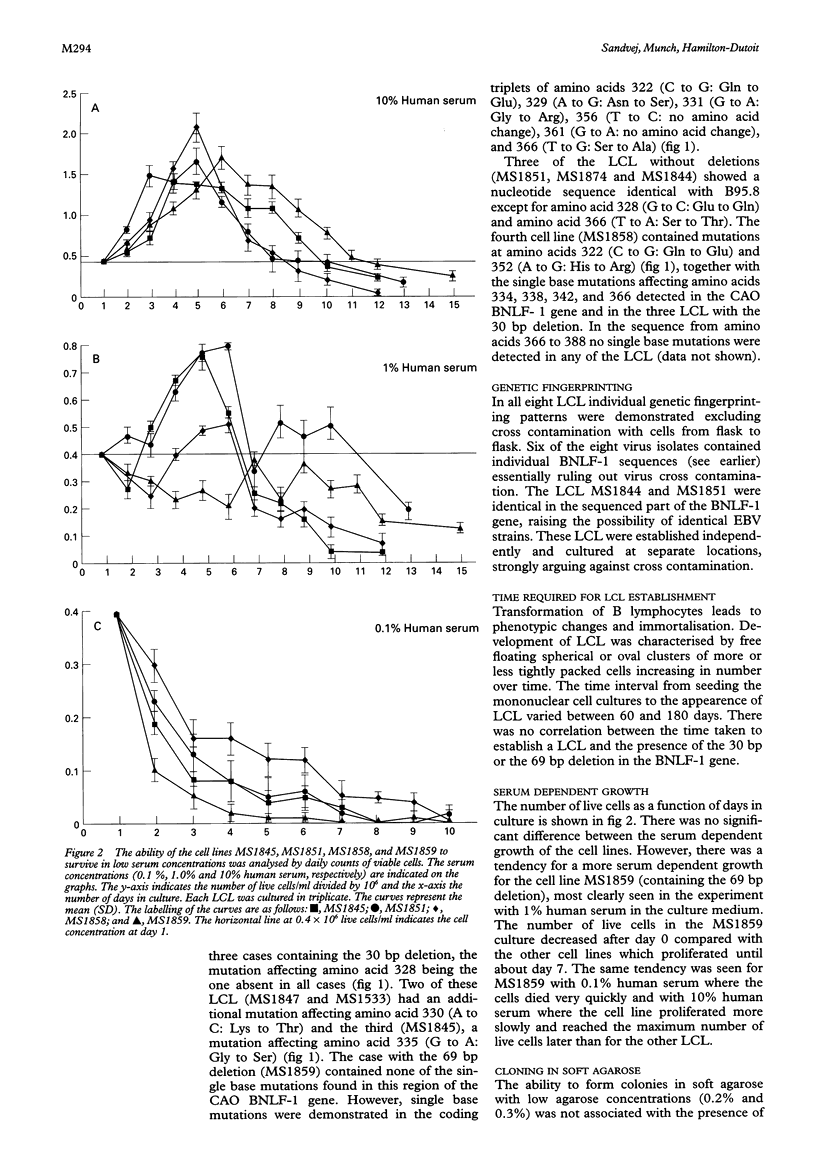
Methods—Eight spontaneous EBV (wild type) infected LCL were established from seven subjects. Deletions and single base mutations in the C-terminus of the BNLF-1 gene were demonstrated using bi-directional solid phase dideoxy sequencing following PCR amplification of viral DNA from the LCL. Tumorigenicity of the LCL was assessed in SCID and nude mice. Serum dependent growth and ability to form colonies in soft agarose were assessed for representative LCL.
Results—All LCL showed sequence differences compared with the prototypic EBV strain B95-8. The 30 bp deletion could be detected in three of eight LCL and a 69 bp deletion (including the 30 bp deletion) was identified in an additional LCL. A range of single base mutations (including those described previously in association with EBV related neoplasias) was also seen in some of the LCL. In transformation studies, the genetic variations did not seem to influence the in vitro behaviour of the LCL. In the tumorigenicity studies, the presence of the 30 bp deletion had no influence on the behaviour of the LCL which were, as expected, tumorigenic in SCID mice but not in nude mice. In contrast, the LCL carrying the 69 bp deletion was tumorigenic in both SCID and nude mice.
Conclusions—Genetic changes described previously in the C-terminus of the LMP-1 gene in various malignancy derived EBV strains are also present frequently in wild type viruses and do not simply define tumour specific EBV strains. Changes within this region may, however, still be important for the tumorigenicity of LMP-1 and thus play a role in EBV oncogenesis.
Keywords: Epstein-Barr virus
Keywords: latent membrane protein-1
Keywords: spontaneous lymphoblastoid cell lines
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen M. L., Tsai C. N., Liang C. L., Shu C. H., Huang C. R., Sulitzeanu D., Liu S. T., Chang Y. S. Cloning and characterization of the latent membrane protein (LMP) of a specific Epstein-Barr virus variant derived from the nasopharyngeal carcinoma in the Taiwanese population. Oncogene. 1992 Nov;7(11):2131–2140. [PubMed] [Google Scholar]
- Dambaugh T., Hennessy K., Chamnankit L., Kieff E. U2 region of Epstein-Barr virus DNA may encode Epstein-Barr nuclear antigen 2. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7632–7636. doi: 10.1073/pnas.81.23.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C. W., Rickinson A. B., Young L. S. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature. 1990 Apr 19;344(6268):777–780. doi: 10.1038/344777a0. [DOI] [PubMed] [Google Scholar]
- Fåhraeus R., Rymo L., Rhim J. S., Klein G. Morphological transformation of human keratinocytes expressing the LMP gene of Epstein-Barr virus. Nature. 1990 May 31;345(6274):447–449. doi: 10.1038/345447a0. [DOI] [PubMed] [Google Scholar]
- Hamilton-Dutoit S. J., Pallesen G., Franzmann M. B., Karkov J., Black F., Skinhøj P., Pedersen C. AIDS-related lymphoma. Histopathology, immunophenotype, and association with Epstein-Barr virus as demonstrated by in situ nucleic acid hybridization. Am J Pathol. 1991 Jan;138(1):149–163. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B., Baichwal V. R. The transforming domain alone of the latent membrane protein of Epstein-Barr virus is toxic to cells when expressed at high levels. J Virol. 1989 Jun;63(6):2469–2475. doi: 10.1128/jvi.63.6.2469-2475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H. E., Morling N. Paternity testing with VNTR DNA systems. II. Evaluation of 271 cases of disputed paternity with the VNTR systems D2S44, D5S43, D7S21, D7S22, and D12S11. Int J Legal Med. 1993;105(4):197–202. doi: 10.1007/BF01642793. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Gajl-Peczalska K. J., Frizzera G., Arthur D. C., Balfour H. H., Jr, McClain K., Simmons R. L., Najarian J. S. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation. Clinical, pathologic, and virologic findings and implications for therapy. Ann Surg. 1983 Sep;198(3):356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. F., Chen F., Zheng X., Ernberg I., Cao S. L., Christensson B., Klein G., Winberg G. Clonability and tumorigenicity of human epithelial cells expressing the EBV encoded membrane protein LMP1. Oncogene. 1993 Jun;8(6):1575–1583. [PubMed] [Google Scholar]
- Hudson G. S., Farrell P. J., Barrell B. G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol. 1985 Feb;53(2):528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen D. S., Henderson S. A., Croom-Carter D., Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995 Feb 2;10(3):549–560. [PubMed] [Google Scholar]
- Kaye K. M., Izumi K. M., Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B. M., Lear A. L., Rowe M., Croom-Carter D., Young L. S., Rookes S. M., Gallimore P. H., Rickinson A. B. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology. 1992 Mar;187(1):189–201. doi: 10.1016/0042-6822(92)90307-b. [DOI] [PubMed] [Google Scholar]
- Knecht H., Bachmann E., Brousset P., Sandvej K., Nadal D., Bachmann F., Odermatt B. F., Delsol G., Pallesen G. Deletions within the LMP1 oncogene of Epstein-Barr virus are clustered in Hodgkin's disease and identical to those observed in nasopharyngeal carcinoma. Blood. 1993 Nov 15;82(10):2937–2942. [PubMed] [Google Scholar]
- Knecht H., Raphaël M., McQuain C., Rothenberger S., Pihan G., Camilleri-Broët S., Bachmann E., Kershaw G. R., Ryan S., Kittler E. L. Deletion variants within the NF-kappa B activation domain of the LMP1 oncogene prevail in acquired immunodeficiency syndrome-related large cell lymphomas and human immunodeficiency virus-negative atypical lymphoproliferations. Blood. 1996 Feb 1;87(3):876–881. [PubMed] [Google Scholar]
- Kunimoto M., Tamura S., Tabata T., Yoshie O. One-step typing of Epstein-Barr virus by polymerase chain reaction: predominance of type 1 virus in Japan. J Gen Virol. 1992 Feb;73(Pt 2):455–461. doi: 10.1099/0022-1317-73-2-455. [DOI] [PubMed] [Google Scholar]
- Li S. N., Chang Y. S., Liu S. T. Effect of a 10-amino acid deletion on the oncogenic activity of latent membrane protein 1 of Epstein-Barr virus. Oncogene. 1996 May 16;12(10):2129–2135. [PubMed] [Google Scholar]
- Miller W. E., Edwards R. H., Walling D. M., Raab-Traub N. Sequence variation in the Epstein-Barr virus latent membrane protein 1. J Gen Virol. 1994 Oct;75(Pt 10):2729–2740. doi: 10.1099/0022-1317-75-10-2729. [DOI] [PubMed] [Google Scholar]
- Moorthy R. K., Thorley-Lawson D. A. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of rat-1 fibroblasts. J Virol. 1993 Mar;67(3):1638–1646. doi: 10.1128/jvi.67.3.1638-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy R. K., Thorley-Lawson D. A. Biochemical, genetic, and functional analyses of the phosphorylation sites on the Epstein-Barr virus-encoded oncogenic latent membrane protein LMP-1. J Virol. 1993 May;67(5):2637–2645. doi: 10.1128/jvi.67.5.2637-2645.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morling N., Hansen H. E. Paternity testing with VNTR DNA systems. I. Matching criteria and population frequencies of the VNTR systems D2S44, D5S43, D7S21, D7S22, and D12S11 in Danes. Int J Legal Med. 1993;105(4):189–196. doi: 10.1007/BF01642792. [DOI] [PubMed] [Google Scholar]
- Nellemann L. J., Møller A., Morling N. PCR typing of DNA fragments of the short tandem repeat (STR) system HUMTH01 in Danes and Greenland Eskimos. Forensic Sci Int. 1994 Sep 6;68(1):45–51. doi: 10.1016/0379-0738(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Pallesen G., Hamilton-Dutoit S. J., Zhou X. The association of Epstein-Barr virus (EBV) with T cell lymphoproliferations and Hodgkin's disease: two new developments in the EBV field. Adv Cancer Res. 1993;62:179–239. doi: 10.1016/s0065-230x(08)60319-x. [DOI] [PubMed] [Google Scholar]
- Peng M., Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in B-cell chronic lymphocytic leukemia cells, T cells, and hematopoietic cell lines: cell-type-independent-induction of CD23, CD21, and ICAM-1. Leukemia. 1993 Jan;7(1):104–112. [PubMed] [Google Scholar]
- Peng M., Lundgren E. Transient expression of the Epstein-Barr virus LMP1 gene in human primary B cells induces cellular activation and DNA synthesis. Oncogene. 1992 Sep;7(9):1775–1782. [PubMed] [Google Scholar]
- Rowe M., Peng-Pilon M., Huen D. S., Hardy R., Croom-Carter D., Lundgren E., Rickinson A. B. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B-cell-specific response that is delayed relative to NF-kappa B activation and to induction of cell surface markers. J Virol. 1994 Sep;68(9):5602–5612. doi: 10.1128/jvi.68.9.5602-5612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sample J., Kieff E. F., Kieff E. D. Epstein-Barr virus types 1 and 2 have nearly identical LMP-1 transforming genes. J Gen Virol. 1994 Oct;75(Pt 10):2741–2746. doi: 10.1099/0022-1317-75-10-2741. [DOI] [PubMed] [Google Scholar]
- Sandvej K., Krenács L., Hamilton-Dutoit S. J., Rindum J. L., Pindborg J. J., Pallesen G. Epstein-Barr virus latent and replicative gene expression in oral hairy leukoplakia. Histopathology. 1992 May;20(5):387–395. doi: 10.1111/j.1365-2559.1992.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Sandvej K., Peh S. C., Andresen B. S., Pallesen G. Identification of potential hot spots in the carboxy-terminal part of the Epstein-Barr virus (EBV) BNLF-1 gene in both malignant and benign EBV-associated diseases: high frequency of a 30-bp deletion in Malaysian and Danish peripheral T-cell lymphomas. Blood. 1994 Dec 15;84(12):4053–4060. [PubMed] [Google Scholar]
- Thymann M., Nellemann L. J., Masumba G., Irgens-Møller L., Morling N. Analysis of the locus D1S80 by amplified fragment length polymorphism technique (AMP-FLP). Frequency distribution in Danes. Intra and inter laboratory reproducibility of the technique. Forensic Sci Int. 1993 Jun;60(1-2):47–56. doi: 10.1016/0379-0738(93)90091-n. [DOI] [PubMed] [Google Scholar]
- Trivedi P., Hu L. F., Chen F., Christensson B., Masucci M. G., Klein G., Winberg G. Epstein-Barr virus (EBV)-encoded membrane protein LMP1 from a nasopharyngeal carcinoma is non-immunogenic in a murine model system, in contrast to a B cell-derived homologue. Eur J Cancer. 1994;30A(1):84–88. doi: 10.1016/s0959-8049(05)80024-3. [DOI] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]
- Wang D., Liebowitz D., Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985 Dec;43(3 Pt 2):831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- Wilson J. B., Weinberg W., Johnson R., Yuspa S., Levine A. J. Expression of the BNLF-1 oncogene of Epstein-Barr virus in the skin of transgenic mice induces hyperplasia and aberrant expression of keratin 6. Cell. 1990 Jun 29;61(7):1315–1327. doi: 10.1016/0092-8674(90)90695-b. [DOI] [PubMed] [Google Scholar]
- Young L. S., Dawson C. W., Clark D., Rupani H., Busson P., Tursz T., Johnson A., Rickinson A. B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988 May;69(Pt 5):1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]



