Abstract
Background
The fornix is a compact bundle of white matter fibers that project from the hippocampus to the mamillary bodies and septal nuclei. Its association with memory, as well as with symptoms in schizophrenia, has been reported in chronic schizophrenia. The purpose of this study is to determine whether or not fornix abnormalities are evident at the onset of schizophrenia.
Methods
Diffusion Tensor Imaging (DTI) and DT tractography were used to evaluate the fornix in 21 patients with first episode schizophrenia (16 males/5 females) and 22 healthy controls (13 males/9 females). Groups were matched on age, gender, parental socioeconomic status, education and handedness. Fractional anisotropy (FA), a measure of white matter integrity, radial diffusivity (RD), thought to reflect myelin integrity, trace, a possible marker of atrophy or cell loss, and axial diffusivity (AD), thought to reflect axonal integrity, were averaged over the entire tract extracted by means of DT tractography, and used to investigate fornix abnormalities in first episode schizophrenia compared with healthy controls.
Results
Significant group differences were found between first episode patients and controls for FA (p=0.0001), RD (p=0.001) and trace (p= 0.006).
Conclusion
These findings suggest abnormalities in the fornix in the early stages of schizophrenia, and further suggest that white matter abnormalities, which are apparent in the early course of the disease, may reflect myelin disturbances.
Keywords: First Episode Schizophrenia, Fornix, Diffusion Tensor Imaging, Fractional Anisotropy, Trace, Axial Diffusivity, Radial Diffusivity
INTRODUCTION
Prior to the introduction of Diffusion Tensor Imaging (DTI), most neuroimaging studies of schizophrenia focused on gray matter (see review in Shenton et al., 2001; Glahn et al., 2008). These findings led to schizophrenia being viewed as a neurodevelopmental disorder (e.g., Rapoport et al., 2005), but with neurodegenerative components (e.g., Andreasen et al., 2011; Vita et al., 2012). The pathogenesis of such brain abnormalities is, nonetheless, still unknown. It is also not clear whether progressive changes reported in gray matter in schizophrenia, are also extant in white matter (e.g., Kasai et al., 2003).
DTI is based on the detection of water diffusion properties (Basser et al., 1994), and provides quantifiable information about white matter tracts, their organization, and their integrity. Research suggests that white matter maturation and myelination processes might be disrupted in schizophrenia, which, in turn, might trigger schizophrenia symptoms. Additional evidence for white matter involvement comes from postmortem and genetic studies, which indicate a disruption of oligodendrocyte function (Uranova et al., 2007; Tkachev et al., 2007). The majority of DTI studies report fractional anisotropy decreases in patients with chronic schizophrenia compared to healthy controls (see reviews in Kubicki et al., 2007; Fitzsimmons et al., 2013).
There are more inconsistencies, however, among studies of first episode schizophrenia (FE, see for example reviews in Kuswanto et al., 2012; Samartzis et al., 2013). In the current study, we focus on the fornix, a compact bundle of white matter fibers that projects from the hippocampus to the mamillary bodies, and is part of the limbic system. The hippocampus has been repeatedly found to be abnormal in schizophrenia (e.g., Heckers et al., 2001), and has been associated with cognitive disturbances in memory in schizophrenia (e.g., Nestor et al., 2007). With respect to postmortem studies of schizophrenia, the fornix has been investigated in only two studies (Chance et al., 1999; Davies et al., 2001), both of which report an increase in fiber density in the left fornix in male subjects and an increase in the cross-sectional area of the fornix in early onset of schizophrenia. Also DTI studies in chronic schizophrenia suggest abnormalities in white matter integrity of the fornix (e.g., Kuroki et al., 2006; Abdul-Rahman et al. 2011), while DTI studies in FE schizophrenia are less consistent, with one study reporting no abnormalities (Kendi et al., 2008), while other studies (e.g., Luck et al., 2010; Davenport at al., 2010) report differences in FA.
The purpose of the current study is to investigate whether or not white matter abnormalities within the fornix are present at illness onset. The current study is unique in that in addition to measuring FA, other measures such as Radial Diffusivity (RD), Trace, and Axial Diffusivity (AD) are also included. RD is purported to be a possible marker of myelin disruption, Trace is purported to be a possible marker of atrophy or cell loss, and AD is purported to be a possible marker of axonal disruption (e.g., Song et al., 2005). Thus by adding these measures, we not only hope to detect fornix abnormalities in FE schizophrenia, which is shown with FA as a sensitive but non-specific measure, but also, if such abnormalities exist, we will be able to describe better their specificity and to microstructural correlates.
METHODS
Subjects
Twenty-one patients (16 males/5 females) diagnosed with first episode schizophrenia and 21 control subjects (13 males/9 females) were recruited as part of the Boston CIDAR Center (www.bostoncidar.org). (See Table 1 for demographic information).
Table 1.
Demographic data
| Mean (standard deviation) | p value | ||
|---|---|---|---|
|
| |||
| Schizophrenia patients n = 21 | Controls n = 22 | ||
| Gender (M/F) | 16/5 | 13/9 | - |
| Age | 21.71 (4.86) | 21.23 (3.29) | 0.701 |
| PSES | 2.19 (1.21) | 1.86 (0.94) | 0.327 |
| Handedness (% right) | 100 | 100 | - |
| WRAT (Wilde Range Achievement Test) | 110.05 (19.11) | 111.73 (17.27) | 0.764 |
The patients were either not medicated (four patients) or medicated with atypical antipsychotic medication (seventeen patients). All medication dosages were converted to chlorpromazine equivalents (Woods 2003). Clinical symptoms were measured using the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 2000). This study was approved by the local IRB committees at all participating institutions.
MRI acquisition and processing
Diffusion Tensor Images were acquired on a 3T GE Echospeed system, using a double echo, echo planar imaging (EPI) DTI Tensor sequence (Alexander et al., 1997) at a resolution of 1.7mm×1.7mm×1.7mm (51 diffusion directions at b=900, 8 baseline scans at b=0, TR=17000 ms, TE=78 ms, FOV=24 cm, 144×144 matrix) at Brigham and Women’s Hospital, Boston.
Before tensor estimation, DICOM data was converted to nrrd format, and data were corrected for eddy current and movement distortions, using Brain Extraction Tool (BET) software (Smith et al., 2002). Tensors were estimated using the Least Squares method (Tristan-Vega, 2009) and the 3DSlicer (www.slicer.org) software version 3.6.4.
Fiber Tracking
In order to extract and measure the fornix, we used a method that is described in detail in our previously published study in chronic patients (Fitzsimmons et al., 2009). Briefly, measurements were obtained using 3Dslicer and diffusion tensor streamline tractography. In order to ensure precise segmentation of the structure, we used a multiple ROI method (e.g., Catani et al., 2002; Jones et al., 2005). Eight separate Regions of Interest (ROIs) were manually defined, by the same rater (JF), blind to diagnosis, in order to tract the desired fibers, as described in Fitzsimmons et al. (2009). Only those pathways passing through all 8 ROIs were retained for further analysis (see Figure 1).
Figure 1.
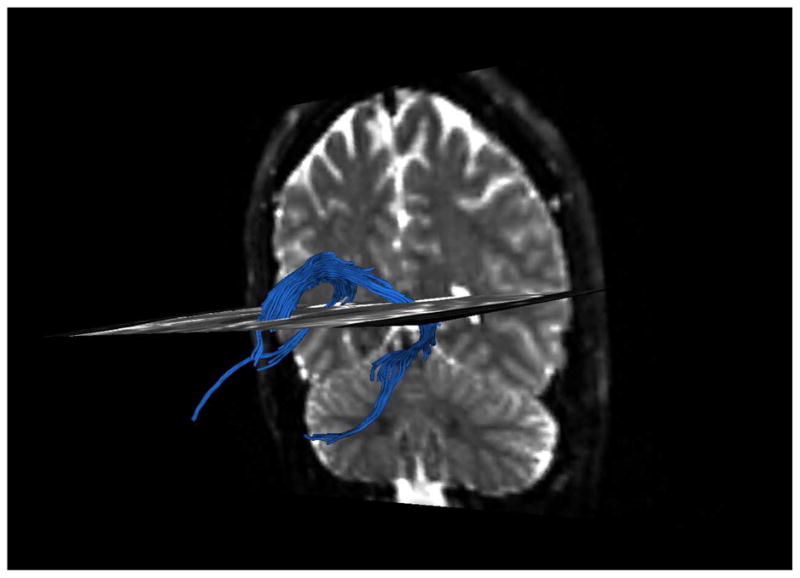
Tracts going through the ROIs.
After fornix tracts were extracted, the average values of FA, RD, Trace and AD were calculated for the entire tract and separately for each side, and subjected to statistical analysis. Inter-rater reliability was also calculated for 5 random cases, drawn by a second rater (TS), and achieved 0.9 for the right and 0.94 for the left fornix.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS V. 20). To test for group differences in FA, RD, Trace and AD within the Fornix, analysis of covariance (ANCOVA) was performed with group as a between-group factor and side as a within group factor, and gender as a regressor. Protected post-hoc independent sample t-tests were used to evaluate differences between groups. Pearson correlations were used to investigate the relationship between the BPRS scores and measures of white matter integrity.
RESULTS
There were no group differences in handedness (all right handed), age, parental social economical status or premorbid IQ (see Table 1). Patients mean duration of illness was 5.62±6.12 months with a mean age of onset of 21.13±4.31. Duration of medication was 9.16±7.71 months.
ANCOVA revealed a significant group effect for fornix with lower FA in FE patients relative to controls (F(1,41)=21; p=0.0001) (Fig 2), higher RD in FE patients relative to controls (F(1,41)=12,04; p=0.001) (Fig 3), and higher Trace in FE patients relative to controls (F(1,41)=8.28; p= 0.006) (Fig 4). There were no group effects for AD (F(1,41)=3.68; p=0.062) (Fig 5).
Fig. 2.
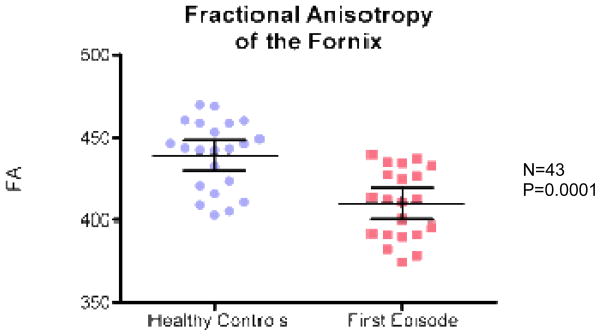
Fractional anisotropy (FA) for FES vs. healthy controls.
Fig. 3.
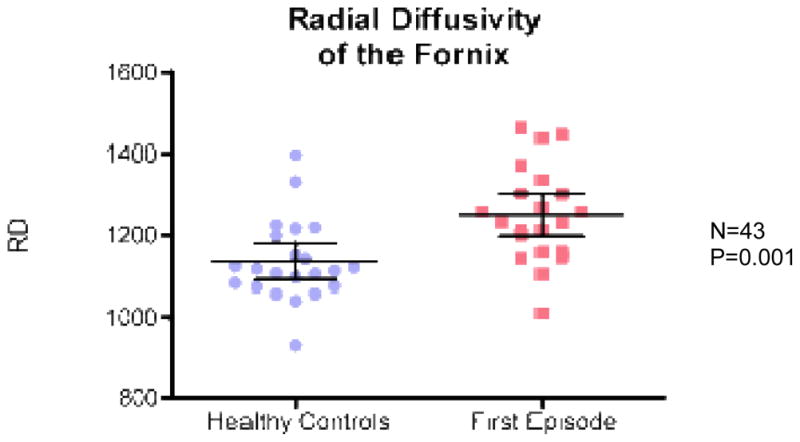
Radial diffusivity (RD) for FES vs. healthy controls.
Fig. 4.
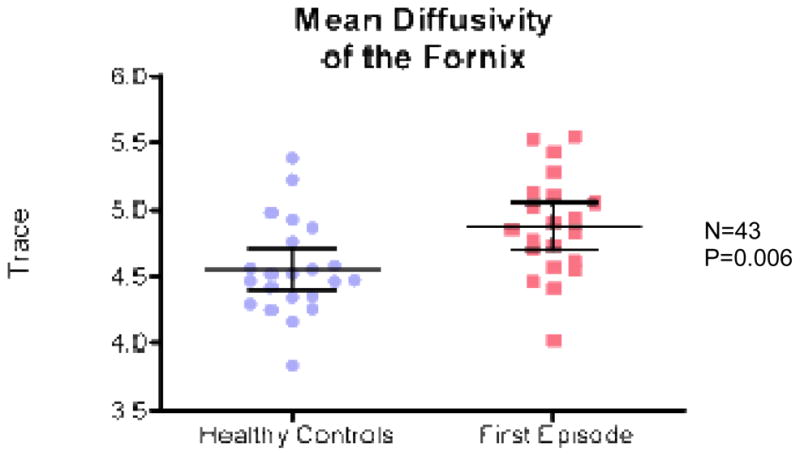
Trace for FES vs. healthy controls.
Fig. 5.
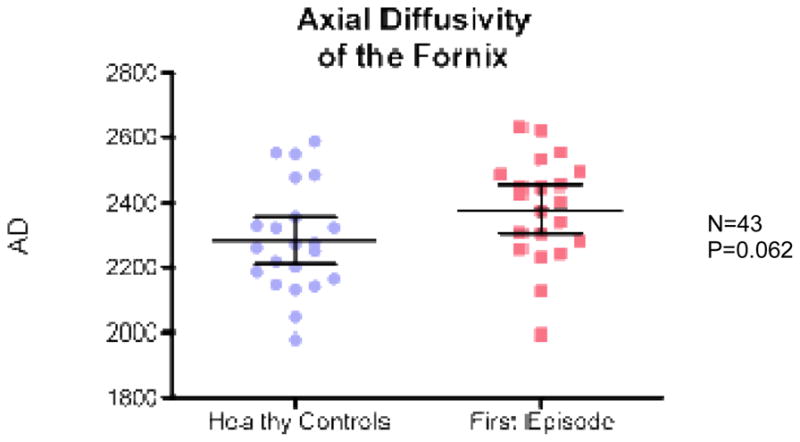
Axial diffusivity for FES vs. healthy controls.
There was no significant effect of side for FA (F(1,41)=0,42; p=0.52), RD (F(1,41)=3.62; p=0.064) or AD (F(1,41)=1.719, p=0.197) but Trace was lower on the left fornix versus right in FE patients (F(1,41)=4.54; p=0.039). There were, however, no group by side interactions for FA (F(1,41)=0.189; p=0.66; RD (F(1,41)=0.58; p=0.45), Trace (F(1,41)=0.115; p=0.292) or AD (F(1,41)=2.08; p=0.157).
There were also no statistically significant correlations between the DTI measures and clinical symptoms or sociodemographic variables.
When we use an ANCOVA design, we used chloropromazine equivalent as a covariate, and the results remained the same.
DISCUSSION
Results of our study are consistent with previous fornix studies in chronic patients that show FA alterations (e.g., Kuroki et al., 2006; Fitzsimmons et al., 2009; Abdul-Rahman et al. 2011). To our knowledge there are only three studies to date investigating the fornix in the early course of schizophrenia. Of these studies, Luck et al. (2010) and Davenport et al. (2010) found lower FA but did not include measures of RD, Trace or AD. Kendi et al. (2008), using FA as a dependent variable, did not report any abnormalities in FE schizophrenia but did report a decrease in fornix volume.
All previous fornix investigations in FE patients have used FA as the dependent imaging measure. FA has been reported to be sensitive to many micro-pathologies, including demyelination, axonal damage, changes in tract orientation, organization and axonal density, but it is not specific to any single one of them. Our study showed a decrease in FA and a concomitant increase in RD and Trace, but no change in AD. This particular pattern of diffusion changes has been previously reported in studies in shiverer mice (Song et al., 2005 and Stricker et al., 2009), and has been associated with myelin loss.
It is, however, notable that while group differences in AD were non-significant, they were at the trend level (p=0.062). This could be due to the fact that RD and AD are not completely independent measures, and thus myelin pathology affects diffusion in all directions (Von Hohenberg at al., 2013). It is also possible that the pathology underlying our results might not be limited to myelin, but may include axons and/or extracellular space.
In conclusion, our findings suggest early alterations of the fornix in first episode schizophrenia, possibly reflecting myelin alterations. Further studies are needed to follow patients from the prodromal period through to first episode to understand further the neurobiological underpinnings of white matter alterations in schizophrenia.
Acknowledgments
Role of Funding
This work was supported, in part, by a T32 Stuart T. Hauser Clinical Research Training Program in Biological and Social Psychiatry (JF), by NIH P50MH080272 (TS, LJS, JG, MK), R01MH050740 (TS, MK), and by a Clinical Translational Science Award UL1RR025758 to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources (LS).
None of the study sponsors had a role in the study design, in the data collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
We thank Joanna Daniluk for administrative support and Tali Swisher, B.A. and Doug Terry, B.A. for their support as research assistants.
Footnotes
Contributors
Jennifer Fitzsimmons, M.D., and Hesham Hamoda M.D., designed the study and wrote the protocol. Jennifer Fitzsimmons, M.D also wrote the first draft of the manuscript. Marek Kubicki, M.D, Ph.D. supervised the MRI data acquisition and processing, and provided guidance on technical aspects of diffusion tensor imaging. He also supervised the statistical analyses and edited multiple iterations of the manuscript. Larry J. Seidman, Raquelle I. Mesholam- Gately, Jill M. Goldstein and Joanne Wojcik, managed the recruitment and collected clinical information of participants. All authors contributed to and have approved the final manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Rahman MF, Qiu A, Sim K. Regionally specific white matter disruptions of fornix and cingulum in schizophrenia. PLoS One. 2011;6(4):e18652. doi: 10.1371/journal.pone.0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Tsuruda JS, Parker DL. Elimination of eddy current artifacts in diffusion-weighted echo-planar images: the use of bipolar gradients. Magn Reson Med. 1997;38(6):1016–1021. doi: 10.1002/mrm.1910380623. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance SA, Highley JR, Esiri MM, Crow TJ. Fiber content of the fornix in schizophrenia: lack of evidence for a primary limbic encephalopathy. Am J Psychiatry. 1999;156(11):1720–1724. doi: 10.1176/ajp.156.11.1720. [DOI] [PubMed] [Google Scholar]
- Clemm von Hohenberg C, Pasternak O, Kubicki M, Ballinger T. White Matter Microstructure in Individuals at Clinical High Risk of Psychosis: A Whole-Brain Diffusion Tensor Imaging Study. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010;181(3):193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DC, Wardell AM, Woolsey R, James AC. Enlargement of the fornix in early-onset schizophrenia: a quantitative MRI study. Neurosci Lett. 2001;301(3):163–166. doi: 10.1016/s0304-3940(01)01637-8. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Curr Opin Psychiatry. 2013;26(2):172–187. doi: 10.1097/YCO.0b013e32835d9e6a. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons J, Kubicki M, Smith K, Bushell G, Estepar RS, Westin CF, Nestor PG, Niznikiewicz MA, Kikinis R, McCarley RW, Shenton ME. Diffusion tractography of the fornix in schizophrenia. Schizophr Res. 2009;107(1):39–46. doi: 10.1016/j.schres.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11(5):520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Jones DK, Catani M, Pierpaoli C, Reeves SJ, Shergill SS, O’Sullivan M, Maguire P, Horsfield MA, Simmons A, Williams SC, Howard RJ. A diffusion tensor magnetic resonance imaging study of frontal cortex connections in very-late-onset schizophrenia-like psychosis. Am J Geriatr Psychiatry. 2005;13(12):1092–1099. doi: 10.1176/appi.ajgp.13.12.1092. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160(1):156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendi M, Kendi AT, Lehericy S, Ducros M, Lim KO, Ugurbil K, Schulz SC, White T. Structural and diffusion tensor imaging of the fornix in childhood- and adolescent-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2008;47(7):826–832. doi: 10.1097/CHI.Ob013e318172ef36. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N, Kubicki M, Nestor PG, Salisbury DF, Park HJ, Levitt JJ, Woolston S, Frumin M, Niznikiewicz M, Westin CF, Maier SE, McCarley RW, Shenton ME. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry. 2006;60(1):22–31. doi: 10.1016/j.biopsych.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto CN, Teh I, Lee TS, Sim K. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin Psychopharmacol Neurosci. 2012;10(1):13–24. doi: 10.9758/cpn.2012.10.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck D, Malla AK, Joober R, Lepage M. Disrupted integrity of the fornix in first-episode schizophrenia. Schizophr Res. 2010;119(1–3):61–64. doi: 10.1016/j.schres.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Kuroki N, Gurrera RJ, Niznikiewicz M, Shenton ME, McCarley RW. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res. 2007;155(1):21–28. doi: 10.1016/j.pscychresns.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr Psychiatry Rep. 2005;7(2):81–82. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White Matter Alterations in Early Stages of Schizophrenia: A Systematic Review of Diffusion Tensor Imaging Studies. J Neuroimaging. 2014;(2):101–10. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage. 2009;45(1):10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Huffaker SJ, Ryan M, Bahn S. Further evidence for altered myelin biosynthesis and glutamatergic dysfunction in schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):557–563. doi: 10.1017/S1461145706007334. [DOI] [PubMed] [Google Scholar]
- Tristan-Vega A, Westin CF, Aja-Fernandez Estimation of fiber orientation probability density functions in high angular resolution diffusion imaging. Neuroimage. 2009;47(2):638–50. doi: 10.1016/j.neuroimage.2009.04.049. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Vikhreva OV, Zimina IS, Kolomeets NS, Orlovskaya DD. The role of oligodendrocyte pathology in schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):537–545. doi: 10.1017/S1461145707007626. [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97(2–3):129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]


