Abstract
Angiotensin II-mediated vascular brain inflammation emerged as a novel pathophysiological mechanism in neurogenic hypertension. However, the precise underlying mechanisms and functional consequences in relation to blood brain barrier integrity and central angiotensin II actions mediating neurohumoral activation in hypertension are poorly understood. Here, we aimed to determine whether blood brain barrier permeability within critical hypothalamic and brainstem regions involved in neurohumoral regulation was altered during hypertension. Using digital imaging quantification following intravascularly injected fluorescent dyes and immunohistochemistry, we found increased blood brain barrier permeability, along with altered key blood brain barrier protein constituents, in spontaneously hypertensive rats within the hypothalamic paraventricular nucleus, the nucleus of the solitary tract, and the rostral ventrolateral medulla, all critical brain regions known to contribute to neurohumoral activation during hypertension. Blood brain barrier disruption, including increased permeability and down-regulation of constituent proteins, was prevented in spontaneously hypertensive rats treated with the AT1 receptor antagonist Losartan, but not with hydralazine, a direct vasodilator. Importantly, we found circulating angiotensin II to extravasate into these brain regions, co-localizing with neurons and microglial cells. Taken together, our studies reveal a novel angiotensin II-mediated feed-forward mechanism during hypertension, by which circulating angiotensin II evokes increased blood brain barrier permeability, facilitating in turn its access to critical brain regions known to participate in blood pressure regulation.
Keywords: Hypertension, Blood brain barrier, Angiotensin II, AT1 receptor, Hypothalamus, Brainstem
INTRODUCTION
Neurogenic hypertension is the most common form of essential hypertension, being a major risk factor for coronary heart disease, congestive heart failure and stroke. Sympathohumoral activation is commonly observed both in human patients and experimental animal models of neurogenic hypertension, including the spontaneous hypertensive rat (SHR), and is known to contribute to morbidity and mortality in this disease. Thus, elucidating its precise underlying mechanisms is of critical importance.
Proper blood pressure regulation by the brain is a highly complex process, which involves sensing of distinct peripheral sensory modalities, which after being integrated within selective neuronal networks, results in specific patterns of autonomic and neurosecretory outflows to peripheral targets, including the general circulation, the heart and kidneys, among others1.
Angiotensin II (AngII) is a key circulating peptide involved in blood pressure regulation. Acting primarily on AT1 receptors (AT1r) in the central nervous system (CNS), AngII triggers adaptive homeostatic responses including stimulation of sympathetic nerve activity, vasopressin release, and drinking behavior2,3. While under normal conditions AngII does not cross the blood brain barrier (BBB), the subfornical organ (SFO) and the area postrema, which reside outside the BBB, senses circulating AngII levels4. From the SFO, information is then conveyed to key autonomic/neurosecretory centers in the hypothalamus and brainstem, including the paraventricular nucleus of the hypothalamus (PVN), the rostral ventrolateral medulla (RVLM) and the nucleus of tract solitarii (NTS)4–6. Interestingly, some of these pathways utilize centrally produced AngII as a neurotransmitter7,8.
While elevated circulating AngII levels, resulting in over-activation of the SFO, PVN and RVLM, is a critical factor contributing to neurohumoral activation in neurogenic hypertension5,9, the precise underlying mechanisms remain elusive.
AngII-mediated vascular brain inflammation has recently emerged as a novel pathophysiological mechanism in neurogenic hypertension10,11. Importantly, the BBB, a physical/metabolic barrier between the CNS and the systemic circulation is disrupted in most inflammatory brain diseases, such as stroke and multiple sclerosis12,13. While recent studies have shown BBB disruption in hypertension14–18, the precise underlying mechanisms, as well as the consequences in relation to AngII actions within cardiovascular-related CNS pathways, remain unknown. Here, we provide evidence supporting a breakdown of the BBB permeability within critical CNS regions involved in cardiovascular control during hypertension. We show that this is mediated by an AngII-AT1r signaling pathway, resulting in increased permeability and access of circulating AngII to neurons and microglial cells within the same key brain regions normally “protected” from circulating AngII actions. Our studies reveal a novel mechanism in neurogenic hypertension, with significant implications to our understanding of how circulating AngII acts in the brain, particularly during hypertension.
METHODS
An expanded Methods section is available in the Online Supplement at http://hyper.ahajournals.org.
Animals and experimental groups
Rats used included spontaneously hypertensive (SHR), Wistar Kyoto (WKY), and Wistar rats either sham-operated or subjected to a partial occlusion of the left renal artery (renovascular hypertensive, RVH)19. Subgroups of SHRs were treated with losartan (20mg/kg/day, starting at 5 to 6-week-old, lasting for 7 weeks) or hydralazin (10mg/kg/day, starting at 8 to 9-week-old animals, lasting for 4 weeks) in drinking water20,21. Systolic blood pressure (BP) was measured using tail cuff method (coda 6; Kent Scientific Corporation, CT). All procedures were approved by the Georgia Regents University Institutional Animal Care and Use Committee Guidelines.
Intravascular injections of fluorescent dextrans and quantification of BBB permeability
Dextran-rhodamine 70-kDa (RHO70; 10mg/mL, 2.86µl/g/each; Sigma-aldrich, MO) and dextran-FITC 10-kDa (FITC10, 10mg/mL, 2.86µl/g/each; Sigma-aldrich, MO), or the fluorescently-labeled angiotensin II (AngIIfluo, 1µmol/L, 2.86µl/g/each; Anaspec, CA), were delivered intracarotidly, and allowed to circulate for 20 min. When indicated, hypertonic mannitol (1.4M, 2ml per 200–250g of animal; Sigma-aldrich, MO)22–24 was infused intracarotidly 5 min prior to the dextrans. Hypothalamic and brainstem sections (40µm) were counterstained (Toto 1:50000).
Immunofluorescence (Table.S1) was performed for cell-type identification, and examined by confocal microscopy (LSM510, Zeiss Microimaging)25. BBB permeability was assessed based on the detection of fluorescent-dye staining within brain microvasculature or parenchyma26. The extravasated FITC10 signal was isolated using a digital subtraction procedure, and the proportion of FITC10-stained pixels in the subtracted image was calculated and compared among experimental groups. A densitometry analysis was used to compare differences in BBB protein constituents or immunoreactivity between WKYs and SHRs and results were expressed as % area within the nucleus occupied by the detected immunoreactivity25.
Statistics
Data are presented as mean±SEM. Unpaired t-tests and one-way analyses of variance, followed by Bonferroni posthoc tests, were used as indicated (Graphpad prism). Values of P<0.05 were considered statistically significant.
RESULTS
Blood pressure measurements
Systolic BP was elevated in SHRs (208.3±4.6 mmHg; 12–13 week-old, n=18), compared to age-matched WKYs (120.3±0.8 mmHg; n=22; P<0.0001). SHRs treated with Losartan (SHR-los; 158.6±7.5 mmHg; n=9) or hydralazine (SHR-hyd; 161.9±6.5 mmHg; n=4) had a significant lower BP compared to control SHRs (P<0.001). Systolic BP in renovascular hypertensive rats (RVH) was also elevated compared to sham-operated rats (201.4±3.5 mmHg and 127.9±1.4 mmHg respectively, n=3 each, P<0.001).
Increased BBB permeability in hypertensive rats
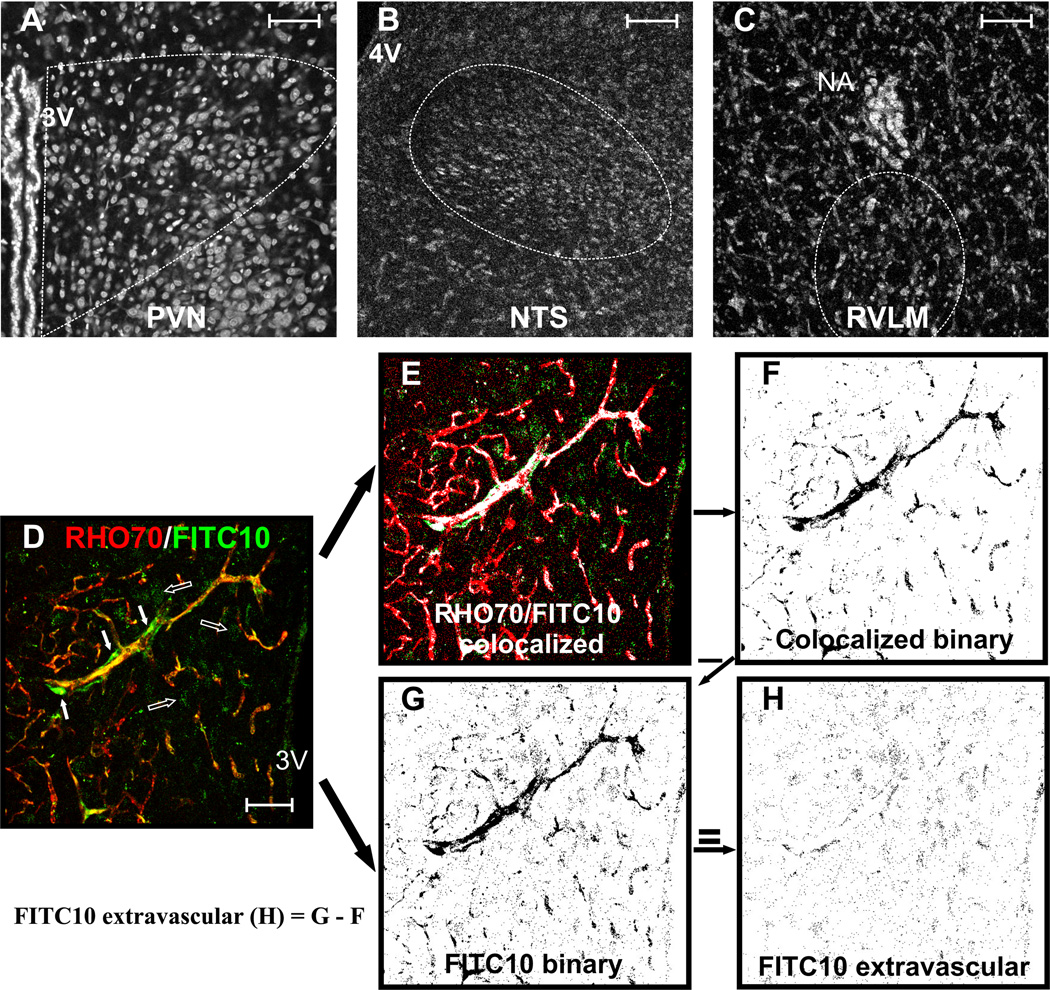
To evaluate altered BBB permeability, we developed an approach based on the simultaneous intracarotid injection of a large-size (RHO70) and a small-size (FITC10) fluorescent-dye, followed by a quantitative assessment of the degree of intravascular and extravasated dyes, within brain sections containing the PVN, NTS or RVLM (counterstained with TOTO, Fig. 1A–C). With an intact BBB, both dyes should be contained and colocalized within the brain vasculature, with minimal staining observed extravascularly. Conversely, with a partially disrupted BBB, the large RHO70 dye should still be contained intravascularly, whereas the smaller FITC10 dye should partially leak into the brain parenchyma. This is shown in the example of Fig.1D (from an SHR rat), in which RHO70 was observed within microvessels, whereas FITC10 was observed both within microvessels (co-localizing with RHO70, yellow staining in Fig.1D, and white in Fig.1E), but also within the brain parenchyma (empty arrows, Fig.1D). To detect and quantify the amount of leaked FITC10, we started with an image containing all the FITC10 signal (intravascular + extravascular, Fig.1G). To detect the FITC contained intravascularly, we used Image J software algorithms (NIH) that detected and isolated the FITC10 colocalized with RHO70 (representing then the intravascular FITC10, shown in the binary image in Fig.1F). Having these two images, we were able then to isolate the extravasated FITC10, by digitally subtracting the intravascular FITC10 image (Fig.1F) from the total FITC10 signal (Fig.1G), resulting in the image shown in Fig.1H, which was used for quantification. To quantify the degree of leaked FITC, the proportion of FITC10-positive pixels in Fig.1H was calculated, and compared among experimental groups.
Figure 1. BBB permeability quantification.
Samples of PVN (A), NTS (B), and RVLM (C) sections (TOTO counterstaining). D, RHO70 (red) and FITC10 (green) staining in the PVN of an SHR. For quantification, pixels containing both signals colocalized are isolated (E, white color) and processed as a binary image (F). Concurrently, a binary image containing only the FITC10 signal is obtained (G). To determine extravasation, image (F) is subtracted from image (G), resulting in a new image containing only the extravascular FITC10 signal (H) from which the density of extravasated FITC10 is calculated. Scale bar: 50µm. 3V, 4V: third and fourth ventricles; NA: nucleus ambiguous.
To validate our approach, we first compared in control rats differences in FITC10 extravasation among brain areas located within and outside the BBB. Whereas we found minimal FITC10 extravasation in the PVN and SON (areas with an intact BBB, Fig.S1-C, D), large amounts were found in the SFO and the area postrema (Fig.S1-A, B), which lack a complete BBB. Furthermore, intracarotid infusion of a hypertonic solution known to disrupt the BBB22–24, resulted in FITC10 extravasation in the PVN of control rats (8.1±0.6 % area, n=6), which appeared as punctate aggregates throughout the parenchyma (Figs.S1,S2 and Figs.2,3), as well as diffuse staining within perivascular cells surrounding capillaries (putative pericytes) (Fig.S2-B).
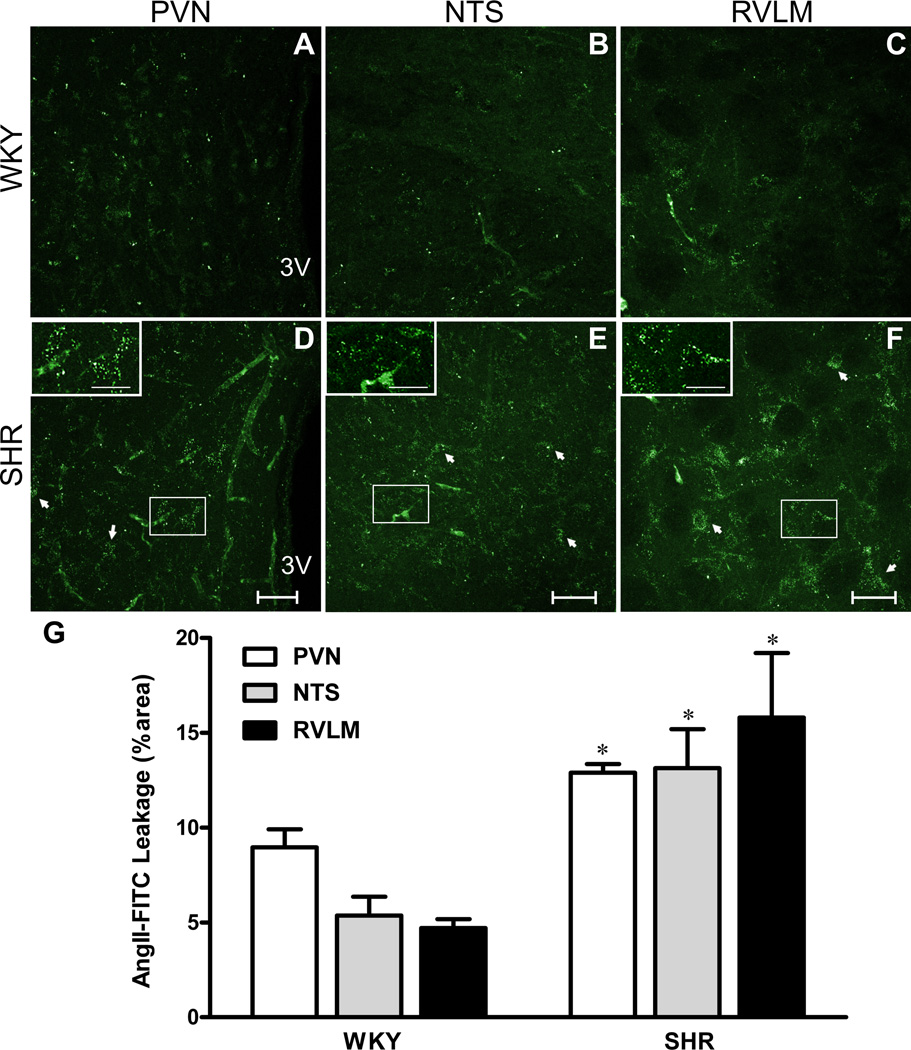
Figure 2. Increased BBB permeability in SHRs is prevented by an AT1 receptor blocker.
Staining for intravascularly-delivered RHO70 (red) and FITC10 (green) within the PVN, NTS and RVLM of WKY (A1,B1,C1) and SHRs (A2,B2,C2). The corresponding extravasated FITC10 are shown in respective panels 3 and 4. A5, B5 and C5: mean extravasated FITC10 (FITC10EV) in WKY, SHR, SHR treated with Losartan (SHR-los) and SHR treated with hydralazine (SHR-hyd). C6, high magnification image of the RVLM (SHR), showing intravascular FITC10 (white arrows) and extravasated FITC10 in neuronal-like profiles (red arrows).***P<0.001 vs WKY; †††P<0.001 vs SHR; n=8 SHR/WKY; n=4 SHR-los/SHR-Hyd. Scale bars: 50µm. 3V: third ventricle.
Figure 3. Circulating AngII leaks through the disrupted BBB in SHRs.
A–F, Intravascularly-delivered AngIIfluo within the PVN, NTS and RVLM of WKYs (A,B,C) and SHRs (D,E,F). Insets show respective squared areas at higher magnification. G, Mean extravasated AngIIfluo. *P<0.05 vs WKY, n=4/group. Scale bar: 50µm; insets: 25µm. 3V: third ventricle.
We found an increased extravasated FITC10 within the PVN (Fig.2A), NTS (Fig.2B) and RVLM (Fig.2C) in SHRs compared to WKYs (~85% increase, P<0.0001 in all cases, Fig.2). Conversely, the larger dye RHO70 remained intravascularly in both groups. Differently from these cardiovascular-related areas, no differences in extravasated FITC10 levels were observed in the basal ganglia, amygdala and somatosensory cortex of SHRs (Table.S2).
Similar to SHRs, we found an enhanced FITC10 leakage into the PVN, NTS and RVLM in RVH, when compared to aged-matched normotensive sham rats (Fig.S3).
Relative contribution of AngII and high blood pressure to altered BBB in SHRs
To gain insights into mechanisms underlying disrupted BBB during hypertension, we assessed the relative contribution of AT1r-mediated AngII signaling pathway, and the effect of high BP itself. We found that the disrupted BBB in SHRs was prevented by losartan treatment (SHR-los, P<0.0001, n=4, Fig.2A5, B5, C5). Conversely, SHRs treated with the vasodilator hydralazine (SHR-hyd) still displayed BBB leakage in the PVN and NTS (Fig.2A5, B5), whereas it was partially improved in the RVLM (P<0.05, Fig.2C5). Given that both treatments reduced BP to a similar extent (see above), these results suggest that an AngII-AT1r signaling pathway, but not high BP per se, contributed to altered BBB integrity in SHRs.
Circulating AngII leaks through the BBB in SHRs, gaining access to hypothalamic and brainstem neurons and microglial cells
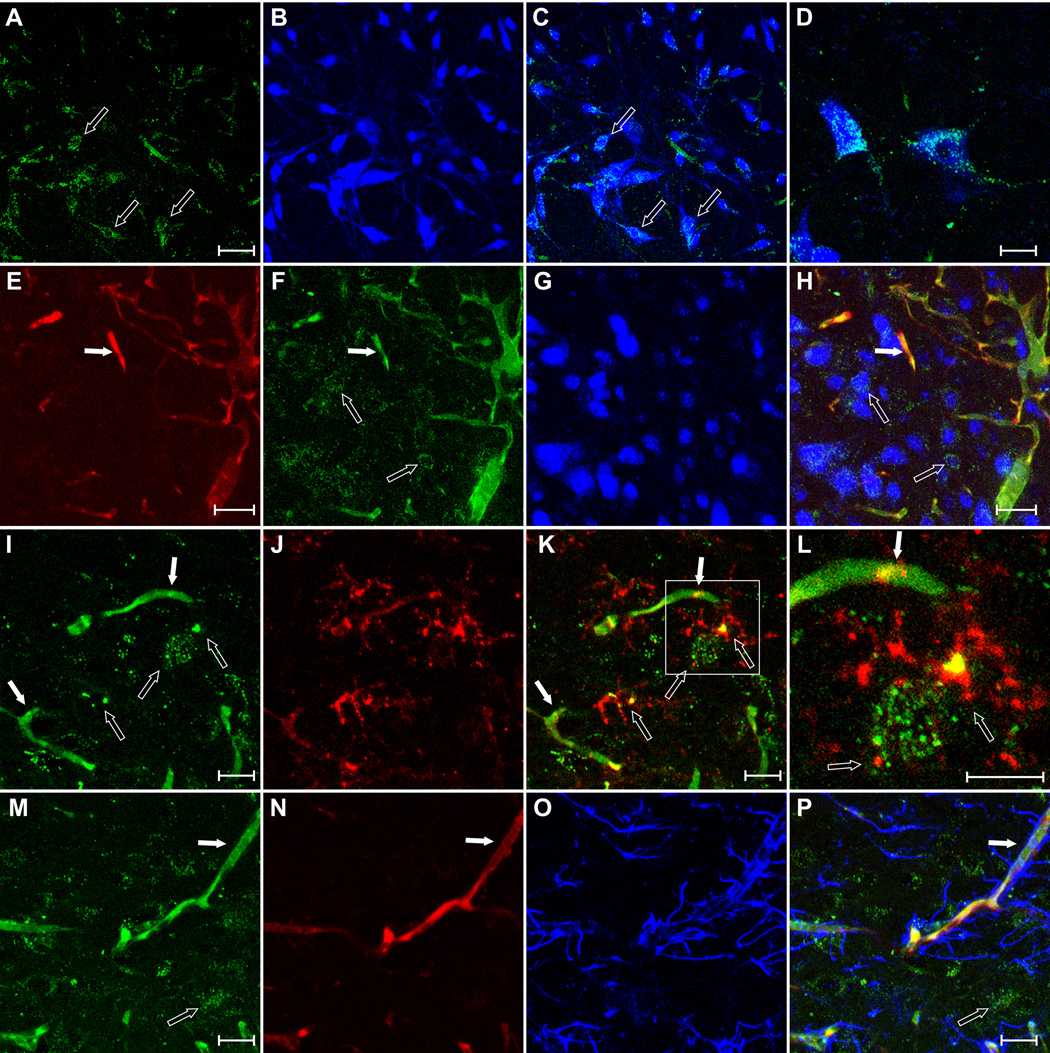
To determine if functionally relevant circulating factors could leak through the disrupted BBB in SHRs, we performed intravascular infusions of a fluorescently-labeled AngII (AngIIfluo; n=4). Similar to FITC10, we found higher levels of extravasated AngIIfluo in the PVN, NTS and RVLM of SHRs, compared to WKYs (P<0.05, Fig.3). We found the leaked AngIIfluo associated with neurons (NeuN immunostaining) and microglia (CD11b immunostaining), but not with astrocytes (S100β and GFAP immunostaining) (Fig.4 and Fig.S4).
Figure 4. Extravasated AngII in SHRs is localized in neurons and microglia.
A–D, intravascularly-delivered AngIIfluo (A) co-localized with the neuronal marker NeuN (B) (C, merged; D, higher magnification) within the RVLM. E–H, RHO70 (E), AngIIfluo (F) and NeuN (G) showing extravasation of the AngIIfluo but not RHO70 and its colocalization with neurons (H) within the PVN. I–L, Leaked AngIIfluo (I) co-localized with the microglial marker CD11b (J) (K, merged; L, higher magnification). M–P, Lack of co-localization between leaked AngIIfluo (M), RHO70 (N) and the astrocytic marker GFAP (O), merged in P. Solid and empty arrows: intravascular and leaked AngIIfluo. Scale bars: 50µm.
Blood Brain Barrier constituents are altered in SHRs
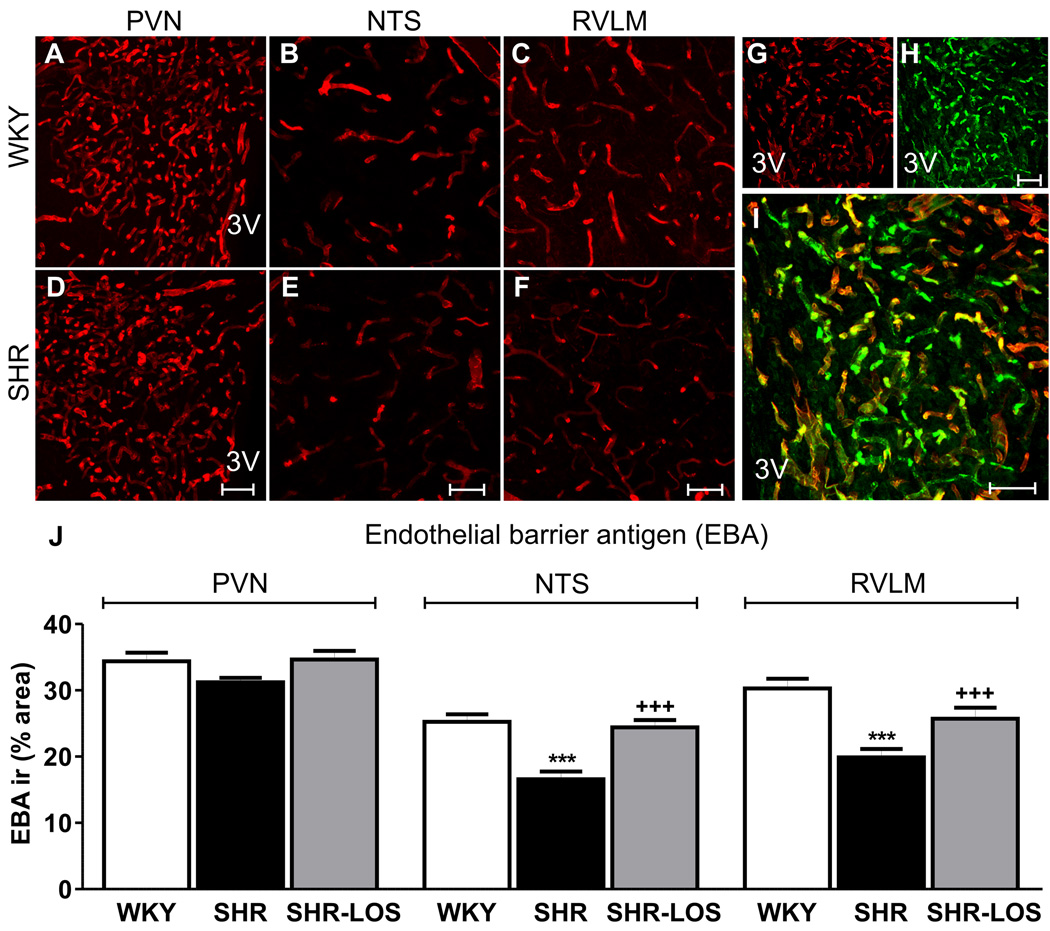
Finally, we assessed whether critical protein constituents of the BBB were altered in the brainstem and hypothalamic nuclei of SHRs. We found that the staining density for endothelial barrier antigen (EBA), a protein expressed in cerebral microvessels with intact BBB27,28, was diminished within the NTS and RVLM of SHRs, compared to WKYs and SHR-los rats P<0.05, n=5 each group, Fig.5). No differences were observed within the PVN (P=0.89). The staining density for transferrin receptor (TfR), also a vascular marker of intact BBB27,29, was also significantly decreased in SHRs compared to WKYs (n=5 in each group) (Fig.S5). Conversely, no differences in EBA and TfR staining intensity were observed (Fig.S6).
Figure 5. AngII-dependent diminished endothelial barrier antigen (EBA) expression in SHRs.
A–F, EBA staining within the PVN, NTS and RVLM of WKYs (A–C; n=5) and SHRs (D–E; n=5). G and H, EBA immunostainning (red) and isolectin IB4 (H) within the PVN of a WKYs. I, merged figure showing co-localization (yellow). J, mean EBA immunoreactivity density in WKY, SHR and SHR-LOS (n=3).***P<0.0001 vs. WKY and +++P<0.0001 vs. SHR. Scale bar: 50µm. 3V: third ventricle.
DISCUSSION
We tested here the hypothesis that disruption of the BBB, particularly in brain areas involved in cardiovascular control, occurs in chronically hypertensive rats. Our results show that in SHRs: 1) the BBB permeability was disrupted within the PVN, NTS and RVLM, major areas that contribute to sympathohumoral activation in SHRs1; 2) AT1r blockade (but not a peripheral vasodilator) prevented the altered BBB integrity; 3) the increased BBB permeability facilitated leakage of circulating AngII into these brain regions; 4) protein constituents of the BBB were down-regulated in an AT1r-dependent manner.
Increased BBB permeability in the PVN, NTS and RVLM during hypertension
Previous studies demonstrated disruption of the BBB in different experimental models of hypertension14–16. However, this is the first detailed study to show altered BBB integrity within hypothalamic and brainstem regions crucial for regulating BP and baroreceptor function, and to investigate underlying mechanisms. We found increased extravasation of a small-size fluorescent dye injected peripherally within the PVN, NTS and RVLM in SHRs, indicative of BBB disruption14. Similar results were observed in renovascular hypertensive rats, indicating that BBB integrity disruption is not a peculiarity of the SHRs. A methodological limitation to consider is that anesthesia could itself alter BBB permeability22. This may explain the presence of a low degree of dye extravasation in normotensive conditions. Nonetheless, given that all experimental groups were subjected to the same anesthetic protocol, this limitation did not prevent us from detecting differences among them. In addition, intracarotid infusions of dyes (particularly AngII in SHRs) may have resulted in further increases in blood pressure of sheer-stress, contributing to the BBB breakdown. We minimized this possibility by infusing small volumes at slow pace (Methods). Moreover, our results showing that the BBB breakdown was prevented by losartan but not hydralazine, despite similar intracarotid infusion protocols and basally lower blood pressure values than in control SHRs, would further argue against this possibility.
While previous studies showed disrupted cerebral vessel microanatomy in hypertensive rats30–32, this is the first study, to the best of our knowledge, to show altered expression of EBA and TfR, two protein markers of intact BBB27–29, during hypertension. Similar findings were previously reported in pathologies involving increased BBB permeability, including encephalopathies33 and sarin toxicity34.
Contribution of AT1r to BBB disruption in SHRs
Circulating AngII, acting on AT1r within brain areas residing outside the BBB, activate central pathways that contribute to enhanced sympathetic outflow, blunted baroreflex sensitivity, and secretion of vasopressin4,9,35,36. Moreover, AngII is a pro-inflammatory signal, acting both centrally and peripherally10,37–39. Since hypertension-associated sheer-stress may also contribute to vascular inflammation40, we assessed the relative contribution of AngII-AT1r signaling and high BP per se to altered BBB permeability in SHRs. We found that AT1r blockade (Losartan) prevented FITC10 leakage into the PVN, RVLM and NTS in SHRs. Conversely, a direct vasodilator that lowered BP in SHRs to a similar extent than losartan (though still significantly higher than WKYs), failed to prevent the BBB breakdown. These results support a major contribution of the AT1r signaling cascade to altered BBB permeability during hypertension. In the RVLM though, partial decrease of BP improved BBB permeability, though to a much less extent than Losartan. Still, whether complete normalization of BP with hydralazine would have fully restored BBB integrity, remains to be determined. The key contribution of AT1r to BBB disruption is consistent with recent studies showing that AngII AT1r modulated paracellular permeability in cultured BBB endothelial cells41, and that chronic AngII infusion lead to an AT1r-mediated increase in BBB permeability, measured in whole mouse brain homogenates16. Finally, it was recently reported that AT1r blockade prevented BBB disruption in the hippocampus of Dahl Salt-sensitive hypertensive rats17.
While several studies support an increased expression of AT1r both in hypothalamic and brainstem areas of SHRs42–45, the specific cell-type location, particularly those contributing to BBB disruption during hypertension, remains unknown. AT1r were reported in neurons, axonal terminals, microglial cells, astrocytes and endothelial cells46–49. Given that neither circulating AngII nor orally administered losartan cross the BBB under normal conditions50–52, it is reasonable to speculate that during the initial phase of the BBB disruption, AT1r located on endothelial cells outside of the BBB are implicated, initiating in turn a cascade of events resulting in early BBB disruption. This is in line with previous studies showing that circulating AngII, acting on endothelial AT1r located outside the BBB, can signal NTS neuronal networks across the BBB49,53, and that endothelial AT1r contribute to endothelial damage and increased endothelial permeability during hypertension47. Given that endothelial AT1r are likely present throughout the brain microvasculature46,47, the regional differences we found may reflect differences downstream to the AT1r themselves. Future studies using cell-type specific AT1r knockouts are warranted to better assess the contribution of cell-type and region-specific AT1r.
Circulating AngII gains access to the hypothalamus and brainstem in hypertensive rats
We found that a fluorescently-labeled form of AngII injected systemically leaked into the PVN, NTS and RVLM of SHRs, indicating that circulating AngII accesses brain areas that are normally excluded from its direct actions. We believe this novel finding has important conceptual and physiological implications. Firstly, given its lipophobic nature, circulating AngII actions on neurohumoral regulation are thought to be mediated via actions within circumventricular organs that reside outside the BBB, such as the SFO4. Within the SFO, circulating AngII stimulates efferent projections to neurosecretory and autonomic neurons in the PVN54,55, which via descending projections to the RVLM, spinal cord and the posterior pituitary, mediate the sympathoexcitatory and neurosecretory effects of circulating AngII55.
Most components of the renin-angiotensin system, including AngII and AT1r, are found in several CNS nuclei, including the PVN, NTS and RVLM36. When microinjected directly into the PVN or RVLM, AngII elicits pressor and sympathoexcitatory responses8,56. Moreover, AT1r blockade in these nuclei attenuated sympathoexcitatory drive in hypertensive rats57,58, whereas it increased baroreflex sensitivity in the NTS59, results that support an enhanced AngII-mediated action within these regions during hypertension. Our studies suggest that extravasation of AngII through the disrupted BBB during hypertension could be an important underlying mechanism leading to exacerbated AngII actions in these brain centers, contributing in turn to neurohumoral activation during hypertension.
In this sense, we found extravasated AngII to localize with neurons and microglial cells, supporting these as potential targets for the extravasated AngII. Since AngII stimulates the activity of PVN60,61, RVLM62 and NTS63 neurons, binding of circulating, extravasated AngII to these neurons may directly contribute to sympathetic outflow and elevated BP during hypertension. This is also supported by a recent study showing an AT1r-mediated activation of bulbospinal, tyrosine hydroxylase RVLM neurons following a hypertonic-induced disruption of the BBB24.
In addition to neurons, AngII can lead to microglia activation, which via release of pro-inflammatory cytokines, may further contribute to hypertension, as recently shown in the PVN10. We did not observe AngII staining in GFAP-stained astrocytes. However, since AT1r were reported in astrocytes49,53,64, the apparent lack of AngII staining in these cells may be due to the fact that GFAP in astrocytes is absent in their fine branch process, which account for the majority of the astrocyte surface65. Thus, it is possible that AngII may be present in astrocyte fine processes, which would be undetected with our approach. To what extent molecules other that AngII can leak through the disrupted BBB in hypertension is at present unknown. Based on leakage differences reported here between RHO70 and FITC10 dextrans, any molecule with a molecular weight less or equal to 10-kDa could in principle transverse the altered BBB in hypertensive rats. Important limitations to be considered in our studies include the possibility that the infusion of AngII itself, by acutely raising BP beyond baseline, could have contributed to the BBB disruption in SHRs. However, the fact that similar effects were observed with the FITC-dextrans would argue against this possibility. Finally, while AngII was given intracarotidly, resulting in the direct delivery of the peptide to the hypothalamus, given its relatively short half-life in the circulation, we cannot determine whether the full length, or a cleaved fragment of peptide, was detected in the brain parenchyma. This could be more likely the case for the brainstem, which is supplied by the vertebral arteries, requiring thus a passage of the peptide through the systemic circulation before reaching brainstem nuclei.
Perspectives
Disruption of the BBB is commonly observed in neuro-inflammatory neurological disorders, including stroke13 and multiple sclerosis12. Similarly, in addition to peripheral vasculature inflammation66, a growing body of evidence supports that a central inflammatory process takes place at early stages in hypertension, contributing in turn to the development/maintenance of neurogenic hypertension10,11. Within this frame, our studies support a novel deleterious AngII-mediated feed-forward mechanism during hypertension, by which AngII, via activation of AT1r, contributes to BBB disruption, enabling its own access to brain areas that are normally “protected” by the BBB, including the PVN, RVLM and NTS. These novel findings are expected to be clinically relevant. Firstly, they suggest that leakage of circulating AngII through the disrupted BBB may be an important pathophysiological mechanism contributing to neurohumoral activation during hypertension. Secondly, our findings add to the growing notion that the properties of the BBB can dynamically change, particularly during disease conditions such as hypertension. Consequently, our current views about sites and mechanisms of actions of both circulating factors and therapeutic agents, known to influence the circulation, need to be revisited to include the possibility of direct CNS actions due to compromised BBB integrity during disease states.
Supplementary Material
Novelty and Significance: 1) What Is New, 2) What Is Relevant?
1. What Is New?
AngII, but not high blood pressure per se, contributes to breakdown of the BBB within hypothalamic and brainstem nuclei during hypertension.
Protein constituents of the BBB within these nuclei are downregulated in an AT1 receptor-dependent manner.
The increased BBB permeability facilitated leakage of circulating AngII into hypothalamic and brainstem nuclei.
2- What Is Relevant?
BP regulation by the brain is strongly influenced by circulating AngII, but its actions are limited to regions located outside the BBB. Our findings showing BBB disruption, and AngII leakage into the brain during hypertension support a novel mechanism by which circulating AngII may contribute to neurohumoral activation in neurogenic hypertension.
3- Summary
Our studies reveal a novel AngII-mediated feed-forward mechanism during hypertension, by which elevated circulating AngII is associated with increased BBB permeability, facilitating its access to critical brain regions involved in the central control of cardiovascular function.
Acknowledgments
Sources of Funding
This work was supported National Heart, Lung, and Blood Institute Grants R01HL085767 to Stern JE and a R01HL089067 to Filosa JA.
Footnotes
Conflict of Interest/Disclosure
None.
REFERENCES
- 1.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 2.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by at1 and at2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl. 1996;3:S99–S104. [PubMed] [Google Scholar]
- 3.Zardetto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann N Y Acad Sci. 1993;689:161–176. doi: 10.1111/j.1749-6632.1993.tb55545.x. [DOI] [PubMed] [Google Scholar]
- 4.Smith PM, Ferguson AV. Circulating signals as critical regulators of autonomic state--central roles for the subfornical organ. Am J Physiol Regul Integr Comp Physiol. 2010;299:R405–R415. doi: 10.1152/ajpregu.00103.2010. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: Implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: Whole-cell properties. Brain Res. 2001;921:78–85. doi: 10.1016/s0006-8993(01)03093-1. [DOI] [PubMed] [Google Scholar]
- 7.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: Functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 8.Tagawa T, Dampney RA. At(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 9.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: Converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 10.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waki H, Gouraud SS, Maeda M, Raizada MK, Paton JF. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir Physiol Neurobiol. 2011;178:422–428. doi: 10.1016/j.resp.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Waubant E. Biomarkers indicative of blood-brain barrier disruption in multiple sclerosis. Dis Markers. 2006;22:235–244. doi: 10.1155/2006/709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Rosenberg GA. Mmp-mediated disruption of claudin-5 in the blood-brain barrier of rat brain after cerebral ischemia. Methods Mol Biol. 2011;762:333–345. doi: 10.1007/978-1-61779-185-7_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayhan WG, Faraci FM, Siems JL, Heistad DD. Role of molecular charge in disruption of the blood-brain barrier during acute hypertension. Circ Res. 1989;64:658–664. doi: 10.1161/01.res.64.4.658. [DOI] [PubMed] [Google Scholar]
- 15.Ueno M, Sakamoto H, Liao YJ, Onodera M, Huang CL, Miyanaka H, Nakagawa T. Blood-brain barrier disruption in the hypothalamus of young adult spontaneously hypertensive rats. Histochem Cell Biol. 2004;122:131–137. doi: 10.1007/s00418-004-0684-y. [DOI] [PubMed] [Google Scholar]
- 16.Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelisch N, Hosomi N, Ueno M, Nakano D, Hitomi H, Mogi M, Shimada K, Kobori H, Horiuchi M, Sakamoto H, Matsumoto M, Kohno M, Nishiyama A. Blockade of at1 receptors protects the blood-brain barrier and improves cognition in dahl salt-sensitive hypertensive rats. Am J Hypertens. 2011;24:362–368. doi: 10.1038/ajh.2010.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues SF, Granger DN. Cerebral microvascular inflammation in doca salt-induced hypertension: Role of angiotensin II and mitochondrial superoxide. J Cereb Blood Flow Metab. 2012;32:368–375. doi: 10.1038/jcbfm.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koprdova R, Cebova M, Kristek F. Long-term effect of losartan administration on blood pressure, heart and structure of coronary artery of young spontaneously hypertensive rats. Physiol Res. 2009;58:327–335. doi: 10.33549/physiolres.931528. [DOI] [PubMed] [Google Scholar]
- 21.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ros production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–H2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 22.Gumerlock MK, Neuwelt EA. The effects of anesthesia on osmotic blood-brain barrier disruption. Neurosurgery. 1990;26:268–277. doi: 10.1097/00006123-199002000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Marchi N, Betto G, Fazio V, Fan Q, Ghosh C, Machado A, Janigro D. Blood-brain barrier damage and brain penetration of antiepileptic drugs: Role of serum proteins and brain edema. Epilepsia. 2009;50:664–677. doi: 10.1111/j.1528-1167.2008.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao ST, May CN. Intra-carotid angiotensin II activates tyrosine hydroxylase-expressing rostral ventrolateral medulla neurons following blood-brain barrier disruption in rats. Neuroscience. 2013;245:148–156. doi: 10.1016/j.neuroscience.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol. 2010;518:567–585. doi: 10.1002/cne.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayhan WG. Leukocyte adherence contributes to disruption of the blood-brain barrier during activation of mast cells. Brain Res. 2000;869:112–120. doi: 10.1016/s0006-8993(00)02376-3. [DOI] [PubMed] [Google Scholar]
- 27.Norsted E, Gomuc B, Meister B. Protein components of the blood-brain barrier (bbb) in the mediobasal hypothalamus. J Chem Neuroanat. 2008;36:107–121. doi: 10.1016/j.jchemneu.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Krum JM. Effect of astroglial degeneration on neonatal blood-brain barrier marker expression. Exp Neurol. 1996;142:29–35. doi: 10.1006/exnr.1996.0176. [DOI] [PubMed] [Google Scholar]
- 29.Hofman P, Hoyng P, vanderWerf F, Vrensen GF, Schlingemann RO. Lack of blood-brain barrier properties in microvessels of the prelaminar optic nerve head. Invest Ophthalmol Vis Sci. 2001;42:895–901. [PubMed] [Google Scholar]
- 30.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 31.Cates MJ, Dickinson CJ, Hart EC, Paton JF. Neurogenic hypertension and elevated vertebrobasilar arterial resistance: Is there a causative link? Curr Hypertens Rep. 2012;14:261–269. doi: 10.1007/s11906-012-0267-6. [DOI] [PubMed] [Google Scholar]
- 32.Lin SZ, Sposito N, Pettersen S, Rybacki L, McKenna E, Pettigrew K, Fenstermacher J. Cerebral capillary bed structure of normotensive and chronically hypertensive rats. Microvasc Res. 1990;40:341–357. doi: 10.1016/0026-2862(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 33.Sternberger NH, Sternberger LA, Kies MW, Shear CR. Cell surface endothelial proteins altered in experimental allergic encephalomyelitis. J Neuroimmunol. 1989;21:241–248. doi: 10.1016/0165-5728(89)90180-x. [DOI] [PubMed] [Google Scholar]
- 34.Abdel-Rahman A, Shetty AK, Abou-Donia MB. Acute exposure to sarin increases blood brain barrier permeability and induces neuropathological changes in the rat brain: Dose-response relationships. Neuroscience. 2002;113:721–741. doi: 10.1016/s0306-4522(02)00176-8. [DOI] [PubMed] [Google Scholar]
- 35.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: Pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 36.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: Recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benicky J, Sanchez-Lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol. 2009;29:781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res. 2012;111:1190–1197. doi: 10.1161/CIRCRESAHA.112.277475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type nad(p)h oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 40.Helderman F, Segers D, de Crom R, Hierck BP, Poelmann RE, Evans PC, Krams R. Effect of shear stress on vascular inflammation and plaque development. Curr Opin Lipidol. 2007;18:527–533. doi: 10.1097/MOL.0b013e3282ef7716. [DOI] [PubMed] [Google Scholar]
- 41.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates bbb permeability via activation of the at(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal D, Welsch MA, Keller JN, Francis J. Chronic exercise modulates ras components and improves balance between pro- and anti-inflammatory cytokines in the brain of shr. Basic Res Cardiol. 2011;106:1069–1085. doi: 10.1007/s00395-011-0231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Raizada MK, Sumners C, Lu D. Angiotensin-II type-1 receptor messenger-rna levels in the brains of normotensive and spontaneously hypertensive rats. Journal of Neurochemistry. 1993;60:1949–1952. doi: 10.1111/j.1471-4159.1993.tb13426.x. [DOI] [PubMed] [Google Scholar]
- 45.Reja V, Goodchild AK, Phillips JK, Pilowsky PM. Upregulation of angiotensin at(1) receptor and intracellular kinase gene expression in hypertensive rats. Clinical and Experimental Pharmacology and Physiology. 2006;33:690–695. doi: 10.1111/j.1440-1681.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Hara Y, Anrather J, Speth RC, Iadecola C, Pickel VM. Angiotensin II subtype 1a (at1a) receptors in the rat sensory vagal complex: Subcellular localization and association with endogenous angiotensin. Neuroscience. 2003;122:21–36. doi: 10.1016/s0306-4522(03)00606-7. [DOI] [PubMed] [Google Scholar]
- 47.Ito H, Takemori K, Suzuki T. Role of angiotensin II type 1 receptor in the leucocytes and endothelial cells of brain microvessels in the pathogenesis of hypertensive cerebral injury. J Hypertens. 2001;19:591–597. doi: 10.1097/00004872-200103001-00011. [DOI] [PubMed] [Google Scholar]
- 48.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 49.Paton JF, Lonergan T, Deuchars J, James PE, Kasparov S. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (epr) spectroscopy. Auton Neurosci. 2006:126–127. 193–201. doi: 10.1016/j.autneu.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 50.Bui JD, Kimura B, Phillips MI. Losartan potassium, a nonpeptide antagonist of angiotensin II, chronically administered p.O. Does not readily cross the blood-brain barrier. Eur J Pharmacol. 1992;219:147–151. doi: 10.1016/0014-2999(92)90593-s. [DOI] [PubMed] [Google Scholar]
- 51.Culman J, von Heyer C, Piepenburg B, Rascher W, Unger T. Effects of systemic treatment with irbesartan and losartan on central responses to angiotensin II in conscious, normotensive rats. Eur J Pharmacol. 1999;367:255–265. doi: 10.1016/s0014-2999(98)00983-2. [DOI] [PubMed] [Google Scholar]
- 52.Wang JM, Tan J, Leenen FH. Central nervous system blockade by peripheral administration of at1 receptor blockers. J Cardiovasc Pharmacol. 2003;41:593–599. doi: 10.1097/00005344-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: Novel implications for neurogenic hypertension. J Mol Med (Berl) 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 54.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: Critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- 56.Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ang II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol. 2002;282:H2039–H2045. doi: 10.1152/ajpheart.00854.2001. [DOI] [PubMed] [Google Scholar]
- 57.Chen AD, Zhang SJ, Yuan N, Xu Y, De W, Gao XY, Zhu GQ. Angiotensin at1 receptors in paraventricular nucleus contribute to sympathetic activation and enhanced cardiac sympathetic afferent reflex in renovascular hypertensive rats. Exp Physiol. 2011;96:94–103. doi: 10.1113/expphysiol.2010.054353. [DOI] [PubMed] [Google Scholar]
- 58.Ito S, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla at1 receptors support blood pressure in hypertensive rats. Hypertension. 2002;40:552–559. doi: 10.1161/01.hyp.0000033812.99089.92. [DOI] [PubMed] [Google Scholar]
- 59.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at at1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol. 1998;275:R1611–R1619. doi: 10.1152/ajpregu.1998.275.5.R1611. [DOI] [PubMed] [Google Scholar]
- 60.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: An in vitro patch-clamp study in brain slices. J Neurophysiol. 2005;93:403–413. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latchford KJ, Ferguson AV. Ang II-induced excitation of paraventricular nucleus magnocellular neurons: A role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol. 2004;286:R894–R902. doi: 10.1152/ajpregu.00603.2003. [DOI] [PubMed] [Google Scholar]
- 62.Li YW, Guyenet PG. Neuronal excitation by angiotensin II in the rostral ventrolateral medulla of the rat in vitro. Am J Physiol. 1995;268:R272–R277. doi: 10.1152/ajpregu.1995.268.1.R272. [DOI] [PubMed] [Google Scholar]
- 63.Barnes KL, DeWeese DM, Andresen MC. Angiotensin potentiates excitatory sensory synaptic transmission to medial solitary tract nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1340–R1353. doi: 10.1152/ajpregu.00505.2002. [DOI] [PubMed] [Google Scholar]
- 64.Fuchtbauer L, Groth-Rasmussen M, Holm TH, Lobner M, Toft-Hansen H, Khorooshi R, Owens T. Angiotensin II type 1 receptor (at1) signaling in astrocytes regulates synaptic degeneration-induced leukocyte entry to the central nervous system. Brain Behav Immun. 2011;25:897–904. doi: 10.1016/j.bbi.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.