Abstract
Background
This study sought to examine the utility of hair testing as a research measure of drug use among individuals with moderate-risk drug use based on the internationally-validated Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST).
Methods
This study is a secondary analysis using baseline data from a randomized trial of brief intervention for drug misuse, in which 360 adults with moderate-risk drug use were recruited from two community clinics in New Mexico, USA. The current study compared self-reported drug use on the ASSIST with laboratory analysis of hair samples using a standard commercially-available 5-panel test with assay screening and gas chromatography/mass spectrometry (GC/MS) confirmation. Both self-report and hair testing covered a 3 month period.
Results
Overall concordance between hair testing and self-report was 57.5% (marijuana), 86.5% (cocaine), 85.8% (amphetamines), and 74.3% (opioids). Specificity of hair testing at standard laboratory cut-offs exceeded 90% for all drugs, but sensitivity of hair testing relative to self-report was low, identifying only 52.3% (127/243) of self-disclosed marijuana users, 65.2% (30/46) of cocaine users, 24.2% (8/33) of amphetamine users, and 2.9% (2/68) of opioid users. Among participants who disclosed using marijuana or cocaine in the past 3 months, participants with a negative hair test tended to report lower-frequency use of those drugs (p< .001 for marijuana and cocaine).
Conclusions
Hair testing can be useful in studies with moderate-risk drug users, but the potential for under-identification of low-frequency use suggests that researchers should consider employing low detection cut-offs and using hair testing in conjunction with self-report.
Keywords: Hair testing, self-report, moderate-risk drug use, brief intervention, primary care
1. INTRODUCTION
Substance abuse treatment in the United States and many other countries is often delivered in a specialty sector, with programs serving patients whose problems have reached a critical threshold of severity. However, the last decade has seen growing integration of substance use services within the larger US healthcare system, with a corresponding shift towards addressing a wider spectrum of substance use problems to intervene before the onset of severe disorders. The screening, brief intervention, and referral to treatment (SBIRT) model promoted by the US federal government has broadened the provision of substance use services to individuals receiving care in mainstream medical settings such as hospitals, emergency departments, and primary care (Madras et al., 2009). Prioritization of behavioral health services within the context of healthcare reform is further expected to broaden eligibility for substance misuse services and encourage their delivery in outpatient and primary care venues (Buck, 2011; Mechanic, 2012). The World Health Organization likewise supports the integration of substance misuse services into primary care, and a multinational trial found that brief intervention led to reductions in illicit drug use risks (Humeniuk et al., 2012).
Within primary care settings, many patients who report illicit drug use may have risky but irregular use patterns, and may not require nor accept specialized drug abuse treatment. Individuals with drug use patterns that place them at a moderate level of risk can be very different from individuals in specialized drug abuse treatment settings, and pose unique challenges for research. Clinical trials of drug abuse interventions often gauge changes in drug consumption using self-report, and rigorous studies often include a biological measure. Use of self-report in addition to toxicology testing has been recommended (Donovan et al., 2012). Urine testing is the most common form of biological testing in drug abuse studies, due to its low cost and widespread clinical use in treatment (Moeller et al., 2008). Although urine testing provides a valuable measure of drug use among patients who use drugs regularly, it has limited utility for those exhibiting more moderate use patterns because of its short detection window (less than a few days for most drugs).
Hair testing is a promising alternative to urine testing, and has found use in a range of clinical, workplace drug testing, and forensic toxicology applications (Curtis and Greenberg, 2008; Klein et al., 2000). Although not without limitations (e.g., variable hair availability/length; participant concerns about cosmetic visibility of sample collection; and higher relative cost), hair testing has several properties that make it potentially well-suited for moderate-risk populations. It has an extended detection window of approximately 1 month per half inch of hair. Thus, a 1.5 inch section of hair captures a 90-day window of drug use. This detection window makes hair testing particularly attractive for studies with individuals whose intermittent and lower frequency drug use patterns resist detection by urine testing. Specimen collection is straightforward, does not pose a biohazard risk or require special storage to avoid spoilage, and is less intrusive than observed urine specimen collection. Given these advantages, it is no surprise that some clinical trials of brief intervention for drug use have begun to use hair testing as an outcome measure (Bernstein et al., 2005; Ondersma et al., 2014; Schwartz et al., in press).
Previous research comparing hair testing to self-report has documented substantial under-reporting of drug use in both youth and adults (Delaney-Black et al., 2010; Fendrich et al., 1999; Grekin et al., 2010; Magura and Kang, 1996). A large epidemiological study with middle-aged men found that hair testing identified more cocaine users, but fewer marijuana users, compared to self-report (Ledgerwood et al., 2008). Other studies have examined the validity of hair testing in controlled settings. For example, a study with ten volunteers in a secure research ward found that concentration of cocaine and its metabolites in hair was correlated with dose level, but affected by melanin content (Scheidweiler et al., 2005). A controlled methamphetamine administration study found good evidence of dose-related detection levels for hair, but noted substantial inter-individual differences (Polettini et al., 2012). Another study with 9 methamphetamine-dependent volunteers concluded that concentrations in hair generally reflect self-reported patterns of usage well, although the authors cautioned against extrapolating findings to light or occasional methamphetamine users (Han et al., 2011). A study with marijuana users found that only 7 of 13 participants who smoked cannabis in a controlled administration setting had a positive hair test (Huestis et al., 2007). Few studies, however, have examined hair testing among out-of-treatment individuals who access the broader healthcare system. A notable exception is a series of studies that examined patterns and predictors of non-disclosure of cocaine use among individuals who disclosed heroin use during an outpatient medical visit (Tassiopoulos et al., 2004, 2006).
The current study extends the literature on hair testing and self-reported drug use by examining their agreement in a sample of adult primary care patients who reported moderate-risk drug use on an internationally-validated screening instrument. The overarching aim of the study is to examine the utility of hair testing as a research measure in this population. Individuals who use drugs at a moderate-risk level have distinct service needs from those with severe substance use problems, and are poised to receive increased attention from clinical researchers given the emphasis on behavioral health integration and adoption of brief intervention services across healthcare settings. Researchers designing clinical services studies are faced with a number of commercially-available options for biological detection of drug use. Potentially important differences can exist in sample processing, analytical procedures, and coverage of different substances between laboratories, and even within the same laboratory across different testing products. In the current study, we examined a standard, commercially-available 5-panel hair test.
2. METHODS
2.1 Parent Study
This study is a secondary analysis using baseline (pre-randomization) data collected for a clinical trial comparing computerized vs. in-person brief intervention for risky drug use. The study was approved by the Institutional Review Boards of Friends Research Institute and Christus Health. All participants provided written informed consent. Additional details about the parent study have been described elsewhere (Schwartz et al., 2014).
2.2 Setting
The study was conducted at two rural health centers in New Mexico, USA.
2.3 Screening and Enrollment
Research assistants approached patients in the clinic waiting rooms and invited them to be screened for a health study. Patients were screened using the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST), an instrument developed and widely disseminated by the World Health Organization. The ASSIST can be used to triage patients into low-, moderate-, and high-risk categories for tobacco, alcohol, cannabis, cocaine, amphetamine-type stimulants, inhalants, sedatives, hallucinogens, and opioids (Humeniuk et al., 2008; Newcombe et al., 2005).
Inclusion criteria were age 18 or older and illicit drug use (including non-medical use of prescription drugs) at a “moderate-risk” level as defined by the ASSIST (i.e., a score of 4-26 for any substance other than tobacco or alcohol). Exclusion criteria were: “high risk” ASSIST score for alcohol or any drug other than tobacco, drug abstinence in the past 3 months, enrollment in substance abuse treatment within the past year, recent receipt of a brief intervention for drug use at the clinic, and plans to move out of state within a year. Among patients eligible for the parent study, 25% declined to participate. Three hundred sixty participants were enrolled, one of whom was subsequently withdrawn due to current enrollment in substance abuse treatment (an exclusion criterion).
Several steps were taken to improve accuracy of self-report. The screening interview with the ASSIST was conducted anonymously, without recording names or identifying information. Screening information was linked with study data only after determining eligibility and obtaining written informed consent. Confidentiality protections were emphasized during the screening introduction, and participants were assured that their responses would not be shared with clinic staff or become part of their medical record.
2.4 Participants
The parent study included 359 participants with moderate-risk drug use, of whom 46% were female, 47% were Hispanic ethnicity, and 90% were White. The mean age was 36.1 years (SD=14.6).
2.5 Measures
2.5.1 ASSIST
The ASSIST was administered during eligibility screening as described above. The ASSIST has established validity for identifying substance use risks (Humeniuk et al., 2008; Newcombe et al., 2005). Past 3 month frequency of use is gauged for each substance, on a response scale of Never, Once or Twice, Monthly, Weekly, and Daily or Almost Daily. Other items tap indicators of problem use (e.g., failed attempts to quit/cut down).
2.5.2 Hair Testing
Hair samples were collected using laboratory-recommended procedures, whereby samples were measured to 1.5 inches from the scalp, corresponding to the 3 month time frame of self-report on the ASSIST. (Participants with insufficient head hair were asked to provide body hair). Hair samples were sent to a commercial laboratory (Confirm Biosciences/Omega Laboratories, Mogadore, OH) and analyzed for presence and quantity of marijuana, cocaine, amphetamines, and opioids (and phencyclidine, for which there were no positives). Although it did not test for all possible drugs, the standard 5-panel test was selected for the parent study because it was readily commercially-available and was thought to cover the most common drugs encountered in primary care. The laboratory analyzed samples using assay screening (Pujol et al., 2007) with confirmation of positives by gas chromatography/mass spectrometry (GC/MS), considered the criterion standard for biochemical verification of substance use (Moeller et al., 2008). To prevent risk of external contamination, hair samples were subjected to a standard wash procedure prior to GC/MS confirmation, and GC/MS was conducted for metabolites of some drugs (marijuana, cocaine, heroin). Screening cut-off levels followed the laboratory’s standard practices for the 5-panel test: 1 pg/mg for marijuana, 500 pg/mg for cocaine and amphetamines, and 300 pg/mg for opioids; GC/MS confirmation cut-offs were: 0.30 pg/mg for carboxy-tetrahydrocannabinol (THC) metabolite, 500 pg/mg for cocaine and 50 pg/mg for cocaine metabolites (benzoylecgonine; norcocaine), 500 pg/mg for amphetamines, methamphetamines, and 3,4-methylenedioxy-N-methylamphetamine (MDMA/Ecstasy), and 300 pg/mg for morphine, codeine, and heroin metabolite 6-monoacetylmorphine. Thus, the test only covered morphine, codeine, and heroin. A test covering other opioids (e.g., oxycodone, methadone, etc.) was not offered at the time the study was planned.
2.6 Analysis Sample
Three hundred thirty six hair specimens from 359 participants in the parent study underwent laboratory processing. Reasons for not obtaining hair samples included participant refusal and insufficient hair quantity. Forty participants who provided body hair were excluded from the analysis due to potentially different growth rates for body vs. scalp hair. Of the 296 scalp hair samples processed by the laboratory, depending on the drug, between 4.7% and 7.1% of the samples could not be analyzed due to insufficient quantity, leaving n=275 samples for marijuana, n=282 samples for cocaine and amphetamines, and n=280 samples for opioids.
2.7 Statistical Analysis
Overall agreement between hair test results and self-reported use of marijuana, cocaine, amphetamines, and opioids was characterized by percent concordance and Kappa (κ) agreement statistics. Sensitivity, specificity, and area under the curve (AUC) were calculated comparing self-report against hair test results, and hair test results against self-report. Participants who denied drug use but had a positive hair test were compared to their concordant counterparts on ethnicity, race, and gender using likelihood-ratio χ2 tests, and on age using independent t-tests. Similar comparisons were examined for participants who reported using a particular drug but had a negative hair test. Among participants who disclosed drug use, differences in self-reported frequency of use were examined based on hair test results, using Mann-Whitney U tests due to the ordinal nature of self-reported frequency of use (i.e., once or twice, monthly, weekly, daily or almost daily). Correlations between quantity of drug in hair and self-reported use frequency for marijuana and cocaine (the drugs with the highest prevalence in the sample by hair test) were characterized using Spearman’s rho (ρ).
3. RESULTS
3.1 Drug Use by Self-Report and Hair Testing
Table 1 shows a detailed summary of drug use by self-report and hair testing. Marijuana had the highest prevalence by either self-report or hair testing. Cocaine was the only drug where more participants were identified as positive by hair test than by self-report. Overall concordance was 57.5% for marijuana, 86.5% for cocaine, 85.8% for amphetamines, and 74.3% for opioids, while κ values for these drugs were .19, .53, .21, and <.01, respectively.
Table 1.
Comparison of drug use by self-report and hair test.
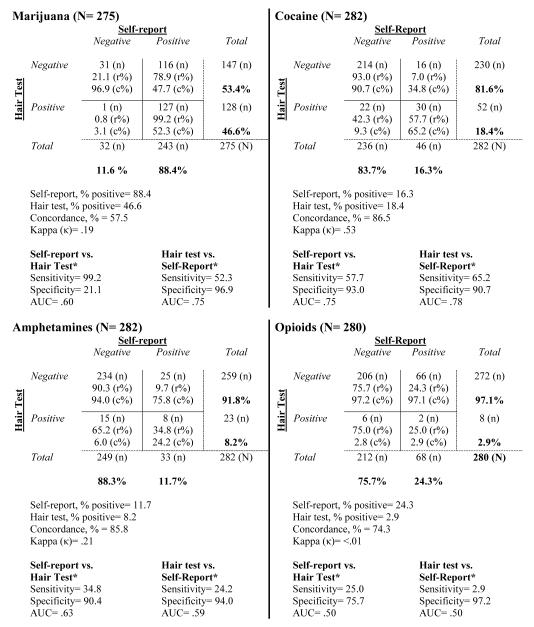
|
Notes: r%= row %; c%= column %. Concordance represents positive-positive + negative-negative matches/total cases. Amphetamines included methamphetamine, amphetamine, and MDMA. Sample for each drug is restricted to those with non-missing hair test data.
The hair test identified only 52.3% (127/243) of participants who reported marijuana use in the past 3 months (self-report +, hair +). In contrast, only 1/32 who denied marijuana use (3.1%) had a marijuana-positive hair test (self-report −, hair +). Most participants with a negative hair test for marijuana actually reported using it in the past 3 months (78.9%; 116/147; hair−, self-report +). For drugs other than marijuana, most participants reported no past 3 month use, and this was confirmed by hair testing in most cases.
Among participants who reported past 3 month cocaine use, 65.2% (30/46) had a cocaine-positive hair test, while 9.3% (22/236) of those who denied cocaine use had a cocaine-positive hair test (self-report −, hair +). Among participants with a cocaine-negative hair test, 7.0% (16/230) reported past 3 month cocaine use (hair−, self-report +).
For amphetamines, only 24.2% (8/33) of participants who reported use had a positive hair test (self-report +, hair +). Among participants who denied amphetamine use, 6.0% (15/249) had an amphetamine-positive hair test (self-report −, hair +). Among participants with an amphetamine-negative hair test, 9.7% (25/259) disclosed using amphetamine-type stimulants (hair −, self-report +).
Only 2.9% (2/68) of participants who reported opioid use had an opioid-positive hair test (self-report +, hair +), while 2.8% (6/212) of those who denied opioid use had a positive hair test (self-report −, hair +). Approximately one quarter (24.3%; 66/272) of participants with a negative hair test reported non-medical opioid use (hair −, self-report +).
There was considerable variability in sensitivity and specificity across drugs, and depending on whether the hair test or self-report was considered the criterion standard. Assuming the hair test as the criterion standard, sensitivity of self-report was excellent for marijuana (99.2%), and poor for cocaine, amphetamines, and opiates (57.7%, 34.8%, and 25.0%, respectively), reflecting in part the high prevalence of self-reported marijuana use in the sample. If self-report is considered the criterion standard, hair testing yielded poor sensitivity, ranging from a high of 65.2% (cocaine) to a low of 2.9% (opiates). Specificity of hair testing against self-report exceed 90% for all drugs.
3.2 Characteristics of Participants with Discordant Self-Report and Hair Tests
3.2.1 Self-Report −, Hair + vs. Concordant
Cocaine non-disclosers were more likely to be Hispanic than the cocaine-concordant (81.8% vs. 41.8%; p< .001). No other significant differences by race, ethnicity, gender, or age were identified between non-disclosers and the concordant.
3.2.2 Self-Report +, Hair Test – vs. Concordant
There were no significant differences in under-identification via hair testing by race or ethnicity. Compared to marijuana-concordant participants, participants who disclosed marijuana use but tested negative were more likely to be female (63.8% vs. 44.9%; p= .002). Participants who reported using amphetamine-type stimulants but had a negative hair test tended to be younger than amphetamine-concordant participants (mean age= 28.0 vs. 35.9 years; p= .01), a pattern also evident for opioids (mean age= 30.4 vs. 37.4 years; p< .001).
3.3 Self-Reported Frequency of Use by Hair Test Results
Among participants who reported using a particular drug, participants with a negative hair test tended to report less frequent use than participants with a positive hair test (p< .001 for marijuana and cocaine). These differences were non-significant for amphetamines (p= .17) and opioids (p= .07), however, cell sizes were very small. All 16 participants who reported cocaine use but tested negative reported using it just once or twice (n=14) or monthly (n=2) in the past 3 months. For amphetamines, 22 of 25 who reported use but tested negative reported using once or twice (n=19) or monthly (n=3) over the past 3 months. For opioids, 52 of 66 participants who reported use but tested negative reported using once or twice (n=30) or on a monthly basis (n=12) in the past 3 months.
3.4 Quantity of Marijuana and Cocaine in Hair
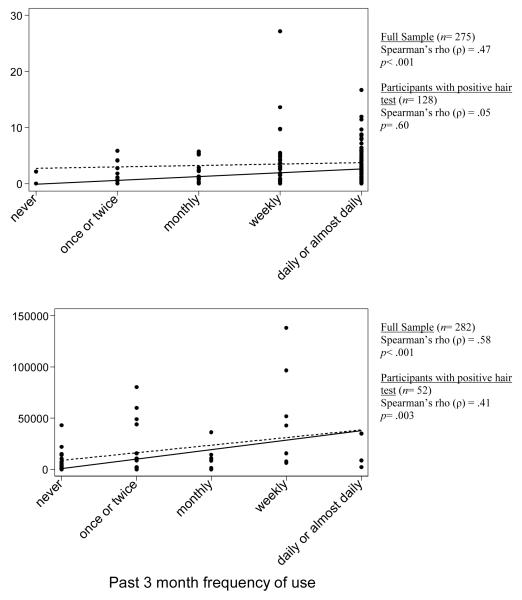
The mean level of THC metabolite in hair increased with each category of self-reported frequency of use in a clear linear progression. The mean concentration of cocaine was highest for the “weekly” category (>50,000 pg/mg on average), which was driven by several outliers. Figure 1 shows scatterplots of levels of drug in hair for marijuana and cocaine. Linear fitted lines are shown for the full sample (solid line) and the subsample of participants with a positive hair test (dashed line). There was a significant correlation between concentration of drug in hair and self-reported frequency of use for marijuana (Spearman’s ρ= .47; p< .001) and cocaine (Spearman’s ρ= .58; p< .001) in the full sample. Due to the sizable number of negative hair tests, the correlation was also tested in the subsamples with positive hair tests for each drug. In this analysis, level of THC metabolite in hair no longer correlated with self-reported frequency of use (Spearman’s ρ= .05; p= .60). Concentration of cocaine in hair continued to correlate with self-reported frequency of use (Spearman’s ρ= .41; p= .003).
Figure 1.
Concentration of drugs in hair by self-reported frequency of use for marijuana and cocaine.
Notes: Solid lines depict linear fitted lines for the full sample (including participants with negative self-report and hair tests). Dashed lines depict the linear fitted line for the subsample with positive hair test results. Similar findings were obtained for cocaine metabolites benzoylecgonine and norcocaine (figures not shown).
4. DISCUSSION
This study examined the utility of hair testing as a research measure among community health center patients with moderate-risk drug use, as determined by an internationally-validated screening instrument (Humeniuk et al., 2008). Although some discrepancy between biological testing and self-report is to be expected, our findings point to discrepancies that were surprising both in their extent and direction. The hair test was largely consistent with self-report for those reporting abstinence over the past 3 months. Relatively few participants who denied using a drug were positive by the hair test: 3-9% of self-reports of abstinence were refuted by the hair test. Nevertheless, self-report had low sensitivity against hair testing for drugs other than marijuana. A large proportion of the relatively few participants who tested positive for cocaine, amphetamines, or opioids denied recent use of those drugs. In a study comparing self-report to hair testing for cocaine among self-disclosed heroin users, Tassiopoulos and colleagues (2004) found that many heroin users with positive hair tests for cocaine denied cocaine use. Compared to marijuana, use of drugs like cocaine, amphetamines, and opioids may be perceived as more stigmatized, and therefore less subject to accurate disclosure.
However, the current study also illustrates that the potential for inaccuracy cuts both ways: a large number of participants reported drug use but had negative hair tests. For marijuana, only about half of self-disclosed users had a positive hair test. Under-identification of drug use by hair testing (or over-reporting) was also widespread for cocaine, amphetamines, and opioids. A study examining the veracity of self-reported heroin and cocaine use in an urban community sample found that self-reports were usually corroborated by hair analysis, and evidence of under-reporting was more common (Fendrich et al., 1999). However, ours was not a community sample, but rather a sample of patients who screened into and enrolled in a research study for moderate-risk drug users; that is, self-reported drug use was an inclusion criterion.
Although the extent of under-identification/over-reporting was surprising, the findings are not unprecedented. In a study with inmates, 43% who reported opiate use had a negative hair test, which the researchers attributed to participants falsely reporting use in hopes of gaining benefits such as entry into a rehabilitation program (Vignali et al., 2012). However, a study with marijuana users found that 38% of hair samples tested negative for marijuana, and even 6/13 participants who smoked marijuana under controlled laboratory conditions tested negative (Huestis et al., 2007).
The first study of brief intervention for drug use to use hair testing found some evidence of over-reporting of heroin and cocaine use at baseline (Bernstein et al., 2005). There are several possible explanations for the inability to identify declared drug users via hair test. Two explanations were put forth in the seminal study by the Bernstein group, which also apply here. First, it is possible that participants were being untruthful in order to gain entry into the study. However, the payment in the present study ($20) was not extravagant, and participants were not told during screening what the study was specifically about or what the eligibility criteria were. Nevertheless, it is possible that information about the study spread to the population by word of mouth. Another possibility is that participants did not accurately recall their drug use patterns, or that the hair test did not overlap perfectly with the self-report time frame (e.g., due to slightly under-length hair samples).
Another possible explanation is that some moderate-risk drug users may consume drugs below the detection limits of the standard hair test. Experts in hair testing have noted that there is insufficient empirical data on minimum detectable dosages for some substances, and that a negative hair test does not necessarily guarantee lack of exposure (Kintz, 2012). Frequency of use is just one of several factors that weigh on the ASSIST’s classification of risk categories. Thus, within our moderate-risk sample there is represented a range of use patterns and use-related problems. It is possible that hair testing at the standard cut-offs was not sufficiently sensitive to capture low levels of drug use or use patterns that were intermittent or sporadic.
We do not believe that the results support replacing urine testing with hair testing as a research measure in all cases; rather, hair and urine testing should be viewed as different tools with unique advantages and disadvantages. The current study provides data on hair testing to inform studies focusing on non-addicted persons with moderate-risk drugs use.
4.1 Limitations
There are several limitations to the present study that should be considered when interpreting the findings. First, marijuana use was highly prevalent in the sample, but prevalence of other drug use was comparatively low. These low base rates reduce confidence in inferences regarding cocaine, amphetamines, and opioids. Nevertheless, these drug use patterns reflect those of the clinic populations from which the sample was drawn.
This study did not aim to identify the best method of drug detection, but rather to evaluate the utility of a readily-available hair test as research measure in a moderate-risk population. Hence, we used the standard commercial 5-panel hair test, with corresponding standard cut-offs for screening and GC/MS confirmation. Future research using hair testing with moderate-risk populations should consider lower drug detection cut-offs. The findings are limited to drugs covered by the 5-panel test, which does not include various substances (e.g., benzodiazepines, various opioids, ethyl glucoronide as a marker of alcohol use).
Possibly, newer and more advanced techniques for hair testing (e.g., Montesano et al., 2014) could have yielded better detection and better concordance with self-report. However, these techniques were not offered by the laboratories we queried.at the time we planned the study. While more advanced approaches are becoming more available, the approach in the current study remains widely used, and our findings provide important data on this readily available approach. It may be of particular interest to clinical drug abuse researchers due to the dissemination of drug misuse screening and brief intervention services across healthcare settings, and increased emphasis on behavioral health integration in primary care. In the coming years, these factors are likely to lead to increased research with individuals who use drugs at a moderate-risk level, and our findings may be of interest to clinical substance use researchers working with these populations.
The discrepancy between hair testing and self-report for opioids may be due in part to the laboratory testing only for morphine, codeine, and heroin metabolites. Non-medical use of prescription opioids is a growing public health problem with massive societal costs, and is being encountered more frequently in primary care settings (Birnbaum et al., 2011; Manubay et al., 2011). Finally, the present study was conducted with community health center patients who disclosed moderate-risk drug use. Although the findings may hold implications for hair drug testing in research studies generally, the results should be interpreted cautiously in light of the particular sample and study limitations.
4.2 Conclusions
Hair testing cannot be considered a panacea for drug detection in individuals with moderate-risk drug use due to potential under-identification, at least at the standard detection thresholds used in this study. Challenges regarding cost, access to sufficient hair of consistent length, the likelihood that some samples will be of insufficient quantity for analysis, and participants’ cosmetic concerns must also be considered. Nevertheless, hair testing has significant utility as an independent biological measure of drug use given the problems of relying exclusively on self-report, and the considerable limitations of urine testing with respect to its short detection window. As noted by Donovan and colleagues (2012), hair testing may best be used in combination with self-report, e.g., to confirm reported abstinence. Even considering its shortcomings, future studies will be stronger if they include hair testing as a measure of drug use alongside self-report.
Acknowledgements
We thank Ms. Kyra Walls for assistance with manuscript preparation. We also thank the staff of the community health centers where the study was conducted.
Role of Funding Source This research was supported by the National Institute on Drug Abuse (R01 DA026003; PI: Robert P. Schwartz). NIDA had no role in the study design, analysis or interpretation of data, in the writing of this report, or in the decision to submit the manuscript for publication.
Footnotes
Contributors JG, RPS, and SGM jointly conceptualized the study. JG supervised data collection, conducted the analyses, and drafted the manuscript. KOG contributed to the statistical analysis plan. RPS, SGM, KOG, and SJO critically revised the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff. (Millwood) 2011;30:1402–1410. doi: 10.1377/hlthaff.2011.0480. [DOI] [PubMed] [Google Scholar]
- Curtis J, Greenberg M. Screening for drugs of abuse: hair as an alternative matrix: a review for the medical toxicologist. Clin. Toxicol. (Phila) 2008;46:22–34. doi: 10.1080/15563650701261462. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Chiodo LM, Hannigan JH, Greenwald MK, Janisse J, Patterson G, Huestis MA, Ager J, Sokol RJ. Just say “I don’t”: lack of concordance between teen report and biological measures of drug use. Pediatrics. 2010;126:887–893. doi: 10.1542/peds.2009-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakim PG, Wells EA. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107:694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendrich M, Johnson TP, Sudman S, Wislar JS, Spiehler V. Validity of drug use reporting in a high-risk community sample: a comparison of cocaine and heroin survey reports with hair tests. Am. J. Epidemiol. 1999;149:955–962. doi: 10.1093/oxfordjournals.aje.a009740. [DOI] [PubMed] [Google Scholar]
- Grekin ER, Svikis DS, Lam P, Connors V, Lebreton JM, Streiner DL, Smith C, Ondersma SJ. Drug use during pregnancy: validating the Drug Abuse Screening Test against physiological measures. Psychol. Addict. Behav. 2010;24:719–723. doi: 10.1037/a0021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han E, Paulus MP, Wittmann M, Chung H, Song JM. Hair analysis and self-report of methamphetamine use by methamphetamine dependent individuals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879:541–547. doi: 10.1016/j.jchromb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gustafson RA, Moolchan ET, Barnes A, Bourland JA, Sweeney SA, Hayes EF, Carpenter PM, Smith ML. Cannabinoid concentrations in hair from documented cannabis users. Forensic Sci. Int. 2007;169:129–136. doi: 10.1016/j.forsciint.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, de Lacerda RB, Ling W, McRee B, Newcombe D, Pal H, Poznyak V, Simon S, Vendetti J. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Kintz P. Value of the concept of minimal detectable dosage in human hair. Forensic Sci. Int. 2012;218:28–30. doi: 10.1016/j.forsciint.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Klein J, Karaskov T, Koren G. Clinical applications of hair testing for drugs of abuse--the Canadian experience. Forensic Sci. Int. 2000;107:281–288. doi: 10.1016/s0379-0738(99)00171-1. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Goldberger BA, Risk NK, Lewis CE, Price RK. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addict. Behav. 2008;33:1131–1139. doi: 10.1016/j.addbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Kang SY. Validity of self-reported drug use in high risk populations: a meta-analytical review. Subst. Use Misuse. 1996;31:1131–1153. doi: 10.3109/10826089609063969. [DOI] [PubMed] [Google Scholar]
- Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim. Care. 2011;38:71–90. vi. doi: 10.1016/j.pop.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic D. Seizing opportunities under the Affordable Care Act for transforming the mental and behavioral health system. Health Aff. (Millwood) 2012;31:376–382. doi: 10.1377/hlthaff.2011.0623. [DOI] [PubMed] [Google Scholar]
- Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin. Proc. 2008;83:66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- Montesano C, Johansen SS, Mielsen MKK. Validation of a method for the targeted analysis of 96 drugs in hair by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2014;88:295–306. doi: 10.1016/j.jpba.2013.08.050. [DOI] [PubMed] [Google Scholar]
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: a randomized trial. J. Subst. Abuse Treat. 2014;46:52–59. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polettini A, Cone EJ, Gorelick DA, Huestis MA. Incorporation of methamphetamine and amphetamine in human hair following controlled oral methamphetamine administration. Anal. Chim. Acta. 2012;726:35–43. doi: 10.1016/j.aca.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol ML, Cirimele V, Tritsch PJ, Villain M, Kintz P. Evaluation of the IDS One-Step ELISA kits for the detection of illicit drugs in hair. Forensic Sci. Int. 2007;170:189–192. doi: 10.1016/j.forsciint.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Cone EJ, Moolchan ET, Huestis MA. Dose-related distribution of codeine, cocaine, and metabolites into human hair following controlled oral codeine and subcutaneous cocaine administration. J. Pharmacol. Exp. Ther. 2005;313:909–915. doi: 10.1124/jpet.104.082388. [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Gryczynski J, Mitchell SG, Gonzales A, Moseley A, Peterson TR, Ondersma SJ, O’Grady KE. Computerized v. in-person brief intervention for drug misuse: a randomized clinical trial. Addiction. 2014 doi: 10.1111/add.12502. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassiopoulos K, Bernstein J, Heeren T, Levenson S, Hingson R, Bernstein E. Hair testing and self-report of cocaine use by heroin users. Addiction. 2004;99:590–597. doi: 10.1111/j.1360-0443.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- Tassiopoulos K, Bernstein J, Heeren T, Levenson S, Hingson R, Bernstein E. Predictors of disclosure of continued cocaine use. Addict. Behav. 2006;31:80–89. doi: 10.1016/j.addbeh.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Vignali C, Stramesi C, Vecchio M, Groppi A. Hair testing and self-report of cocaine use. Forensic Sci. Int. 2012;215:77–80. doi: 10.1016/j.forsciint.2011.05.007. [DOI] [PubMed] [Google Scholar]