Abstract
Objective
Myositis is characterized by severe muscle weakness. We and others have previously shown that endoplasmic reticulum (ER) stress plays a role in the pathogenesis of myositis. The present study was undertaken to identify perturbed pathways and assess their contribution to muscle disease in a mouse myositis model.
Methods
Stable isotope labeling with amino acids in cell culture (SILAC) was used to identify alterations in the skeletal muscle proteome of myositic mice in vivo. Differentially altered protein levels identified in the initial comparisons were validated using a liquid chromatography tandem mass spectrometry spike-in strategy and further confirmed by immunoblotting. In addition, we evaluated the effect of a proteasome inhibitor, bortezomib, on the disease phenotype, using well-standardized functional, histologic, and biochemical assessments.
Results
With the SILAC technique we identified significant alterations in levels of proteins belonging to the ER stress response, ubiquitin proteasome pathway (UPP), oxidative phosphorylation, glycolysis, cytoskeleton, and muscle contractile apparatus categories. We validated the myositis-related changes in the UPP and demonstrated a significant increase in the ubiquitination of muscle proteins as well as a specific increase in ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1) in myositis, but not in muscle affected by other dystrophies or normal muscle. Inhibition of the UPP with bortezomib significantly improved muscle function and also significantly reduced tumor necrosis factor α expression in the skeletal muscle of mice with myositis.
Conclusion
Our findings indicate that ER stress activates downstream UPPs and contributes to muscle degeneration and that UCHL-1 is a potential biomarker for disease progression. UPP inhibition offers a potential therapeutic strategy for myositis.
Emerging evidence indicates that class I major histocompatibility complex (MHC) overexpression on skeletal muscle fibers leads to an accumulation of unfolded proteins in the endoplasmic reticulum (ER) and activates an unfolded protein response and ER overload response in the affected muscle (1). Several studies have confirmed that ER stress contributes to disease pathology in myositic muscle (2–5). In a previously described conditional class I MHC overexpression mouse model (6), clinical, biochemical, histologic, and immunologic features similar to those of human myositis are produced. This mouse model is useful for identifying and investigating downstream ER stress pathways in muscle. Since major changes in myositic muscle occur at the protein level, a quantitative proteomic technique that enables identification of alterations in protein between normal and diseased muscle is an ideal strategy for the study of myositis.
Stable isotope labeling with amino acids in cell culture (SILAC) has been used successfully for precise quantification of altered proteins and has been extensively used in in vitro cell culture systems (7,8). More recently, Kruger et al extended the SILAC technique to in vivo systems using mice labeled with 13C6-Lys (9). We have generated 13C6-Lys–labeled C57BL/6 mice in which the whole mouse proteome of all tissues and organs is fully labeled (≥96% labeling efficiency) with the heavy lysine (SILAC mice) (10,11).
In the present study, use of the SILAC strategy allowed us to identify both known and previously unknown disease-specific protein modulations and pathways in class I MHC–transgenic mice. We found that the ubiquitin proteasome pathway (UPP) is highly active in myositic muscle, and inhibition of this pathway using bortezomib, an inhibitor of evolutionarily conserved 26S proteasome, resulted in decreased muscle inflammation and improved muscle function in mice with experimental myositis (12).
MATERIALS AND METHODS
Animals
All animals were handled according to the local Institutional Animal Care and Use Committee (IACUC) guidelines under IACUC-approved protocols. SILAC mice (also referred to as labeled mice), mice with myositis (C57BL/6 class I MHC–overexpressing double-transgenic [C57BL/ 6-HT]; also referred to as HT mice or as unlabeled mice), and single-transgenic control mice (C57BL/6-H or -T; also referred to as H or T mice or as unlabeled mice) available in-house were used for all experiments. SILAC mice were generated as described earlier (9). Briefly, C57BL/6 mice were fed a custom diet containing “heavy” 13C6-Lys (Cambridge Isotope Laboratories) for 2 generations; in parallel, HT mice and H or T mice were fed unlabeled custom feed (12C6-Lys; Cambridge Isotope Laboratories). Labeling efficiency for the whole mouse proteome with the l-lysine (13C6) was ≥96% (11). The generation and genotyping of the HT mice have been described previously (6). Doxycycline withdrawal at 5 weeks of age results in up-regulation of class I MHC and development of muscle disease at ~16 weeks of age, in double-transgenic HT mice but not in single-transgenic H or T mice. Most of the present experiments were performed on 16–18-week-old mice. Female mice develop consistently severe and early disease whereas male mice develop disease at a later age (6); hence, to reduce variation, we used only female mice for these experiments.
Sample collection
Sixteen-week-old female SILAC mice, C57BL/6-HT mice (myositis), and C57BL/6-H or T mice (control) were perfused with phosphate buffered saline and killed via CO2 administration. Quadriceps muscle was harvested, flash-frozen in liquid nitrogen–chilled isopentane, and stored at −80°C until use.
Mass spectrometry (MS) analysis and proteomic profiling
These analyses were performed in two phases: a discovery phase followed by validation. A schematic representation of the experimental methodology is available at http://gen medmanuscriptsuppleinfo.cnmcresearch.org.
Discovery and validation phases
Aliquots of protein extracts (50 μg each) from the quadriceps of unlabeled H or T mice or unlabeled HT mice were mixed at 1:1 with protein extract from the muscle of a SILAC mouse and processed for proteome profiling. To increase the robustness of the analyses and quantitatively validate the data obtained in the initial experiments, a spike-in SILAC strategy was used. An independent set of HT mice and H or T mice (3 per group) that had been maintained on normal mouse feed were used for these experiments. Muscle lysates from individual HT and H or T mice were spiked with equal amounts of lysate from a SILAC mouse and then used for further downstream sample preparation and MS analyses.
Sample preparation and MS analyses
Sample preparation and MS analysis were performed as described previously (13). Briefly, total protein lysates were prepared from quadriceps muscle using radioimmunoprecipitation assay buffer (50 mM Tris HCl [pH 8.0] with 150 mM sodium chloride, 1.0% Igepal CA-630 [Nonidet P40], 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate [SDS]) with protease inhibitors (Halt protease inhibitor cocktail 100×). Aliquots (50 μg each) of labeled and unlabeled protein extracts were mixed 1:1 and then resolved by SDS–polyacrylamide gel electrophoresis. Gels were stained with Bio-Safe Coomassie (Bio-Rad), and each lane was cut into 30–35 serial slices. Proteins in each gel slice were in-gel digested with trypsin. Peptides from each band were analyzed using LTQ-Orbitrap-XL, as described previously (10,11,13).
Database search and SILAC ratio measurement
Protein identification and quantification were performed using IP2 version 1.01 (http://www.integratedproteomics.com/) as described previously (11). Briefly, protein identification parameters include a mass tolerance of ±30 parts per million for MS and ±1.5 Da for MS/MS, and a protein false discovery rate of <1%. Ratios of unlabeled to labeled peptides were determined using an extracted ion chromatogram (Census version 1.77 and IP2). The validity of the data was checked using regression correlations of >0.5 for each peptide pair. Mean relative ratios were calculated from the individual ratios obtained for unlabeled-to-labeled proteins; i.e., the ratios in unlabeled HT versus SILAC mice and unlabeled H or T versus SILAC mice, and the significant protein alterations in HT versus H or T mice were determined and assigned to a specific pathway based on function, using UniProt. The percentage of proteins assigned to each pathway was then determined.
Validation of potential candidates using immunoblotting
Immunoblotting was performed as described previously (14,15). Primary antibodies specific for ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1) (1:1,000; Cell Signaling Technology), ubiquitin (1:1,000, Cell Signaling Technology), glucose regulatory protein 78 (Grp78) (1:1,000 Epitomics), dystrophin (1:1,000, Novocastra), and dysferlin (1:1,000, Santa Cruz Biotechnology), and the corresponding horseradish peroxidase–conjugated polyclonal rabbit or mouse secondary antibodies (Dako) were used; β-actin was used as loading control. Densitometric analysis was carried out on a Bio-Rad GS-800 calibrated densitometer with the Quantity One software package. Data are presented as ratios of the optical density of each specific protein to the corresponding β-actin sample.
Effect of blocking of proteasome with inhibitor (bort-ezomib) in HT mice
Experimental design
Female HT mice (myositis) and H or T mice (control) (total 13 mice) were generated through in-house breeding and genotyped at 21 days of age. Experiments were performed when the mice were 16–18 weeks of age (i.e., 12–15 weeks after transgene induction). Mice were randomly assigned to 1 of 3 groups: 1) control (H or T; n = 5), 2) myositis, untreated (untreated HT; n = 4), and 3) myositis, bortezomib-treated (bortezomib-treated HT; n = 4). Bortezomib was injected intraperitoneally at 0.75 mg/kg body weight for 4 weeks (2 injections per week). At the end of the trial, mice were killed via CO2 administration and various muscle tissues were harvested for functional studies, flash-frozen in liquid nitrogen–chilled isopentane, and stored at −80°C until use. Body weight (gm) and individual muscle weights (mg) were recorded.
Proteasomal activity
Quadriceps muscle lysates were prepared using 300 μl of lysis buffer (10 mM HEPES [pH 7.9] at 4°C, 1.5 mM magnesium chloride, 10 mM potassium chlo-ride, and 0.5 mM dithiothreitol). Proteasomal activity was monitored using a 20S proteasome activity assay kit (no. APT280; Chemicon International) according to the instructions of the manufacturer. Protein concentrations were estimated with a BCA Protein Assay kit (Pierce), and the values obtained (relative fluorescence units [RFU]) were normalized to the protein concentration. Data were expressed as RFU/μg of protein.
In vitro muscle function tests
Muscle function tests were performed on isolated extensor digitorum longus muscle as described previously (16,17). Maximal force generated by the extensor digitorum longus was represented as mN. The force produced after a fatigue protocol was measured as the percent of basal force.
Measurement of inflammation markers
Quadriceps tissue was obtained from H or T, untreated HT, and bortezomib-treated HT mice. RNA was isolated using TRIzol according to the protocol recommended by the manufacturer (Life Technologies). Complementary DNA was synthesized using a High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (no. 4374966; Life Technologies). Quantitative polymerase chain reaction was performed using an Applied Biosystems 7900HT Real-Time PCR machine. TaqMan probe sets TNFa (Mm00443260_g1), ICAM1 (Mm00516023_m1), and HPRT (Mm01545399_m1) were obtained from Applied Biosystems. The relative expression of each transcript was calculated according to the ΔΔCt method with hypoxanthine guanine phosphoribosyltransferase as the internal reference, and fold changes were expressed as the mean ± SEM.
Histologic analysis
Muscle tissue (extensor digitorum longus) was obtained from all mice used in the drug trial. Formalin-fixed tissue samples were sectioned and stained with hematoxylin and eosin as described previously (18). Histologic analysis was performed under blinded conditions, and staining was scored on a scale of 0–5; sections with the highest number of inflammatory infiltrates were assigned a score of 5, and sections with no inflammatory cells were assigned a score of 0. Representative images (20×) were obtained using a Nikon Eclipse E800 microscope fitted with a SPOT digital color camera with SPOT advanced software (Diagnostic Instruments). Muscle regeneration was assessed using an antibody against embryonic myosin heavy chain (1:20; Developmental Studies Hybridoma Bank) and the corresponding anti-mouse secondary antibody, and quantified. The mean ± SEM number of embryonic myosin heavy chain–positive fibers per section was calculated.
Statistical analysis
To identify proteomic modulations that differed significantly between HT (myositis) and H or T (control) mice, the nonparametric Wilcoxon rank sum test was used. Other statistical analyses were performed using either Student's t-test or one-way analysis of variance (with Bonferroni correction post-test) where appropriate. Calculations were performed with GraphPad Prism version 5.
RESULTS
SILAC strategy enables accurate proteome profiling in the muscle of mice with myositis
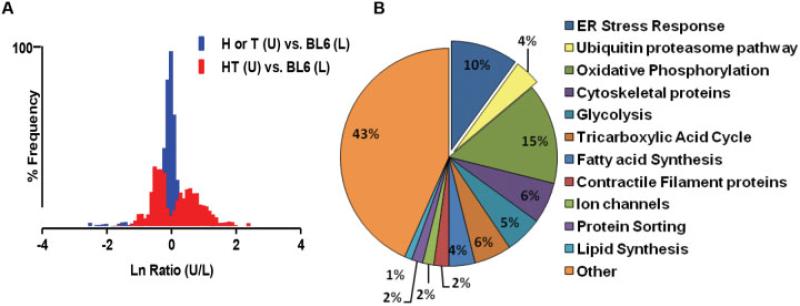
Doxycycline withdrawal in HT mice results in up-regulation of class I MHC (H-2Kb); therefore, the SILAC technique could be validated by detection of class I MHC protein specifically in HT mouse muscle but not in control mouse muscle. Elution profiles of SILAC-labeled and unlabeled peptide pairs for H-2Kb indeed did reveal the expected protein alterations, providing proof of principle that the technique enabled accurate detection. Representative profiles are available at http://genmed manuscriptsuppleinfo.cnmcresearch.org. The protein ratio distribution of unlabeled myositic muscle and SILAC-labeled normal muscle revealed a broader distribution of up-regulated and down-regulated proteins in the myositic muscle, suggesting highly altered protein expression in myositic muscle. In contrast, a comparison of SILAC-labeled normal muscle versus unlabeled normal muscle showed a narrow protein ratio distribution, suggesting minimal differences in the muscle proteomes (Figure 1A).
Figure 1.
Differentially modulated proteins and pathways involved in experimental myositis in mice, identified by proteomic profiling using stable isotope labeling with amino acids in cell culture (SILAC). Quadriceps muscle was harvested from SILAC-treated (labeled [L]) C57BL/6 (BL6) mice, from age-matched major histocompatibility complex–overexpressing double-transgenic (HT) myositic mice that had not undergone SILAC (unlabeled [U]), and from age-matched single-transgenic (H or T) control mice that had not undergone SILAC (n = 3 per group). A, Log-transformed protein ratio distribution in muscle from unlabeled normal mice versus normal SILAC mice and in unlabeled mice with myositis versus normal SILAC mice, showing a broader distribution of up-regulated and down-regulated proteins in the myositic muscle. The ratios of unlabeled-to-labeled peptide pairs were obtained using IP2 software. B, Proteins with significant modulations (≥1.5-fold) annotated to specific pathways based on function, using the UniProt knowledge database. The proportion of proteins annotated to each pathway (as a percentage of the total number of proteins) is shown. ER = endoplasmic reticulum. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38180/abstract.
Global proteomic analysis of quadriceps muscle identified a mean ± SEM of 490 ± 90 proteins with ≥2 unique peptides. Validation using a spike-in strategy in an independent set of mice identified 178 proteins whose levels were significantly altered (by a factor of at least ±1.5) in myositic muscle compared to control muscle (see Supplementary Table 1, on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38180/abstract). We recently reported a significant down-regulation of AMP deaminase-1 (AMPD1), a muscle-specific rate-limiting enzyme, in the purine nucleotide cycle of HT mouse skeletal muscle (19). In the present study we have confirmed that the relative abundance of AMPD1 is decreased by 3.5-fold in HT mouse muscle relative to control muscle (representative elution profiles available at http://genmedmanuscriptsuppleinfo.cnmcresearch.org). Experiments using the SILAC mouse model also confirmed that the ER stress protein Grp78 is significantly up-regulated (15-fold) in HT mice relative to control mice, as previously described (1).
Furthermore, significant up-regulation of other ER stress-related proteins, such as protein disulfide isomerase A3 (PDIA3), eukaryotic elongation factor 1A2, calnexin, and heat-shock proteins (HspB1, Hsp7C), was observed in HT mouse muscle (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.38180/abstract). Other proteins that were notably up-regulated included UCHL-1, major vault protein, and annexin; the most drastically down-regulated proteins included titin, actinin, myosin binding proteins, and myosins. Overall, the data indicate that proteomic profiling using the SILAC mouse model identifies expected protein alterations in myositic muscle.
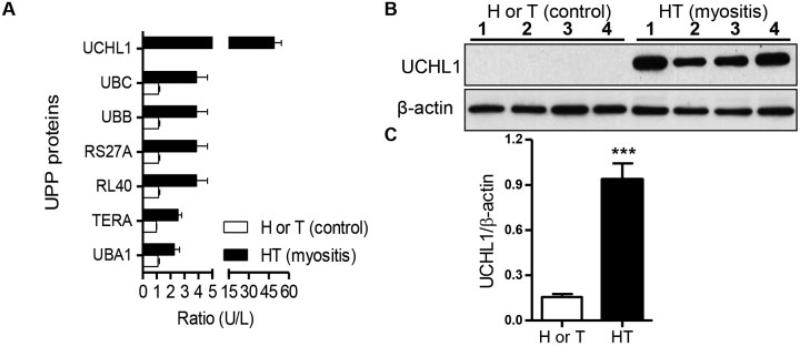
Up-regulation of the ER stress-associated degradation (ERAD) pathway and the UPP in myositic muscle
Annotation of identified proteins indicated that pathways such as the ER stress, ubiquitin proteasome, oxidative phosphorylation, cytoskeletal protein, and glycolysis pathways were significantly altered in myositic muscle (Figure 1B), providing evidence of perturbation of metabolic pathways in myositis. A closer examination of the profiles revealed that several members of the UPP family i.e., UCHL-1, ubiquitin 60S ribosomal protein L40, and polyubiquitins B and C, were significantly up-regulated (>2-fold) in HT mice compared to control mice (Figure 2A). Similarly, proteins involved in all components of the ERAD pathway were also significantly up-regulated (>2-fold) in HT mice. These included heat-shock proteins (Hsp90 and Hsp70 families), PDIA3, calnexin, and valosin-containing protein (Supplementary Table 1, http://onlinelibrary.wiley.com/doi/10.1002/art.38180/abstract). An unlabeled peptide of UCHL-1, one of the highly up-regulated UPP proteins in myositic muscle, was observed in myositic mice (doubly charged m/z, 839.92), but the corresponding heavy peptide (at m/z 842.93) was not (representative MS results shown at http://genmedmanuscriptsuppleinfo.cnmcresearch.org).
Figure 2.
Up-regulation of the ubiquitin proteasome pathway (UPP) in myositic muscle. Quadriceps muscle was obtained from SILAC-treated mice, HT mice that had not undergone SILAC, and H or T mice that had not undergone SILAC (n = 3 per group), and mass spectrometry analysis was performed. A, Mean ± SEM ratios of UPP proteins in muscle from unlabeled versus labeled myositic mice and from unlabeled versus labeled control mice. B, Homogenates of quadriceps muscle lysates from 4 H or T mice and from 4 HT mice, immunoblotted with anti–ubiquitin carboxyl-terminal hydrolase isozyme L1 (anti–UCHL-1) antibody or with β-actin as a loading control. C, Mean ± SEM ratio of UCHL-1 to β-actin, calculated by densitometric analysis using Quantity One software. *** = P < 0.001 versus H or T mice, by Student's t-test. UBC = ubiquitin C; RS27A = ubiquitin 40S ribosomal protein S27A; RL40 = ubiquitin 60S ribosomal protein L40; TERA = transitional endoplasmic reticulum ATPase (see Figure 1 for other definitions).
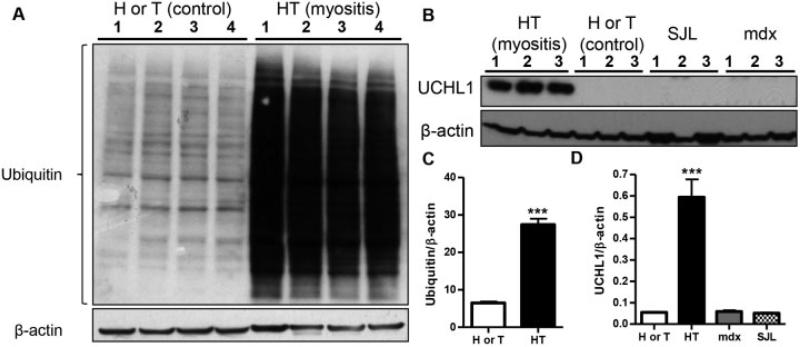
UCHL-1 up-regulation is specific to myositis and not seen in other myopathies
Since the UPP was up-regulated, we sought to validate the global ubiquiti-nation status as well as UCHL-1 expression in myositic and dystrophic (dystrophin- and dysferlin-deficient) muscle, by immunoblotting. We found a significant up-regulation (P < 0.001) of UCHL-1 (Figures 2B and C) and ubiquitination of proteins (Figures 3A and C) in myositic muscle, especially during the symptomatic phase of the disease. To ascertain whether the UCHL-1 up-regulation is specific to myositis, we examined muscle in 2 dystrophic mouse models (dysferlin-deficient [SJL/J] mice and dystrophin-deficient [mdx] mice) and found that UCHL-1 was not expressed in either of these 2 myopathies (Figures 3B and D). Since UCHL-1 is highly expressed in the brain, we investigated its expression in the brain tissue of HT and H mice. We found no significant differences between the affected and control strains (details available at http://genmedmanuscriptsuppleinfo.cnmcresearch.org), indicating that up-regulation of UCHL-1 is disease- and muscle-specific in this model.
Figure 3.
Expression of ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1) is specific to myositis muscle and does not occur in other types of dystrophic muscle. A, Quadriceps muscle lysates from 4 H or T mice and from 4 HT mice were obtained, and homogenates were immunoblotted for ubiquitin. B, Another set of quadriceps muscle lysates was obtained from 16-week-old H or T mice, HT mice, and age- and sex-matched SJL/J and mdx mice (n = 3 per group). The homogenates were immunoblotted with UCHL-1 or with β-actin as a loading control. C and D, The ratios of the respective proteins to β-actin from the experiments described in A and B, respectively, were calculated. Values are the mean ± SEM. *** = P < 0.001 versus other groups, by Student's t-test (C) or one-way analysis of variance (D). See Figure 1 for other definitions.
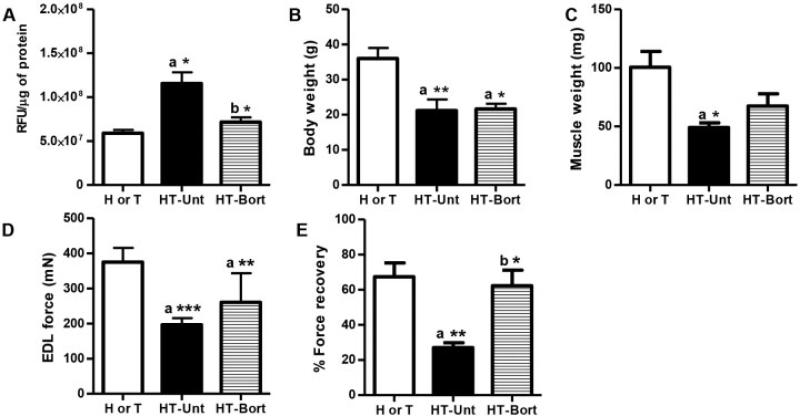
Effect of bortezomib on proteasomal activity in myositic muscle
To assess the effect of proteasome pathway blockade in HT mice, we treated these mice with bortezomib, a 26S proteasomal inhibitor, for 4 weeks. Proteasomal activity in the quadriceps muscle of untreated HT mice was significantly increased (by 45%; P < 0.05) when compared to that in muscle from H or T control mice (Figure 4A). Treatment of HT mice with bortezomib significantly reduced (by 33%; P < 0.05) the proteasomal activity in the quadriceps muscle when compared to levels in untreated HT mice (Figure 4A).
Figure 4.
Bortezomib (Bort) treatment improves muscle function in the mouse model of myositis. Major histocompatibility complex–overexpressing double-transgenic (HT) myositic mice and single-transgenic (H or T) control mice were divided into 3 groups: H or T (n = 5), untreated (Unt) HT (n = 4), and bortezomib-treated HT (n = 4). Bortezomib (0.75 mg/kg body weight) was injected intraperitoneally twice a week for 4 weeks. A, Proteasomal activity in quadriceps muscle lysates, shown as relative fluorescence units (RFU) normalized to protein units. B, Body weight. C, Gastrocnemius muscle weight. D, Maximal muscle force, determined by in vitro testing of extensor digitorum longus (EDL) muscles. E, Percent force recovery for extensor digitorum longus muscle. Values are the mean ± SEM. * = P < 0.05; ** = P < 0.01; *** = P < 0.001 versus H or T control mice (a) or versus untreated HT mice (b), by one-way analysis of variance.
Effect of bortezomib on body and muscle weights
Body weight and muscle weight were significantly lower in untreated HT mice than in control mice (P < 0.01 and P < 0.05, respectively) (Figures 4B and C). Bortezomib-treated HT mice did not exhibit a significant increase in body weight when compared to untreated HT or control mice (Figure 4B). The weight of gastrocnemius muscle from the bortezomib-treated HT mice was 37% higher compared to the untreated group, but the difference was not statistically significant (Figure 4C). To ascertain whether the increase in muscle mass was reflected in the levels of cytoskeletal structural proteins, we performed immunoblotting for dysferlin and dystrophin proteins. Bortezomib treatment did not alter the levels of these cytoskeletal structural proteins (details available at http://genmedmanuscriptsuppleinfo.cnmcresearch.org).
Effect of bortezomib on muscle function and muscle inflammation
Maximal force in the muscle of untreated HT mice was significantly lower than that in muscle of control mice (P < 0.001) (Figure 4D). Although maximal force in the muscle of bortezomib-treated HT mice was 33% higher than in untreated HT mice, the difference was not statistically significant. We also did not identify a significant difference in specific force between the bortezomib-treated and untreated groups (mean ± SEM 229.8 ± 7.89 kN/m2 in the H or T group, 173.6 ± 7.86 kN/m2 in the untreated HT group, and 167.8 ± 18.6 kN/m2 in the bortezomib-treated HT group). Notably, the percent force recovery was higher in bortezomib-treated HT mice than in untreated HT mice (P < 0.05) (Figure 4E).
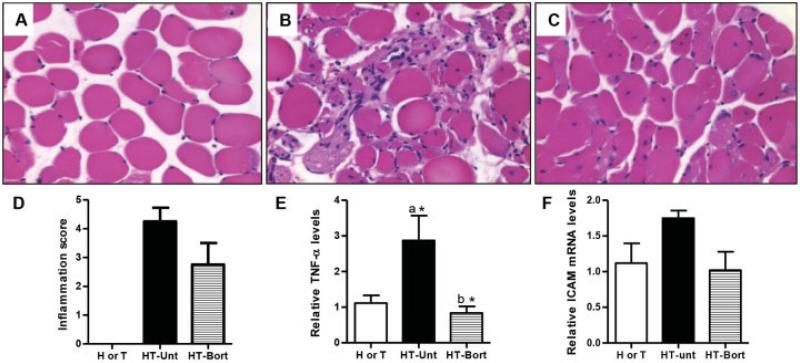
Hematoxylin and eosin staining revealed significant muscle degeneration and inflammation in HT mouse muscle (Figures 5B and D) but not in H or T mouse muscle (Figures 5A and D). Bortezomib treatment resulted in a 35% reduction in the level of inflammation in HT mice (P not significant) (Figures 5C and D). HT mice showed a significantly increased level of transcripts of the proinflammatory tumor necrosis factor α (TNFα) when compared to controls (P < 0.05). The reduced inflammation in the bortezomib-treated HT mice corresponded to a significant reduction in TNFα transcript levels in these mice (P < 0.05) (Figure 5E). Levels of intercellular adhesion molecule 1 transcripts did not differ significantly between the groups (Figure 5F).
Figure 5.
Bortezomib treatment decreases muscle inflammation in the mouse model of myositis. Myositic (HT) mice and control (H or T) mice were divided into 3 groups: H or T (n = 5), untreated HT (n = 4), and bortezomib-treated HT (n = 4). Bortezomib (0.75 mg/kg body weight) was injected intraperitoneally twice a week for 4 weeks. Extensor digitorum longus muscles were collected in formalin, and tissue sections were stained with hematoxylin and eosin. A–C, Representative photomicrographs of muscle from an H or T mouse (A), an untreated HT mouse (B), and a bortezomib-treated HT mouse (C). Original magnification × 20. D, Inflammation scores of stained sections (on a scale of 0–5 [0 = no inflammation, 5 = highest inflammation]), determined under blinded conditions. E and F, Levels of tumor necrosis factor α (TNFα) transcript (E) and intercellular adhesion molecule 1 (ICAM-1) transcript (F) in quadriceps muscle lysates, determined by quantitative polymerase chain reaction and expressed as the fold change in relation to hypoxanthine guanine phosphoribosyltransferase. Values in D–F are the mean ± SEM. * = P < 0.05 versus H or T control mice (a) or versus untreated HT mice (b), by Wilcoxon rank sum test. See Figure 4 for other definitions.
Effect of bortezomib on Grp78 levels and muscle regeneration in HT mice
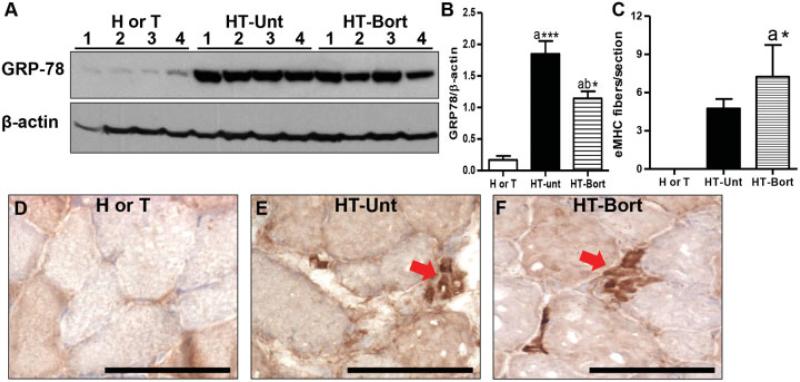
We measured levels of the ER stress sensor Grp78/BiP and found that bortezomib treatment significantly reduced Grp78 levels in comparison to those in untreated HT mice or control H or T mice (Figures 6A and B). In addition, bortezomib treatment increased the number of embryonic myosin heavy chain–positive muscle fibers (by ~50% compared to untreated HT mouse muscle) (Figures 6C–F), suggesting an increase in regeneration in treated HT mice.
Figure 6.
Bortezomib treatment decreases glucose regulatory protein 78 (Grp78) levels and increases regeneration in myositic mouse muscle. A, Immunoblotting for Grp78 in quadriceps muscle lysates from 4 control (H or T) mice, 4 untreated myositic (HT) mice, and 4 bortezomib-treated HT mice. B, Quantification of Grp78 levels in relation to β-actin levels. C, Number of embryonic myosin heavy chain (eMHC)–positive fibers per section. Values in B and C are the mean ± SEM. * = P < 0.05; *** = P < 0.001 versus H or T control mice (a) or versus untreated HT mice (b), by one-way analysis of variance (B) or Wilcoxon rank sum test (C). D–F, Representative photomicrographs showing staining for embryonic myosin heavy chain (arrows) in muscle from an H or T mouse (D), an untreated HT mouse (E), and a bortezomib-treated HT mouse (F). Bars = 100 μM. See Figure 4 for other definitions.
DISCUSSION
In the present study, we have demonstrated that novel pathways such as the UPP contribute to muscle pathology in the mouse myositis model. Inhibition of the UPP using a proteasome inhibitor in vivo in mice with myositis led to histologic and functional improvements. These data suggest that UPP is an appropriate therapeutic target in myositis.
To understand the pathogenetic mechanisms involved in myositis, we performed an unbiased quantitative exploratory proteomic analysis of quadriceps muscle in a mouse model of myositis, using in vivo SILAC and MS. To date, the majority of studies exploring perturbed molecular mechanisms in myositic muscle have been conducted at the messenger RNA (mRNA) transcript level (1,5,19,20). There have been a few proteomic studies of sporadic inclusion body myositis (IBM) and hereditary IBM in which the investigators used label-free proteomic approaches (21–23); however, precise quantification of protein modulations is technically chal lenging when label-free proteomic approaches are used. Application of the SILAC strategy enabled precise quantification of alterations in protein such as H-2Kb in diseased versus healthy muscle, and our initial proteomic profiling experiments demonstrated the effectiveness of this SILAC mouse strategy. Alterations in protein levels were observed in diseased mice as compared with normal mice, suggesting an underlying pathology in HT mice. Similar proteomic analyses performed using dystrophin-deficient mdx mice have demonstrated up-regulation of integrin-linked kinase and actin cytoskeleton pathway proteins in the diseased mice (11). These findings suggest that the SILAC mouse strategy can be used to detect disease-specific protein modulations in skeletal muscle.
With the spike-in SILAC strategy using an independent set of normal and diseased mice, we identified 178 significantly modulated proteins, a larger number than has been identified earlier. For example, investigators in one study conducted a proteomic analysis of IBM muscle using 2-dimensional electrophoresis and reported 22 differentially modulated proteins (21). In another study, analyses of hereditary IBM samples were conducted using 2-dimensional electrophoresis in combination with iTRAQ, and 41 differentially modulated proteins were found (23). These results suggest the superiority of in vivo labeling over other proteomic strategies without labeling. Some of the limitations of this technique include impracticality of its use in in vivo human studies and difficulties in detecting posttranslational modifications.
Our proteomic profiling using SILAC mice revealed marked (≥1.5-fold) down-regulation of structural proteins, such as myosins, actinins, and titin, in HT mice. This is consistent with the previously reported 40% reduction in body and muscle mass in mice with myositis (6). It is generally difficult to compare mouse tissue to human samples; however, there is some degree of concordance with respect to pathways identified in human samples because of the heterogeneity and chronicity of the human disease. Down-regulation of structural proteins (notably titin) in the perifascicular atrophic fibers of dermatomyositis (DM) patient samples has been reported (22).
Our proteomic profiling studies also identified significant up-regulation (≥1.5-fold) of heat-shock proteins (HspB7, HspB1, HspB6, Hsp74, and Hsp7C), superoxide dismutase, and peroxiredoxin, indicating disturbances in stress response pathways as well as redox signaling mechanisms in the affected muscle. An earlier study conducted on muscle biopsy samples from patients with sporadic IBM also revealed up-regulation of redox proteins and heat-shock proteins. Up-regulation of the glycolytic enzymes enolase 1 and enolase 3β was observed in muscle from these patients as well (21). In contrast, we observed a significant down-regulation of enolase (−2.8-fold) in HT mice. Moreover, in another proteomic profiling study of IBM, down-regulation of enolase, fast-twitch sarcomeric proteins, as well as glycolytic enzymes was found (24). Given the number of factors that differed between these studies, it would be difficult to pinpoint the reasons for the discordant results.
Proteomic analyses using the SILAC mouse model showed that several ER stress-associated proteins were up-regulated in HT mice; this finding is consistent with the results of earlier studies by our group and others (1–5). These results indicate that chronic activation of ER stress occurs in myositic muscle. Annotation of differential protein modulations to specific pathways provides an indication of what mechanisms are perturbed at the tissue level. ER stress, oxidative phosphor-ylation, cytoskeletal protein, and glycolytic pathways were found to be significantly involved. An earlier study that included proteomic profiling of bronchoalveolar lavage fluid in 3 different subsets of patients with polymyositis/DM also showed disturbances in general metabolism, cytoskeletal protein, and immunologic response pathways (25). The perturbations in the glycolytic and mitochondrial energy metabolic pathways are consistent with the underlying muscle weakness in myositis. These findings suggest that metabolic changes in other tissues affect the skeletal muscle; however, metabolic changes within the affected skeletal muscle in myositis have not been fully explored.
The presence of UCHL-1 in myositis but not in other dystrophies and its concomitant appearance with symptoms suggest that this molecule may serve as a surrogate marker for disease progression. Although relatively little is known about UCHL-1, it has recently been suggested to play a role in synaptic dysfunction and memory loss. Enhanced UCHL-1 expression has been reported to have a protective effect on memory loss in β-amyloid–induced Alzheimer's disease in mice (26). A lack of released ubiquitin and a local deficiency of ubiquitin for other cellular functions may explain the enhanced expression of UCHL-1. In any case, it is important to understand the mechanism of action and mode of regulation of this protein in the pathology of myositis. Modulation of ubiquitination pathway proteins has been mentioned in relation to hereditary IBM (23), but their pathogenic relevance has not been studied. Another study also demonstrated an increase in the transcripts of ubiquitin-modifying enzymes (Ube1L, Ube2L6) in DM muscle (22), suggesting a potential role of the UPP in the pathology of myositis. Our findings also indicate that ER stress and ERAD pathways (Grp78, heat-shock proteins, valosin-containing protein, PDIA3, calnexin) are linked with the UPP in myositic skeletal muscle. Our data are consistent with those from a previous study by Nogalska et al, which showed that an essential player in the ERAD pathway, homocysteine-induced endoplasmic reticulum protein, is up-regulated in the muscle of patients with sporadic IBM (27).
Comparison of SILAC profiling with our previous mRNA profiling data from the skeletal muscle of HT mice suggests that there is 41% overall concordance between protein and mRNA expression levels (see http://genmedmanuscriptsuppleinfo.cnmcresearch.org). This provides validation of the data using 2 independent cohorts.
Levels of ER stress in humans with myositis and in the class I MHC mouse model have been compared and appear to be similar with respect to Grp78 expression (1), suggesting striking similarities between human and mouse myositic muscle disease. Herein we propose that overexpression of class I MHC on the affected myofibers leads to an accumulation of MHC molecules in the ER, which, in turn, induces ER stress response and activates downstream ERAD and UPP, ultimately affecting calcium homeostasis and energy metabolism and causing muscle degeneration (28) (see Supplementary Figure 1, on the Arthritis & Rheumatism web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38180/abstract).
The role of the UPP in the pathogenesis of several diseases, including arthritis, neurodegenerative disorders, cachexia, and multiple myeloma, has been recognized (29–32). Since bortezomib is the inhibitor of evolutionarily conserved 26S proteasome and is active both in human and in murine cells (33,34), we treated the mice with this drug. Treatment with bortezomib at 0.75 mg/kg significantly reduced the proteasomal activity in the quadriceps muscle of HT mice, indicating that the concentrations used in our study had adequate bioavail-ability and therapeutic efficacy (35).
Although the treatment did not significantly affect body weights or levels of structural proteins such as dystrophin and dysferlin, we found an increase in the mass of certain muscles. This observation is consistent with findings of a previous study in which MG132, a reversible inhibitor of proteasome, increased tibialis anterior muscle mass without affecting body weight (36). Our data also suggest that bortezomib treatment preserves muscle function, as indicated by the increase in the percent force recovery in treated versus untreated HT mice. Caron et al reported a similar improvement in muscle function after treatment with MG132 (36). However, we did not observe an increase in maximal or specific force in HT mice upon treatment with bortezomib. This discrepancy in the lack of improvement in maximal and specific force but significant increase in force recovery after fatigue can be explained by fiber type switching (fast to slow). We have previously reported fiber type switching in this mouse model (19) and speculate that it might be affected by bortezomib.
Bortezomib treatment reduced inflammation as well as the transcript level of TNFα (an NF-κB pathway target gene) in the affected muscle. Attenuation of NF-κB activation and inflammation and improvement in disease phenotype in response to treatment with a proteasome inhibitor have also been reported in a peptidoglycan–polysaccharide–induced polyarthritis model (37). Our data also suggest that bortezomib significantly reduced the levels of Grp78 in the muscle of HT mice. It is possible that bortezomib decreases overall protein synthesis, resulting in reduced protein load in the ER and ER stress in myositic muscle. The molecular mechanism by which bortezomib enhances muscle regeneration is unclear; however, one possibility is that inhibition of proteasome activity by bortezomib results in reduced NF-κB levels by inhibition of IκB degradation (38–40). Furthermore, bortezomib has been implicated in blocking of the transmigration of lymphocytes via the inhibition of NF-κB and, in turn, the reduction of adhesion molecules needed for transmigration into the affected tissue (41,42). The above data indicate that the beneficial effects of bortezomib are dependent not exclusively on the inhibition of proteasome activity but also on its antiinflammatory properties and, taken together with our present results, suggest that this drug is a potential therapeutic option for autoimmune myositis.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Dr. Deborah McClellan for editing the manuscript.
Supported by the NIH (Intellectual and Developmental Disabilities Research Center [IDDRC] grant P30-HD-40677, National Center for Medical Rehabilitation Research/National Institute of Neurological Disorders and Stroke core grant 2R24-HD-050846-06, IDDRC core grant 5P30-HD-040677-10, and National Center for Research Resources grant UL1-RR-031988 [George Washington University–Children's National Medical Center, Clinical and Translational Science Institute]). Dr. Rayavarapu's work was supported by the Association Francaise Contre les Myopathies (Pre-Doctoral Fellowship). Dr. Nagaraju's work was supported by the NIH (grants R01-AR-050478, 5U54-HD-053177, and K26-OD-011171), the Muscular Dystrophy Association, and the US Department of Defense (grant W81XWH-05-1-0616).
Footnotes
Presented by Dr. Rayavarapu in partial fulfillment of the requirements for a PhD degree, George Washington University, Washington, DC.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Nagaraju had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Rayavarapu, Hathout, Nagaraju. Acquisition of data. Rayavarapu, Coley, Van der Meulen, Cakir, Tappeta, Kinder, Dillingham, Brown.
Analysis and interpretation of data. Rayavarapu, Brown, Hathout.
REFERENCES
- 1.Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–35. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 2.Vitadello M, Doria A, Tarricone E, Ghirardello A, Gorza L. Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res Ther. 2010;12:R52. doi: 10.1186/ar2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogalska A, Engel WK, Askanas V. Increased BACE1 mRNA and noncoding BACE1-antisense transcript in sporadic inclusion-body myositis muscle fibers—possibly caused by endoplasmic reticulum stress. Neurosci Lett. 2010;474:140–3. doi: 10.1016/j.neulet.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol. 2004;164:1–7. doi: 10.1016/S0002-9440(10)63089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CK, Knopp P, Moncrieffe H, Singh B, Shah S, Nagaraju K, et al. Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis: implications for juvenile myositis. Am J Pathol. 2009;175:1030–40. doi: 10.2353/ajpath.2009.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A. 2000;97:9209–14. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–86. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 8.Blagoev B, Kratchmarova I, Ong SE, Nielsen M, Foster LJ, Mann M. A proteomics strategy to elucidate functional protein-protein interactions applied to EGF signaling. Nat Biotechnol. 2003;21:315–8. doi: 10.1038/nbt790. [DOI] [PubMed] [Google Scholar]
- 9.Kruger M, Moser M, Ussar S, Thievessen I, Luber CA, Forner F, et al. SILAC mouse for quantitative proteomics uncovers kindlin-3 as an essential factor for red blood cell function. Cell. 2008;134:353–64. doi: 10.1016/j.cell.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Medikayala S, Defour A, Rayavarapu S, Brown KJ, Hathout Y, et al. Use of quantitative membrane proteomics identifies a novel role of mitochondria in healing injured muscles. J Biol Chem. 2012;287:30455–67. doi: 10.1074/jbc.M112.354415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rayavarapu S, Coley W, Cakir E, Jahnke V, Takeda S, Aoki Y, et al. Identification of disease specific pathways using in vivo SILAC proteomics in dystrophin deficient mdx mouse. Mol Cell Proteomics. 2013;12:1061–73. doi: 10.1074/mcp.M112.023127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–80. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang A, Williamson CD, Wong DS, Bullough MD, Brown KJ, Hathout Y, et al. Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics. 2011;10:M111.009936. doi: 10.1074/mcp.M111.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alger HM, Rayavarapu S, Nagaraju K. Measurement of activation of the endoplasmic reticulum stress response in autoimmune myositis. Methods Enzymol. 2011;489:207–25. doi: 10.1016/B978-0-12-385116-1.00012-1. [DOI] [PubMed] [Google Scholar]
- 15.Alger HM, Raben N, Pistilli E, Francia DL, Rawat R, Getnet D, et al. The role of TRAIL in mediating autophagy in myositis skeletal muscle: a potential nonimmune mechanism of muscle damage. Arthritis Rheum. 2011;63:3448–57. doi: 10.1002/art.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahnke VE, van der Meulen JH, Johnston HK, Ghimbovschi S, Partridge T, Hoffman EP, et al. Metabolic remodeling agents show beneficial effects in the dystrophin-deficient mdx mouse model. Skelet Muscle. 2012;2:16. doi: 10.1186/2044-5040-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayavarapu S, van der Meulen JH, Gordish-Dressman H, Hoffman EP, Nagaraju K, Knoblach SM. Characterization of dysferlin deficient SJL/J mice to assess preclinical drug efficacy: fasudil exacerbates muscle disease phenotype. PLoS One. 2010;5:e12981. doi: 10.1371/journal.pone.0012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coley W, Rayavarapu S, Pandey GS, Sabina RL, van der Meulen JH, Ampong B, et al. The molecular basis of skeletal muscle weakness in a mouse model of inflammatory myopathy. Arthritis Rheum. 2012;64:3750–9. doi: 10.1002/art.34625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nader GA, Dastmalchi M, Alexanderson H, Grundtman C, Gernapudi R, Esbjornsson M, et al. A longitudinal, integrated, clinical, histological and mRNA profiling study of resistance exercise in myositis. Mol Med. 2010;16:455–64. doi: 10.2119/molmed.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Yin C, Okamoto H, Jaffe H, Oldfield EH, Zhuang Z, et al. proteomic analysis of inclusion body myositis. J Neuropathol Exp Neurol. 2006;65:826–33. doi: 10.1097/01.jnen.0000228204.19915.69. [DOI] [PubMed] [Google Scholar]
- 22.Salajegheh M, Kong SW, Pinkus JL, Walsh RJ, Liao A, Nazareno R, et al. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann Neurol. 2010;67:53–63. doi: 10.1002/ana.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sela I, Milman Krentsis I, Shlomai Z, Sadeh M, Dabby R, Argov Z, et al. The proteomic profile of hereditary inclusion body myopathy. PLoS One. 2011;6:e16334. doi: 10.1371/journal.pone.0016334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker KC, Kong SW, Walsh RJ, Salajegheh M, Moghadaszadeh B, Amato AA, et al. Fast-twitch sarcomeric and glycolytic enzyme protein loss in inclusion body myositis. Muscle Nerve. 2009;39:739–53. doi: 10.1002/mus.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passadore I, Iadarola P, di Poto C, Giuliano S, Montecucco C, Cavagna L, et al. 2-DE and LC-MS/MS for a comparative proteomic analysis of BALf from subjects with different subsets of inflammatory myopathies. J Proteome Res. 2009;8:2331–40. doi: 10.1021/pr800943t. [DOI] [PubMed] [Google Scholar]
- 26.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, et al. Ubiquitin hydrolase Uch-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–88. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Nogalska A, Engel WK, McFerrin J, Kokame K, Komano H, Askanas V. Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers. J Neurochem. 2006;96:1491–9. doi: 10.1111/j.1471-4159.2006.03668.x. [DOI] [PubMed] [Google Scholar]
- 28.Henriques-Pons A, Nagaraju K. Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis. Curr Opin Rheumatol. 2009;21:581–7. doi: 10.1097/BOR.0b013e3283319265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsten K, de Vrij FM, Verhoef LG, Fischer DF, van Leeuwen FW, Hol EM, et al. Mutant ubiquitin found in neurodegenerative disorders is a ubiquitin fusion degradation substrate that blocks proteasomal degradation. J Cell Biol. 2002;157:417–27. doi: 10.1083/jcb.200111034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 31.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–46. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 32.Medina R, Wing SS, Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995;307:631–7. doi: 10.1042/bj3070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton TM, Gannavarapu A, Blaney SM, D'Argenio DZ, Plon SE, Berg SL. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother Pharmacol. 2006;58:13–23. doi: 10.1007/s00280-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 34.Sayers TJ, Brooks AD, Koh CY, Ma W, Seki N, Raziuddin A, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–10. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 35.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–55. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 36.Caron AZ, Haroun S, Leblanc E, Trensz F, Guindi C, Amrani A, et al. The proteasome inhibitor MG132 reduces immobilization-induced skeletal muscle atrophy in mice. BMC Musculoskelet Disord. 2011;12:185. doi: 10.1186/1471-2474-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, et al. Role of the proteasome and NF-κB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–6. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amiri KI, Horton LW, LaFleur BJ, Sosman JA, Richmond A. Augmenting chemosensitivity of malignant melanoma tumors via proteasome inhibition: implication for bortezomib (VELCADE, PS-341) as a therapeutic agent for malignant melanoma. Cancer Res. 2004;64:4912–8. doi: 10.1158/0008-5472.CAN-04-0673. [DOI] [PubMed] [Google Scholar]
- 39.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-κB, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–28. [PubMed] [Google Scholar]
- 40.Thaloor D, Miller KJ, Gephart J, Mitchell PO, Pavlath GK. Systemic administration of the NF-κB inhibitor curcumin stimulates muscle regeneration after traumatic injury. Am J Physiol. 1999;277:C320–9. doi: 10.1152/ajpcell.1999.277.2.C320. [DOI] [PubMed] [Google Scholar]
- 41.Jobin C, Hellerbrand C, Licato LL, Brenner DA, Sartor RB. Mediation by NF-κB of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779–87. doi: 10.1136/gut.42.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial–cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186:517–27. doi: 10.1084/jem.186.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.