Abstract
Background
Current state of knowledge suggests that disruption of neuronal information integration may be a unitary mechanism of anesthetic-induced unconsciousness. A neural system central for information integration is the thalamocortical system whose specific and nonspecific divisions may play the roles for representing and integrating information; respectively. How anesthetics affect the function of these systems individually is not completely understood. We studied the effect of propofol on thalamocortical functional connectivity in the specific and nonspecific systems using functional magnetic resonance imaging.
Methods
Eight healthy volunteers were instructed to listen to and encode 40 English words during wakeful baseline, light sedation, deep sedation, and recovery in the scanner. Functional connectivity was determined as the temporal correlation of blood oxygen level-dependent signals with seed regions defined within the specific and nonspecific thalamic nuclei.
Results
Thalamocortical connectivity at baseline was dominantly medial and bilateral frontal and temporal for the specific system and medial frontal and medial parietal for the nonspecific system. During deep sedation, propofol reduced functional connectivity by 43% (specific) and 79% (nonspecific), a significantly greater reduction of connections in the nonspecific than in the specific system and in the left hemisphere than in the right. Upon regaining consciousness, functional connectivity increased by 58% (specific) and 123% (nonspecific) during recovery, exceeding their values at baseline.
Conclusions
Propofol conferred differential changes in functional connectivity of the specific and nonspecific thalamocortical systems. The changes in nonspecific thalamocortical connectivity may correlate with loss and return of consciousness.
Introduction
How general anesthetics suppress consciousness is not fully understood. Recent conceptual approaches focus on the effect of anesthetics on integrated information as defining property of human consciousness.1,2 According to a theory, the brain’s capacity for information integration3,4 is determined by the repertoire of its states (information) and the causal interaction of its elements (integration). A reduction of either component, for example due to anesthesia, could result in a reduction of the level of consciousness. Accordingly, general anesthetics exert a preferential effect on integrative processes of the brain, as opposed to simply a block of sensory transmission or sensory reactivity during reduced consciousness.1 As we have proposed, under general anesthesia, information in the brain may be "received but not perceived.”5
It has also been suggested that the thalamocortical system plays a central role in information integration in the brain.6–8 In particular, the two major divisions of the thalamus, the specific relay nuclei and the more diffusely projecting “nonspecific” nuclei, may collaborate to accomplish this task;9–11 with the specific system responsible for the transmission and encoding of sensory and motor information and the nonspecific system engaged in the control of cortical arousal and temporal conjunction of information across distributed cortical areas.11 These considerations emphasize the importance of the nonspecific thalamocortical system in information integration, and raise the possibility that its dysfunction may be a primary, and possibly unitary, mechanism of anesthetic-induced unconsciousness.
Previous studies implied a critical involvement of the nonspecific (intralaminar) thalamic nuclei in the loss and recovery of consciousness in patients in vegetative12 or minimally conscious state,13 and in anesthetized animals.14 One of these studies also emphasized the importance of intralaminar thalamocortical functional connectivity in the recovery of consciousness.12 However, the role of nonspecific thalamic nuclei under general anesthesia in humans has not been investigated. Therefore, the goal of this work was to examine the effect of propofol sedation on functional connectivity of the specific and nonspecific thalamocortical systems.
We chose to investigate thalamocortical functional connectivity using blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) in healthy human volunteers. Functional connectivity has been defined as the temporal correlation of BOLD signals among spatially remote regions of the brain,15 and is currently a favored approach of neuroimaging. To learn about functional connectivity changes upon loss of consciousness, we targeted an anesthetic depth at which response to verbal commands and auditory verbal memory were suppressed, but auditory cortical sensory reactivity was preserved. We hypothesized that, under this condition, nonspecific thalamocortical connectivity would be disrupted more than specific thalamocortical connectivity, consistent with a failure of cortical integration but not sensory information transmission. Functional connectivity was evaluated in four conditions (wakeful baseline, light sedation, deep sedation, and recovery) while volunteers listened to word lists that were later used to assess their implicit memory. Seed regions used for functional connectivity analysis were manually defined within the specific (medial dorsal, ventral lateral, ventral posterior, etc.) and nonspecific (centromedian and parafascicular) thalamic nuclei. We show that propofol sedation indeed produced distinct changes in the functional connectivity of the two divisions of the thalamocortical system, consistent with their postulated roles in information and integration in specific states of human consciousness.
Materials and Methods
Study Participants
Eight volunteers of both genders (four men and four women; ages 24 to 42; body mass index <25) provided written informed consent to participate in this study. Experimental protocols were approved by the Institutional Review Board of the Medical College of Wisconsin (Milwaukee, WI). The study participants were native English speakers from Medical College of Wisconsin communities, free of drug administration and with no history of neurological or psychiatric conditions or structural brain abnormalities.
Auditory Verbal Memory Task and Propofol Administration
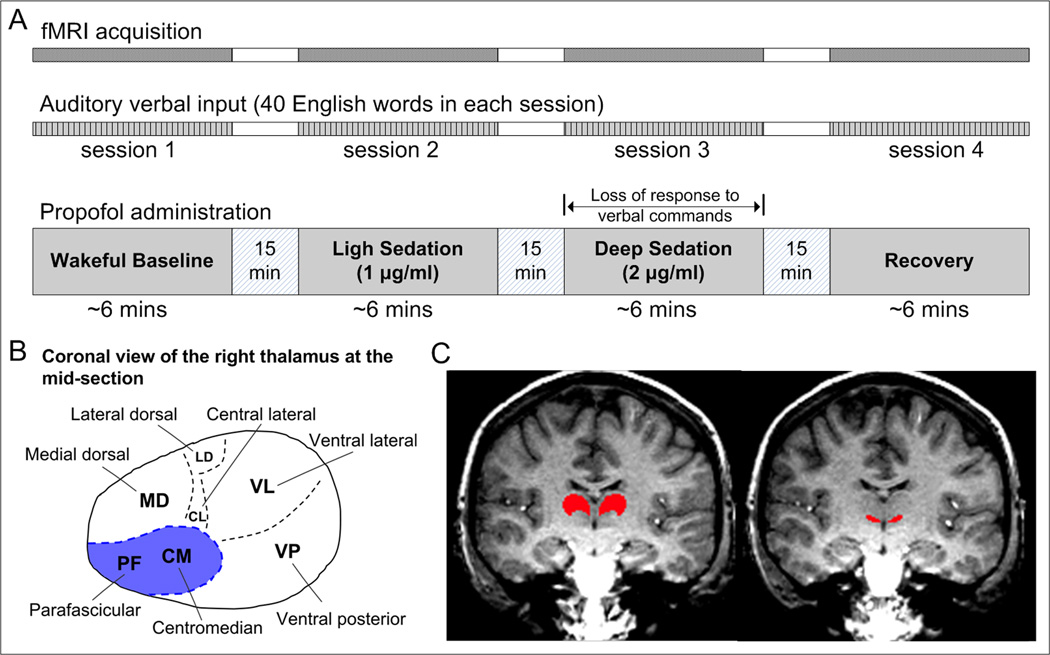
Lying in the scanner, all participants were instructed to listen to and encode a distinct set of 40 high-frequency English words (nouns) presented during each of the four experimental sessions in four different states of consciousness: wakeful baseline, light sedation, deep sedation, and recovery (Fig. 1A). The rate of word presentation was about seven words per minute with a random inter-stimulus interval during each of the approximately 6-min scanning sessions. BOLD time course data were obtained from the entire duration of word presentation without interruption.16 Participants were informed that their recall/recognition performance of words heard during each of these experimental sessions would be assessed after scanning. The experimental sessions were separated by approximately 15 minutes for experimental preparations.
Figure 1.
Experimental paradigm and the seeding of the specific and nonspecific thalamic nuclei for functional connectivity analysis. (A) Study participants underwent fMRI scans while listening to and encoding a distinct set of English words presented during each of the four experimental sessions in wakeful baseline, light sedation, deep sedation, and recovery. Propofol was administrated by computer-controlled intravenous infusion to target plasma concentrations as indicated. (B) The distribution of the thalamic nuclei in a coronal plane of the right thalamus. Two intralaminar nuclei (centromedian and parafascicular) indicated by the shaded area were used as a seed to calculate nonspecific thalamocortical connectivity. The rest of the thalamus was used as a seed for calculating specific thalamocortical connectivity. (C) Examples of the specific and nonspecific thalamic seeds (red area) overlaid the anatomical image of a coronal slice in one subject.
The anesthetic agent, propofol, was administered by a bolus followed by a target-controlled continuous infusion (STANPUMP).17 For light sedation, we targeted plasma concentration of 1 µg/ml. For deep sedation, the target was 2 µg/ml plasma concentration. The latter dose was chosen to achieve the desired endpoints of unresponsiveness to verbal commands and global loss of memory.18 A behavioral assessment of the level of consciousness was performed before each fMRI scan. Study participants were asked to respond to questions like “how are doing?,” “take a deep breath,” and “can you squeeze my hand?” by the anesthesiologist through a speaker (the one used in the task or at the bedside of the scanner between recording sessions). During light sedation, participants in general had lethargic responses to the questions. During deep sedation, participants showed no response to verbal commands. Once a desired sedative state was achieved, the next fMRI scan was initiated. During the scan, the sedation level was maintained by computer-controlled infusion with the preset plasma concentration. Immediately after the scanning of the last sedated state, the administration of propofol was stopped. Upon the confirmation that participants had recovered responsiveness to verbal commands, the last scanning session (defined as “recovery”) was initiated. Every participant had two intravenous catheters, one for propofol administration and another for the withdrawal of blood samples for measuring propofol plasma concentration. However, due to a problem with red blood cell lysis, the plasma propofol concentrations could not be determined in this study. The order of light and deep sedation was counterbalanced in participants, with four participants receiving the low-dose before a high-dose, and the other four receiving them in a reversed order. Standard American Society of Anesthesiologists monitoring was performed during the experiment, including electrocardiogram, noninvasive blood pressure cuff, pulse oximetry, and end-tidal carbon dioxide gas analysis.
The auditory verbal material was presented using a headphone set (Koss Corporation, Milwaukee, WI) designed to work in the magnetic resonance scanner environment. The word lists were matched for the maximum number of letters, frequency of usage in English, concreteness, and imageability (Paivio, Yuille, and Madigan norms for 925 nouns),19 and were presented in a counterbalanced order across participants. Approximately 20 to 30 minutes after the completion of all the experiments (after study participants had been taken out of the scanner), all participants completed a free-recall test followed by a forced-choice recognition test. The time separation between the memory tests and the last scan was intended to neutralize the primacy and recency effects that occur when the recognition memory test is administered immediately following the stimulus presentations. In the forced-choice recognition test, participants were presented auditorily with 320 words, of which 160 words were what they had heard during the experiment, and the other 160 words were foils or distractors. Participants were required to press a button if they thought they heard the word previously and another button if the word was new. Participants were instructed to make a decision regarding every presented word as quickly as possible. Each participant’s residual memory to words heard during each of the four experimental sessions was assessed by a few performance indices: the percentage of recalled words, the recognition ratio vs. chance, and the discriminability index (d’). Of these, the value of d’, computed from the hit (correct recognition) rate and false-alarm rate, provides a criterion-independent measure of the internal response of participants (i.e., regardless of how conservative or liberal participants are in making decisions).20 A d’ equal to zero indicates same hit and false alarm rates, and a d’ value significantly greater than zero indicates a higher hit than a false-alarm rate (a d’ value of 3 represents minimal overlap between the probability of the occurrence of hit and false alarm).
Magnetic Resonance Imaging Acquisition
Imaging acquisition was performed using a 1.5 Tesla GE Signa scanner (General Electric Medical Systems, Milwaukee, WI) with a locally designed gradient and radio frequency coil. Potential head movements were minimized using a bite-bar system developed at the Medical College of Wisconsin. Functional echo-planar images were obtained using whole-brain imaging in the sagittal plane during each task session (repetition time, 2000 msec; echo time, 40 ms; thickness, 6 mm; in-plane resolution, 3.75 × 3.75 mm; 22 slices; flip angle, 90 degrees; field of view, 24 cm; matrix size, 64*64). High-resolution spoiled-gradient-recalled anatomical images were always acquired after the third experimental session for each participant (repetition time, 24 ms; echo time, 5ms; slice thickness, 1.2 mm; flip angle, 40°; field of view, 24 cm; matrix size, 256*128).
Defining the Specific and Nonspecific Thalamic Seed Regions
The specific and nonspecific thalamic nuclei to be used as seeds for functional connectivity analysis were determined in the coronal plane of each individual's high-resolution spoiled-gradient-recalled images after transformation into the standard Talairach space. Specifically, the nonspecific seed mainly consisted of the intralaminar nuclei, including the centromedian and parafascicular thalamic nuclei located at the ventromedial corners of the left and right thalami (Fig. 1B). Anatomical references that could be used to enhance the accuracy of the definition of the nonspecific thalamic seed included the lateral maximum stretch point of the third ventricle, red nucleus, and the interthalamic adhesion. These referential structures were identifiable in the high-resolution anatomical images of each participant by properly adjusting the brightness and contrast of image pixels. The remaining parts of the thalamus were used as an aggregate for seeding the specific thalamic connections (Fig. 1C).
Data Preprocessing
Imaging data analysis was conducted using the software packages Analysis of Functional NeuroImages (AFNI, Bethesda, MD) and Matlab (The MathWorks, Natick, MA). The high-resolution anatomical images were first manually transformed into the standard Talairach space, followed by co-registering the functional data to the Talairach space in 2-mm cubic voxels (adwarp in AFNI). Subsequent data preprocessing included despiking, detrending (3dDetrend in AFNI, using the Legendre polynomials with an order of 3), and motion correction (3dvolreg in AFNI, obtaining three translational and three rotational parameters for each image). The first four points of the time series of each voxel were discarded to reduce the transient effects. To minimize contaminating signals from the white matter and the cerebrospinal fluid, we extracted the average BOLD signal from these areas, defined manually through each individual's anatomical images. We then constructed eight regressors using the signals corresponding to the six motion parameters (obtained during the volume registration), white matter, and cerebrospinal fluid signals for the subsequent analysis.
Thalamocortical Connectivity Analysis
After data preprocessing, the event-related fMRI time series of all task sessions were analyzed by general linear regression (3dDeconvolve in AFNI). The regression analysis takes into consideration the eight regressors defined to remove the potential noise artifacts from the motion, white matter, and cerebrospinal fluid. The residual signals were considered representative of the task-induced BOLD responses with the potential contamination minimized. In the next step, the averaged voxel time courses of the predefined specific and nonspecific thalamic seed regions were used separately to perform voxel-wise Pearson cross-correlation (3dfim+ in AFNI) of the whole brain. The Fisher transformation, m = 0.5*ln(1 + r)/(1− r), was applied to the obtained correlation coefficients (r) to normalize the output. Spatial smoothing of the m-values was performed using a 3.5-mm full-width half-maximum Gaussian kernel filter in order to compensate for intersubject variability.
Regional distribution of thalamocortical functional connectivity
To evaluate the distribution of the specific and nonspecific thalamocortical functional connections across anatomically defined brain regions, we multiplied the obtained functional connectivity maps with a brain mask provided in AFNI (tt_n27_ez_ml.tlrc). The mask provides a reference template that partitions the whole brain into 116 anatomical regions in a voxel-wise manner in the Talairach space.21 The corresponding voxel count of each region is taken as a quantitative measure of regional thalamocortical functional connections in each state of consciousness. We defined two indices, IA and IB, to represent the normalized changes in the voxel count of thalamocortical functional connectivity by contrasting the wakeful states (baseline and recovery) to deep sedation (E. 1) and the recovery to wakeful baseline (E. 2), respectively:
| (E. 1) |
| (E. 2) |
where W, S, and R represent the region-specific voxel count of specific or nonspecific thalamocortical functional connections in wakeful baseline, deep sedation, and recovery, respectively.
Exclusion of Imaging Data at Low Propofol Dose from Analysis
From the group analysis of the hemodynamic activation of the low propofol dose, we could identify a distinct pattern of cerebral hemodynamic responses, particularly in the dorsal medial prefrontal cortex.16 This was presumably associated with propofol-induced excitation, which is common at low anesthetic dose.22 Behavioral observations during experiments and post-scan memory tests corroborated a certain degree of excitation during the low propofol dose. Because of the difficulty in interpreting the state of consciousness and the relatively large inter-individual variability at this dose, the imaging data obtained at the low propofol dose were not fully analyzed. For referential information, we provided the thalamocortical connectivity maps obtained during the low propofol dose in Supplemental Digital Content 1, Figure S1. These results should be interpreted with caution in the above-mentioned context.
Statistical Analysis
The group effects of connectivity maps were evaluated by one-sample t-tests followed by transformation to z-scores. We report results considered significant at p<0.05 after correction for multiple comparisons (AlphaSim in AFNI, a minimum cluster thresholding of 179 voxels of 2-mm cubic in the Talairach space). The program AlphaSim considers both voxel probability thresholding and minimum cluster-size thresholding to estimate the probability of false-positive detection from the frequency count of cluster sizes in each image. The underlying principle of this method is that true regions of activation or connection will tend to occur over contiguous voxels, whereas noise has much less of a tendency to form clusters. The minimum cluster size threshold (179) was obtained based on a combined mask of the specific and nonspecific thalamic functional connections in normal, wakeful subjects in resting state, which we reported previously.23 To provide a level of inference about the results, we performed a leave-one-out test on the participants’ connectivity analysis in each condition. The computation generates a collection of maps corresponding to eight overlapping subsets of participants and performs statistical comparisons (paired t-test, group dependent) between the maps in the different conditions.
Results
Propofol-Induced Changes of Cognitive Performance and BOLD Activation
All participants produced purposeful responses to verbal commands during wakeful baseline, light sedation, and recovery. All responses were absent during deep sedation. The post-scan memory tests showed that participants could freely recall a significant percentage of words heard in the scanner at baseline (9% ± 0.3), light sedation (10% ± 0.5), and recovery (14% ± 0.35), but not during deep sedation (2% ± 0.15, not significant). The auditory forced-choice recognition tests performed outside the scanner showed the same trend. Participants were able to distinguish target words from foils heard at baseline (d', 0.9 ± 0.25), light sedation (d', 0.6 ± 0.24), and recovery (d', 1.5 ± 0.42), but not the words heard during deep sedation (d', 0.2 ± 0.15). The results indicate that participants at least partially maintained the capability of verbal auditory processing and memory in light sedation, whereas these functionalities were suppressed in deep sedation. For further details regarding the task and performance results, we refer the reader to our former publication.16
In the baseline condition, auditory verbal stimuli induced significant, mainly left-lateralized BOLD activations in multiple temporal and frontal regions and prominent negative BOLD responses in the posterior cingulate cortex and the precuneus. During deep sedation, most of the activations were suppressed, except in a few brain areas in the superior temporal gyrus, centered on the primary auditory cortex. In addition, the negative BOLD effects expanded in the frontal, temporal, and occipital lobes. After the participants regained behavioral responsiveness, brain activation maps were restored to a pattern similar to those observed at baseline.16
Specific and Nonspecific Thalamocortical Functional Connectivity at Wakeful Baseline
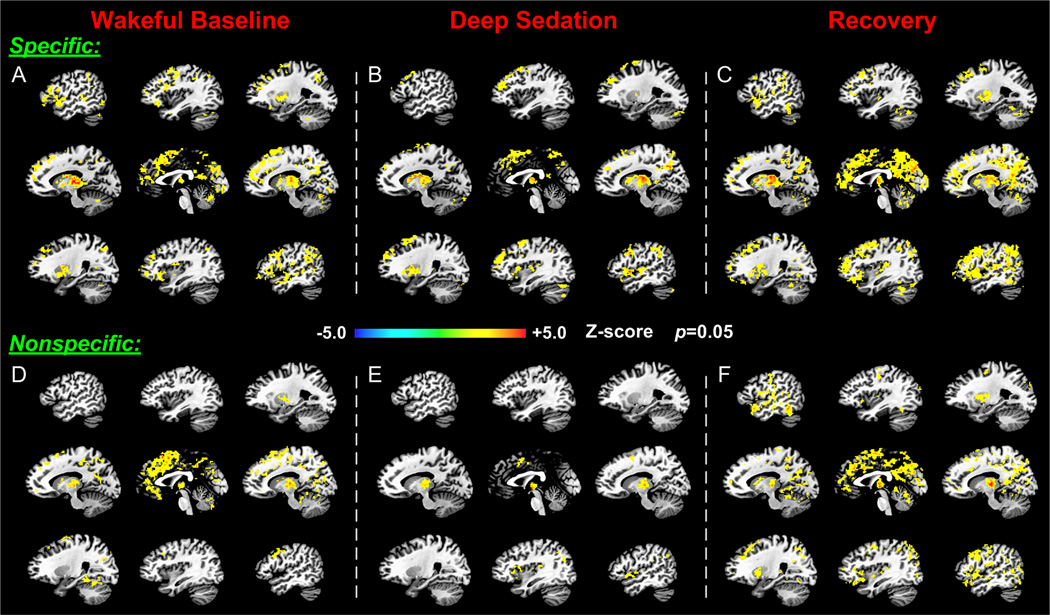
Thalamocortical functional connectivities showed distinct spatial distributions within the two thalamic divisions (Fig. 2). In the baseline condition, specific and nonspecific connections were widely distributed, forming large clusters particularly in the frontal lobe (including the prefrontal and cingulate cortices) and the parietal lobe (Fig. 2, A and D). In both thalamocortical divisions, the frontal cluster extended from the ventral medial frontal cortex up to the posterior segment of the dorsal medial frontal cortex. The functional connectivity of the dorsal and medial frontal cortices was more prominent in the nonspecific than in the specific system. In contrast, functional connectivity of the specific thalamus in the bilateral frontal and temporal cortices, especially in the left-lateralized inferior frontal gyrus and superior temporal gyrus was more prevalent than those associated with the nonspecific thalamus. The specific thalamocortical connections also formed pronounced clusters in the primary visual cortex, whereas the nonspecific connections did not. Functional connections in the areas of the parietal default mode network (i.e., the precuneus, posterior cingulate cortex, and retrosplenial cortex) were expressed in both thalamocortical divisions. (See also Supplemental Digital Content 1, Figure S2, which describes the full correlation maps obtained by averaging across participants.)
Figure 2.
Differential modification of specific and nonspecific thalamocortical functional connectivity across the states of consciousness. The upper three panels (A, B, and C) and the lower three panels (D, E, and F) illustrate respectively the significant specific and nonspecific thalamic connections derived from one-sample t-tests in the states of wakeful baseline, deep sedation, and recovery. Note the different distributions of specific and nonspecific thalamocortical connectivity at baseline and the greater reduction in nonspecific vs. specific connectivity in deep sedation.
Specific and Nonspecific Thalamocortical Functional Connectivity in Deep Sedation
The two thalamocortical systems exhibited substantially different changes in functional connectivity in deep sedation as compared to the wakeful baseline (Fig. 2, B and E). The extent of connections as measured by the number of significantly correlated voxels was reduced in both the specific and the nonspecific systems across the whole brain. However, the nonspecific connections were substantially more suppressed, leaving only a few scattered spots of connectivity in the middle cingulate and premotor areas. Changes in the specific thalamic connectivity were more modest both in terms of the brain regions involved and of the overall voxel count. During recovery, the specific and nonspecific connections were restored to a similar spatial distribution to that seen in the baseline condition. Nevertheless, the extent of connectivity in both systems was generally increased compared to baseline (Fig. 2, C and F).
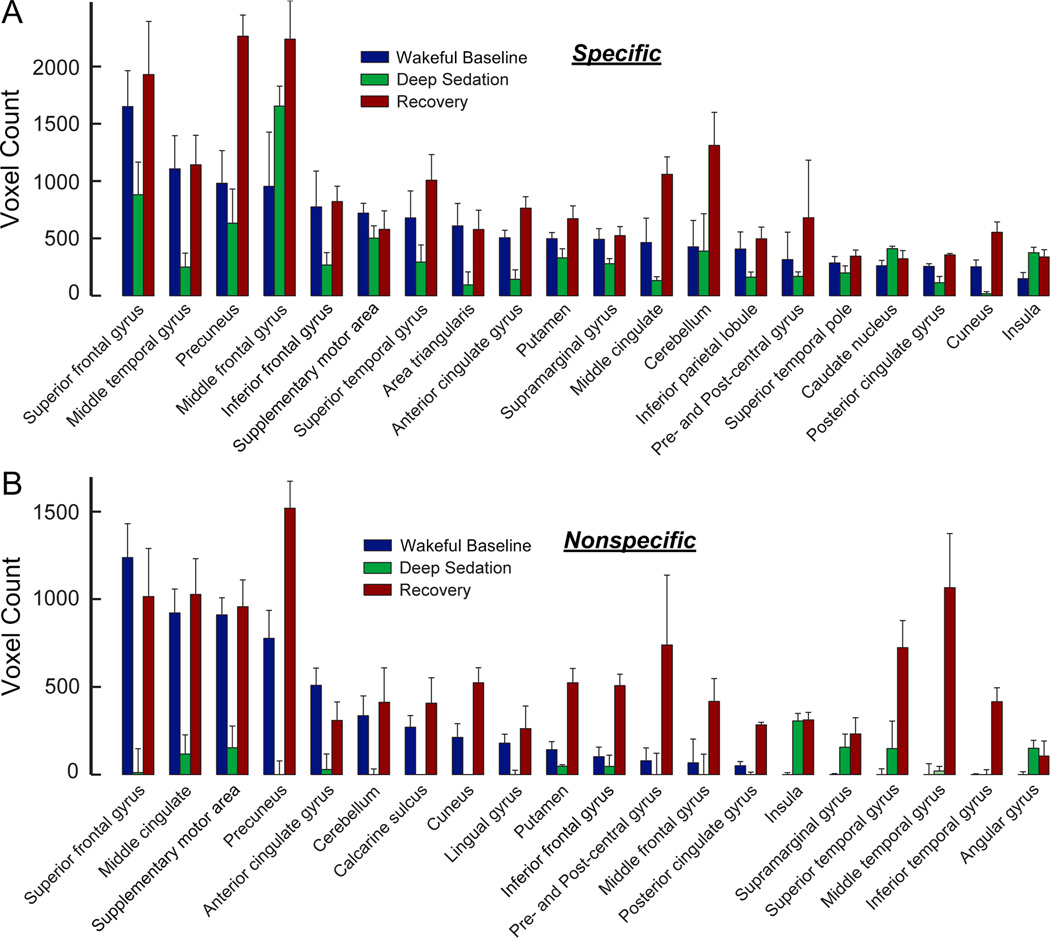
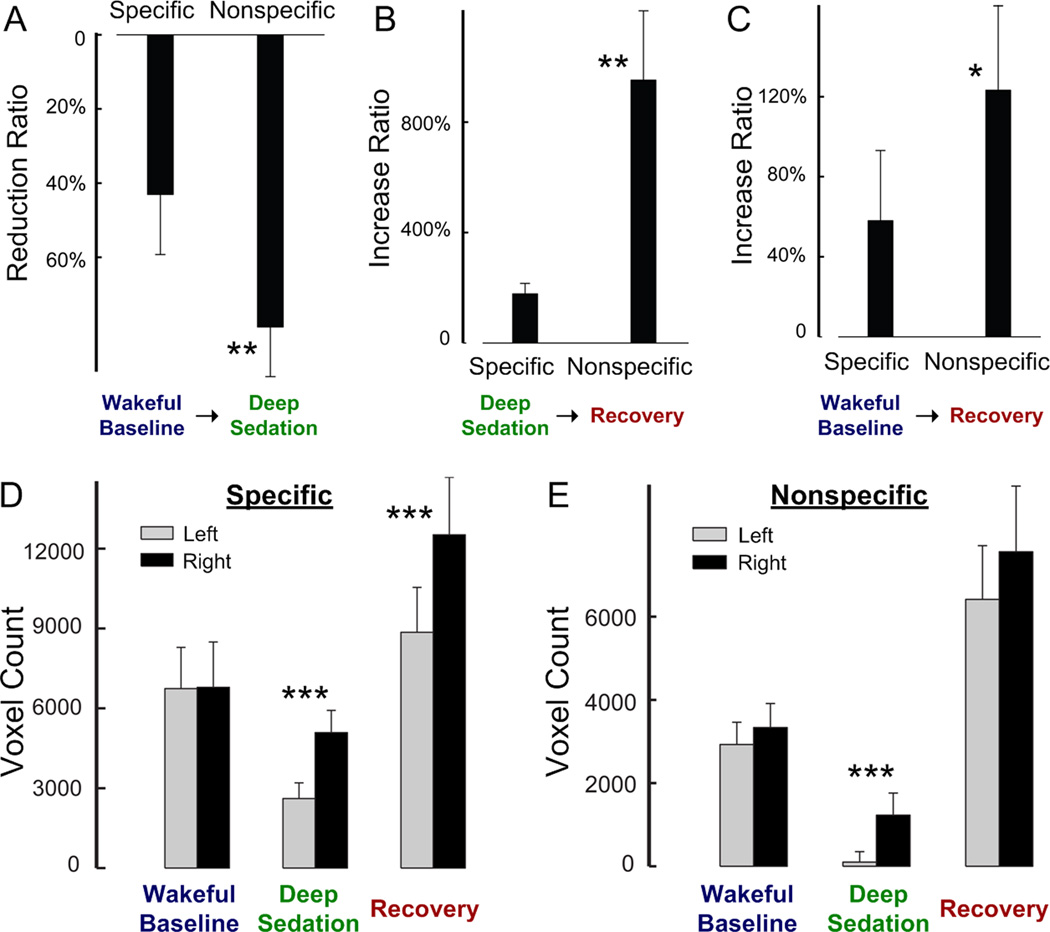
The region-specific changes of functional connectivity of the two thalamic divisions across wakeful baseline, deep sedation, and recovery were quantified by calculating the number of significantly correlated voxels for 20 anatomical regions that covered most of the major structures of the brain (Fig. 3). The results confirmed that during deep sedation, specific connectivity was moderately (Fig. 3A) and nonspecific connectivity was substantially reduced (Fig. 3B). Connectivity was then restored in recovery. Combining the voxel count data from all regions revealed an overall reduction from wakeful baseline to deep sedation that was significantly larger (p < 0.01, group comparison through a leave-one-out test) in the nonspecific system (79±13%) than in the specific system (43±16%) (Fig. 4A). Likewise, the overall increases in connectivity from deep sedation to recovery and from baseline to recovery were both significantly larger (p < 0.01 and p < 0.05) in the nonspecific (952±251% and 123±42%, respectively) than in the specific system (178±38% and 58±35%, respectively) (Fig. 4, B and C).
Figure 3.
Regional distributions of specific (A) and nonspecific (B) thalamocortical functional connectivity as measured by voxel count in Figure 2. Twenty anatomical regions that primarily account for the distribution of functional connections across the states of wakeful baseline, deep sedation, and recovery were selected and displayed from the left to right in a descending order according to the voxel count of wakeful baseline.
Figure 4.
Comparison of the changes of the specific and nonspecific thalamocortical functional connectivities across the states of consciousness. (A) The reduction ratio wakeful baseline to deep sedation. (B) The increase ratio from deep sedation to recovery. (C) The increase ratio from wakeful baseline to recovery. (D) Comparison of the extent of functional connectivity between the left and right hemispheres in the three states of consciousness for the specific system. (E) The same for the nonspecific system. Note the hemispheric asymmetry of connectivity in deep sedation. *: p < 0.05, **: p < 0.01, ***: p <0.001, paired t-test via a leave-one-out test analysis.
We also examined the inter-hemispheric asymmetry of thalamic functional connections (Fig. 4, D and E). The baseline distribution of the specific and nonspecific thalamocortical connections extended over nearly equal number of voxels in the left and right hemispheres. However, in deep sedation, the extent of connectivity was significantly less (p < 0.001) in the left hemisphere than in the right hemisphere at 50% in the specific system and 8% in the nonspecific system. Upon recovery, the inter-hemispheric balance of connectivity was restored in the nonspecific thalamocortical system, but not in the specific system. These results suggest that the inter- hemispheric balance of nonspecific thalamocortical connectivity correlates with the loss and return of consciousness better than that of specific thalamocortical connectivity.
Region-specific Quantification of Sedation vs. Recovery
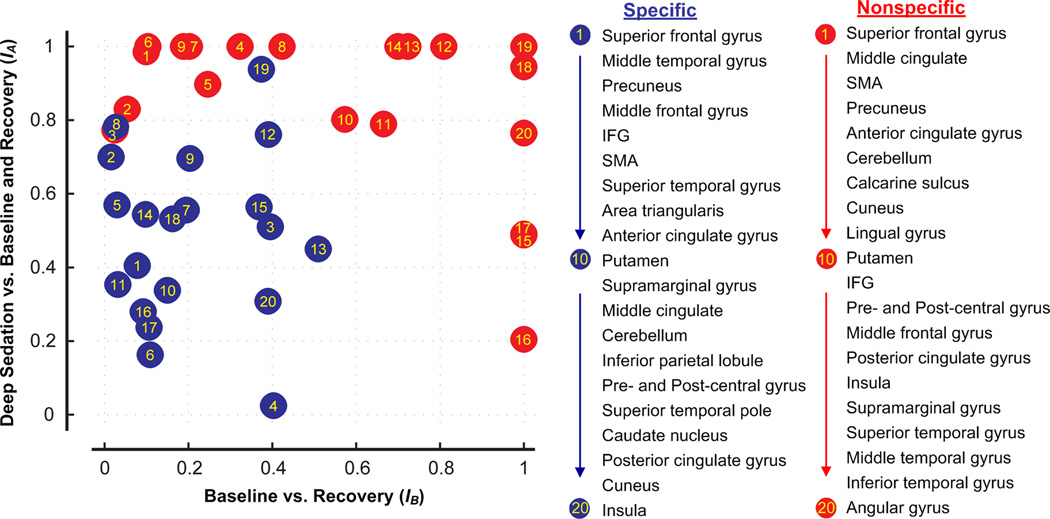
For further illustration of the region-specific changes of functional connectivity in the two thalamocortical systems, we calculated the two indices, IA (E. 1) and IB (E. 2). For each selected brain region, IA expresses the reduction in the count of connected voxels during deep sedation relative to those of wakeful baseline and recovery. IB expresses the relative difference in connectivity between recovery and wakeful baseline. The values of both indices range from 0 to 1, indicating a magnitude of change from small to large, respectively. Brain regions associated with the specific and nonspecific systems are then identified by the two indices as two dimensions in a scatter plot (Fig. 5). The plot shows that brain regions in the specific and nonspecific systems are strongly segregated, expressing a difference in their relative change in connectivity during sedation and recovery. Specifically, the brain regions involved in the nonspecific thalamic functional connectivity undergo a greater decrease during sedation and a greater increase during recovery than the brain regions involved in specific connectivity.
Figure 5.
Regional association of thalamocortical functional connectivity with changes of the state of consciousness. Indices IA (E.1)and IB (E. 2) quantify the reduction in connectivity during sedation relative to wakeful baseline and sedation (symmetric component of change) and the relative difference between in connectivity between recovery and wakeful baseline (asymmetric component of change). Note the clearly segregated distribution of brain regions with specific (blue) and nonspecific (red) thalamic connectivity, implying a qualitatively different behavior between the two systems. The changes in the connectivity of the nonspecific regions show a greater association with alterations of the state of consciousness than those of specific connectivity.
Finally, the issue was raised if the relatively large volume of the specific thalamic seed region rendered the specific system less sensitive to propofol than the nonspecific system that was based on a significantly smaller seed volume. To investigate this possibility, additional connectivity analysis was conducted using a reduced volume of the specific seed. Specifically, we subsampled the original specific seed at random so that the number of voxels contained was identical to that in the nonspecific seed for each participant. The results fully confirmed the findings previously obtained with the original specific seed (see Supplemental Digital Content 1, Figure S3, which describes the functional connectivity maps of the reduced specific seed).
Discussion
Current investigations into the neurophysiological mechanisms of consciousness and of general anesthesia mutually inform each other,1,24 with the neuronal effects of anesthetics considered in the light of the neural correlates of consciousness, and vice versa. In the framework of the information integration theory of human consciousness,3,4 we hypothesize that the two necessary properties of consciousness, information and integration, are sustained respectively by the specific and nonspecific divisions of the thalamocortical system10,11 and that anesthetics may exert a differential effect on these systems as they suppress consciousness. Our results show that using a level of propofol sedation characterized by preserved cortical sensory reactivity,16 the specific thalamocortical network is moderately affected, whereas the nonspecific thalamocortical network is severely suppressed and subsequently reactivated after recovery of consciousness.
There is substantial evidence for the role of the thalamocortical system in integrative information processing in the brain.6,8,11,25–27 The rich and reciprocal nature of thalamocortical interconnectivity involving differential thalamic divisions establishes oscillatory circuits across multiple cortical layers. Such organizational structures put the thalamocortical networks in a unique and crucial position for implementing integrative functionalities necessary for the development of conscious experience. Consistent with the neuroanatomical and neurofunctional features of the thalamus, converging evidence from brain lesion,28 anesthesia,27 electrophysiology,11 and stimulation13 studies suggests that the thalamocortical system is essential in the maintenance and modulation of the state of consciousness.29
Previous investigations into the mechanism of anesthetic-induced unconsciousness have examined the possible interruption of thalamocortical information transfer within the relay nuclei30,31 or the failure of nonspecific thalamocortical functional connections in enabling the conscious state.12,32–34 Nonspecific thalamocortical connections that originate from the matrix cells of the thalamus are particularly dense in the intralaminar nuclei and have been implicated in supporting conscious experience.11,32,33,35,36 An early publication37 that dates back to 1953 already indicated that electrical potentials in the central medial thalamus evoked by peripheral afferent stimulation in awake monkeys were blocked in anesthesia while laterally conducted impulses reached the sensory cortex unimpaired. Subsequent studies showed that a selective lesion in the medial/intralaminar thalamus invariably caused loss of consciousness.28,32,35 Pharmacological or electrical stimulations to certain intralaminar nuclei helped restore wakeful behavior in anesthetized animals14 and, in one instance, in a minimally conscious patient.13 An anesthetic effect on the nonspecific thalamus is suggested by a shift from γ-rhythms to frontal α-rhythms in the electroencephalogram by propofol. The 40 Hz γ-band synchrony of thalamocortical circuits has been proposed as a neural correlate of consciousness.11 In addition, anesthetics preferentially suppress feedback vs. feedforward signaling in cortical electrophysiological recordings.38,39 It has been proposed that feedforward pathways alone are insufficient for conscious perception that also requires reentrant top-down feedback that mediates conscious integration.38,40–42 Whether such selective changes in corticocortical interactions are a manifestation of direct cortical or thalamic anesthetic effects is not yet clear.
A general observation from most previous studies1,5,24 is that during anesthetic sedation, the reactivity primary sensory cortices, e.g., the visual43 and auditory,16,44,45 to external stimuli is preserved, while higher order integrative processing of the stimuli is suppressed. This is consistent with a differential effect of anesthetics on the known functional partitions of the thalamocortical system. That is, the specific thalamic system that encodes and relays sensory information is essentially preserved in anesthesia, whereas the nonspecific thalamic system that facilitates temporal conjunction, binding, and integration of information in the brain11 is consistently disrupted during suppressed consciousness.
Previous reports regarding the proposed role of thalamocortical connectivity in anesthetic suppression of consciousness have been conflicting. For example, White and Alkire26 and Boveroux et al.46 suggested a significant suppression of the thalamocortical connectivity, whereas Mhuircheartaigh et al.47 reported relatively preserved thalamocortical connectivity during propofol sedation. The lack of consensus may be related to the just demonstrated differential effect of anesthetic on specific and nonspecific thalamic networks. It is possible that further differentiation of thalamic nuclei may also be necessary. In the current study, only two nonspecific (intralaminar) thalamic nuclei were considered as a seed for connectivity analysis because they admitted a relatively clear identification from the anatomical images obtained at 1.5 Tesla. Also, the specific nuclei were lumped together with no further differentiation into sensory, motor and other nuclei. It is known that higher order thalamic relay nuclei may mediate cortico-cortical communication linking primary and higher order sensory cortex.48 Thus, a more refined differentiation of thalamocortical functional connectivity at higher field strength may bring further insight into the differential connectivity of individual thalamic nuclei in the future.
A notable result of our current study was that during deep sedation, thalamocortical functional connectivity increased in a number of brain regions. These regions included the middle frontal gyrus as part of the specific system (Fig. 3A) and the insula, superior temporal gyrus, and angular gyrus as part of the nonspecific system (Fig. 3B). The mechanism of these connectivity increases is currently unclear. One is tempted to speculate that it may result from the work of a compensating mechanism by which the brain strives to maintain its state of activity, perhaps, by activating alternative neuronal pathways in the face of the pharmacological challenge by propofol. Alternatively, it is possible that increased functional connectivity above the awake baseline may reflect undifferentiated, stereotypical correlations that do not represent meaningful communication. A better understanding of the neural correlates of BOLD functional connectivity will be necessary to determine which of these alternatives is correct.
Although we demonstrated statistically significant differences in the effect of propofol on the two thalamocortical systems, the degree of reduction in the specific and nonspecific thalamic connections necessary to diminish consciousness cannot be determined from the current study. Obtaining this information would require a dose-dependent study with anesthetic depths graded at a finer scale. In addition, one should consider other factors such as the experimental protocol (e.g., resting state46 vs. task47), the anesthetic drug (propofol vs. other agents26), the magnetic field strength of the scanner (affecting the signal-to-noise ratio), etc. Nevertheless, the current study highlights the differential effects of propofol on the two thalamic divisions at least at a deep sedative level.
Similar to all other fMRI studies, our results are constrained by the methodological limitation that they indirectly reflect the neuronal mass activity.49 In addition, the experimental protocol included a memory task and auditory stimulation that may have modified the connectivity patterns as compared to those observed in the task-free “resting state” condition. An assessment of functional connectivity during sensory activation is not without precedence. Previously, Mhuircheartaigh et al. (2010) examined functional connectivity from data obtained during auditory or somatosensory stimulation.47 We also showed connectivity results of the same type of analysis with task effect regressed out of the data.50 The patterns and changes of thalamocortical connectivity maps across the states of consciousness showed little difference as compared with the current results derived from data containing task effect.
Two additional observations in our results deserve comments. First, we saw a prominent left-lateralized suppression of thalamic functional connections in deep sedation, especially in the nonspecific system. This is consistent with the left-lateralized distribution of neural structures involved in verbal processing and memory.51,52 Second, the dramatic increase in the extent of functional connections in both thalamic divisions during recovery relative to the wakeful baseline was unexpected. One would have anticipated an incomplete recovery within the time frame of the study due to a lingering drug effect, seen as emergence delirium. Nevertheless, our results are confirmed by recent studies. For example, the strength of corticocortical connectivity derived from electroencephalogram recordings showed a precipitous increase upon the regaining of consciousness from anesthesia.53 Likewise, brain activity in the anterior cingulate cortex revealed a significant anesthetic-independent increase at the emergence of consciousness as characterized by positron emission tomography imaging.54,55 While the cause for these large increases in connectivity is unclear, they may indicate the presence of a nonlinear process in regaining consciousness.56 They may also be related to the phenomenon of hysteresis commonly observed in general anesthesia. Hysteresis describes the existence of differential characteristic paths of anesthetic-modulated transitions toward and away from unconsciousness. It implies a more stringent energy barrier for regaining consciousness from the anesthetized state, as opposed to losing it from wakeful baseline. A recent study shows that hysteresis cannot be fully explained by pharmacokinetics, as it reflects an inherent property of the central nervous system to resist transitions in the state of arousal, recently coined as “neural inertia.”57 It is therefore conceivable that the restoration of consciousness in recovery requires more extensive neural processing than its maintenance at wakeful baseline. We speculate that awakening after anesthesia is a constructive process that relies on transiently increased neural communication as the brain “reboots” itself to consciousness.
In summary, we demonstrated a differential effect of propofol on specific and nonspecific thalamocortical functional connectivities consistent with their presumed roles in information and integration as necessary conditions of the conscious state. The findings support the theory that cortical information disintegration, as opposed to an inhibition of sensory reactivity, is the common mechanism of general anesthesia. They also strengthen the view that the nonspecific thalamocortical system may play an essential role in the neural basis of consciousness.
Supplementary Material
Summary Statement.
Deep sedation with propofol preferentially inhibits nonspecific vs. specific thalamocortical functional connectivity suggesting loss of higher integration of auditory information.
What We Already Know about This Topic.
Disruption of cortical information integration may account for the loss of consciousness during general anesthesia. The thalamocortical system most likely plays a pivotal role in this process.
What This Article Tells Us That is New
Propofol sedation leads to a preferential inhibition of nonspecific vs. specific thalamocortical functional connectivity in the human brain suggesting a primary role for the nonspecific system in the breakdown of information integration and fading consciousness.
Acknowledgement
We express our thanks to Stephen M. Rao, PhD, ABPP-Cn, Ralph and Luci Schey Chair and Director of the Schey Center for Cognitive Neuroimaging at Cleveland Clinic, for his invaluable advice to the original study. We thank Ms. Carrie M. O’Connor, MA, Department of Biophysics, Medical College of Wisconsin, for editorial assistance.
Funding Sources: Research reported in this publication was supported by the National Institutes of Health under Award Numbers P01-MH51358, R01-MH57836, M01-RR00058, R01-GM056398, NIH AG20279 and the W. M. Keck Foundation (Los Angeles, California). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of the Keck foundation.
Footnotes
Meeting at which part of the work has been presented: The 14th Annual Meeting of the Association for the Scientific Study of Consciousness, Toronto, Canada, June 24–27, 2010 and the 15th World Congress of Anaesthesiologists, Buenos Aires, Argentina, March 25–30, 2012.
References
- 1.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, Bruno MA, Laureys S, Phillips C, Pelegrini-Issac M, Maquet P, Benali H. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 5.Hudetz AG. Suppressing Consciousness: mechanisms of general anesthesia. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25:196–204. [Google Scholar]
- 6.Alkire MT. In: Anesthesia and the thalamocortical system, Suppressing the mind: anesthetic modulation of memory and consciousness. Hudetz AG, Pearce RA, editors. New York, NY: Humana Press; 2010. [Google Scholar]
- 7.Edelman GM. Naturalizing consciousness: a theoretical framework. Proc Natl Acad Sci U S A. 2003;100:5520–5524. doi: 10.1073/pnas.0931349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tononi G, Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 9.John ER. The neurophysics of consciousness. Brain Res Brain Res Rev. 2002;39:1–28. doi: 10.1016/s0165-0173(02)00142-x. [DOI] [PubMed] [Google Scholar]
- 10.Llinas R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- 11.Llinas R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 13.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O'Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 14.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21385. 10.1002/hbm.21385 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shafer SL. STANPUMP User’s Manual. Stanford, CA: Stanford University; 1996. [Google Scholar]
- 18.Purdon PL, Pierce ET, Bonmassar G, Walsh J, Harrell PG, Kwo J, Deschler D, Barlow M, Merhar RC, Lamus C, Mullaly CM, Sullivan M, Maginnis S, Skoniecki D, Higgins HA, Brown EN. Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Ann N Y Acad Sci. 2009;1157:61–70. doi: 10.1111/j.1749-6632.2008.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 1968;76(Suppl):1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- 20.Wickens TD. Elementary Signal Detection Theory. New York: Oxford University Press; 2002. [Google Scholar]
- 21.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008;28:13488–13504. doi: 10.1523/JNEUROSCI.3536-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Liu X, Song W, Yang Y, Zhao Z, Ling F, Hudetz AG, Li SJ. Specific and nonspecific thalamocortical functional connectivity in normal and vegetative states. Conscious Cogn. 2011;20:257–268. doi: 10.1016/j.concog.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mashour GA. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103:975–982. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- 25.Alkire MT, Haier RJ, Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious Cogn. 2000;9:370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- 26.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 27.Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res. 2005;150:229–244. doi: 10.1016/S0079-6123(05)50017-7. [DOI] [PubMed] [Google Scholar]
- 28.Posner JB, Plum F. Plum and Posner's Diagnosis of Stupor and Coma. 4th edition. New York, USA: Oxford University Press; 2007. [Google Scholar]
- 29.Tononi G, Laureys S. In: The neurology of consciousness: An overview, The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Laureys S, Tononi G, editors. Elsevier Ltd.; 2008. [Google Scholar]
- 30.Angel A. The G.L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991;76:1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- 31.Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829:77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 32.Bogen JE. On the neurophysiology of consciousness: I. An overview. Conscious Cogn. 1995;4:52–62. doi: 10.1006/ccog.1995.1003. [DOI] [PubMed] [Google Scholar]
- 33.Bogen JE. Some neurophysiologic aspects of consciousness. Semin Neurol. 1997;17:95–103. doi: 10.1055/s-2008-1040918. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama K, Muteki T, Shimoji K. Halothane-induced hyperpolarization and depression of postsynaptic potentials of guinea pig thalamic neurons in vitro. Brain Res. 1992;576:97–103. doi: 10.1016/0006-8993(92)90613-e. [DOI] [PubMed] [Google Scholar]
- 35.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 36.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24:595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 37.French JD, Verzeano M, Magoun HW. A neural basis of the anesthetic state. AMA Arch Neurol Psychiatry. 1953;69:519–529. doi: 10.1001/archneurpsyc.1953.02320280107010. [DOI] [PubMed] [Google Scholar]
- 38.Hudetz AG. Are we unconscious during general anesthesia? Int Anesthesiol Clin. 2008;46:25–42. doi: 10.1097/AIA.0b013e3181755db5. [DOI] [PubMed] [Google Scholar]
- 39.Ku SW, Lee U, Noh GJ, Jun IG, Mashour GA. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K. Preserved feed forward but impaired top-down processes in the vegetative state. Science. 2011;332:858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- 41.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee U, Kim S, Noh GJ, Choi BM, Hwang E, Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18:1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, Owen AM, Menon DK. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104:16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plourde G, Belin P, Chartrand D, Fiset P, Backman SB, Xie G, Zatorre RJ. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology. 2006;104:448–457. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 47.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 50.Hudetz AG, Liu X, Lauer KK, Li SJ. Propofol-induced changes of brain activation and thalamocortical connectivity - interpreted from information and integration. Proceeding of the 14th Annual Meeting of the Association for the Scientific Study of Consciousness (ASSC14); Toronto, Canada. 2010. [Google Scholar]
- 51.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 53.Lee U, Muller M, Noh GJ, Choi B, Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–881. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- 54.Langsjo JW, Alkire MT, Kaskinoro K, Hayama H, Maksimow A, Kaisti KK, Aalto S, Aantaa R, Jaaskelainen SK, Revonsuo A, Scheinin H. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langsjo JW, Alkire MT, Kaskinoro K, Hayama H, Maksimow A, Kaisti KK, Aalto S, Aantaa R, Jaaskelainen SK, Revonsuo A, Scheinin H. Returning from Oblivion: Imaging the Neural Core of Consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steyn-Ross ML, Steyn-Ross DA, Sleigh JW. Modelling general anaesthesia as a first-order phase transition in the cortex. Prog Biophys Mol Biol. 2004;85:369–385. doi: 10.1016/j.pbiomolbio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Friedman EB, Sun Y, Moore JT, Hung HT, Meng QC, Perera P, Joiner WJ, Thomas SA, Eckenhoff RG, Sehgal A, Kelz MB. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.