Abstract
Aim—To describe a method for amplifying human papilloma virus (HPV) in situ hybridisation (ISH) signals.
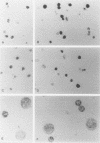
Methods—Three human cervical cell lines, namely CaSKi, HeLa and SiHa, containing different copy numbers of integrated HPV DNA were studied. Following ISH, catalysed reporter deposition (CARD), based on the deposition of biotinylated tyramine at the location of the DNA probe, was used to amplify the ISH signal.
Results—Using CARD-ISH, one to three HPV type 16 copies were detected in situ both in cell suspensions and paraffin wax sections of SiHa cells. CARD-ISH can also be used to detect oncogenic HPV DNA sequences, such as HPV types 16 and 18, in routinely processed formalin fixed, paraffin wax embedded cervical specimens.
Conclusions—CARD-ISH is a fast and highly sensitive ISH method for the routine detection of low copy number HPV DNA sequences in cervical cell lines and routinely processed tissue sections. Application of this technology also enables the routine detection and cellular localisation of other viral DNA sequences present at copy numbers below the detection limit of conventional ISH methods.
Keywords: in situ hybridisation
Keywords: signal amplification
Keywords: cell lines
Keywords: human papilloma virus
Keywords: cervix uteri
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992 Oct;40(10):1457–1463. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989 Dec 20;125(1-2):279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991 Mar 1;137(1):103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kerstens H. M., Poddighe P. J., Hanselaar A. G. A novel in situ hybridization signal amplification method based on the deposition of biotinylated tyramine. J Histochem Cytochem. 1995 Apr;43(4):347–352. doi: 10.1177/43.4.7897179. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Friedman D., Richart R. M. In situ hybridization analysis of human papillomavirus DNA segregation patterns in lesions of the female genital tract. Gynecol Oncol. 1990 Feb;36(2):256–262. doi: 10.1016/0090-8258(90)90184-m. [DOI] [PubMed] [Google Scholar]
- Nuovo G. J., Gallery F., MacConnell P., Becker J., Bloch W. An improved technique for the in situ detection of DNA after polymerase chain reaction amplification. Am J Pathol. 1991 Dec;139(6):1239–1244. [PMC free article] [PubMed] [Google Scholar]
- Nuovo G. J., MacConnell P., Forde A., Delvenne P. Detection of human papillomavirus DNA in formalin-fixed tissues by in situ hybridization after amplification by polymerase chain reaction. Am J Pathol. 1991 Oct;139(4):847–854. [PMC free article] [PubMed] [Google Scholar]
- Pontén J., Adami H. O., Bergström R., Dillner J., Friberg L. G., Gustafsson L., Miller A. B., Parkin D. M., Sparén P., Trichopoulos D. Strategies for global control of cervical cancer. Int J Cancer. 1995 Jan 3;60(1):1–26. doi: 10.1002/ijc.2910600102. [DOI] [PubMed] [Google Scholar]
- Raap A. K., van de Corput M. P., Vervenne R. A., van Gijlswijk R. P., Tanke H. J., Wiegant J. Ultra-sensitive FISH using peroxidase-mediated deposition of biotin- or fluorochrome tyramides. Hum Mol Genet. 1995 Apr;4(4):529–534. doi: 10.1093/hmg/4.4.529. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H., Bauer H. M., Hoover R. N., Glass A. G., Cadell D. M., Rush B. B., Scott D. R., Sherman M. E., Kurman R. J., Wacholder S. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J Natl Cancer Inst. 1993 Jun 16;85(12):958–964. doi: 10.1093/jnci/85.12.958. [DOI] [PubMed] [Google Scholar]
- Siadat-Pajouh M., Ayscue A. H., Periasamy A., Herman B. Introduction of a fast and sensitive fluorescent in situ hybridization method for single-copy detection of human papillomavirus (HPV) genome. J Histochem Cytochem. 1994 Nov;42(11):1503–1512. doi: 10.1177/42.11.7930533. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Melchers W. J., Mullink H., Meijer C. J., Struyk A., Quint W. G., van der Noordaa J., ter Schegget J. Sensitivity of in situ detection with biotinylated probes of human papilloma virus type 16 DNA in frozen tissue sections of squamous cell carcinomas of the cervix. Am J Pathol. 1988 Jun;131(3):587–594. [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- de Roda Husman A. M., Walboomers J. M., van den Brule A. J., Meijer C. J., Snijders P. J. The use of general primers GP5 and GP6 elongated at their 3' ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995 Apr;76(Pt 4):1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]