SUMMARY
Inactivation of the Pten tumor suppressor negatively regulates the PI3K-mTOR pathway. In a model of cutaneous squamous cell carcinoma (SCC), we demonstrate that deletion of Pten strongly elevates Fgf10 protein levels without increasing Fgf10 transcription in vitro and in vivo. The translational activation of Fgf10 by Pten deletion is reversed by genetic disruption of the mTORC1 complex, which also prevents skin tumorigenesis in Pten mutants. We further show that ectopic expression of Fgf10 causes skin papillomas, while Pten deletion-induced skin tumors are inhibited by epidermal deletion of Fgfr2. Collectively, our data identify autocrine activation of FGF signaling as an essential mechanism in promoting Pten-deficient skin tumors.
INTRODUCTION
Squamous cell carcinoma (SCC) is the second most common form of skin cancer (Ridky and Khavari, 2004). Mouse genetic studies have demonstrated that skin SCC can be induced by epidermal deletion of Pten, a critical tumor suppresser gene implicated in multiple types of cancer (Suzuki et al., 2003). Consistent with these data, germline mutations in various types of the PTEN harmartoma tumor syndrome (PHTS) are known to predispose patients to skin tumorigenesis (Eng, 2003). In addition to gene deletion, Pten function can be manipulated in cancer cells by other mechanisms such as transcription, microRNA, translation, protein stability, promoter methylation, and by various post-translational events (Song et al., 2012). Indeed, although somatic PTEN mutations have not been found in human SCCs, PTEN expression is shown to be frequently down regulated by a GRHL3-mediated microRNA network (Agrawal et al., 2011; Darido et al., 2011; Stransky et al., 2011). Skin SCC observed in Pten deficient animals has been reproduced by transgenic overexpression of activated AKT or RHEB in the epidermis and blocked by inhibition of class Ia PI3K (Lu et al., 2010; Segrelles et al., 2007; Wang et al., 2013), demonstrating the importance of PI3K-AKT-mTOR signaling in Pten-induced tumorigenesis. Recent evidence suggests that mTOR signaling controls the translation of nearly all mRNAs to some extent, but its direct targets critical for tumorigenesis remain poorly understood (Hsieh et al., 2012; Thoreen et al., 2012).
FGF signaling is required for epidermal growth, skin barrier formation and hair cycle activation (Greco et al., 2009; Grose et al., 2007; Yang et al., 2010). Up-regulation of ligands FGF2, 7, 10 and 22, and their activation of FGFR2 signaling has been shown to initiate keratinocyte proliferation in diseased states such as acne, psoriasis and wound healing (Braun et al., 2004). On the other hand, although activating mutations in FGFR3 have been shown to underlie benign skin tumors, including epidermal nevi or seborrhoeic keratosis, epidermal deletion of Fgfr2 is shown to sensitize animals to chemical-induced skin papillomas and SCCs (Grose et al., 2007; Logie et al., 2005). Therefore, FGF signaling plays highly context-dependent roles in skin homeostasis and tumorigenesis (Turner and Grose, 2010). In this study, we show that Pten loss in keratinocytes activates the translation of Fgf10 in an mTOR-dependent manner. The resulting induction of FGF signaling promotes skin papilloma formation, which can be reversed by genetic ablation of Fgfr2 in epidermis. Our results thus reveal an autocrine FGF signaling network induced by Pten-mTOR signaling essential for skin tumorigenesis.
RESULTS
A mouse model of Pten-regulated skin tumorigenesis
We generated an epidermal-specific ablation of Pten using Le-Cre driver, which is known to be expressed in the ocular surface but is also reportedly active in the anterior mandibular ectoderm (Ashery-Padan et al., 2000; Miller et al., 2006; Pan et al., 2008). Indeed, one month old Le-Cre;Ptenflox/flox (PtenCKO) animals exhibited skin swelling both around the eyelid and in the cheek (Fig. 1A and B, arrows). Histological sections revealed epidermal hyperplasia, hypergranulosis and hyperkeratosis, which progressed to papilloma in severely affected animals (Fig. 1C and D, arrows, and data not shown). By crossing with R26R Cre reporter, we showed that the Cre-positive cells stained by X-gal were restricted to the epidermis and to hair follicles, confirming the specificity of Le-Cre to skin (Fig. 1E and F, arrows). Consistent with these results, PtenCKO epidermis displayed a marked reduction in Pten staining and strong increases in cell proliferation markers Ki67 and phospho-Histone H3 (Fig. 1G-J and data not shown). K14-positive keratinocytes remained within the single basal cell layer attached to the basement membrane; however, the K10-labeled suprabasal layer was greatly expanded (Fig. 1K and L, arrows). These hyperplastic proliferation phenotypes resembled the onset of SCC previously described in a pan-keratinocyte Pten deletion model (Suzuki et al., 2003).
Figure 1. Pten loss initiates hyperkeratosis in the facial epidermis.
(A and B) 4 week old Le-Cre;Ptenflox/flox or PtenCKO mice displayed hyperkeratosis in cheek and eyelid epidermis (arrows). (C and D) Paraffin sections of the skin revealed the expansion of epidermal layers in PtenCKO mice (arrow). (E and F) X-gal staining indicated specific activity of Le-Cre;R26R in the epidermis (arrows). (G-L) PtenCKO mice exhibited a loss of Pten from the epidermis (H, arrow), increased Ki67 staining denoting proliferation of the basal layer (J, arrow) and expansion of the suprabasal layer shown by K10 positive staining (L, arrow).
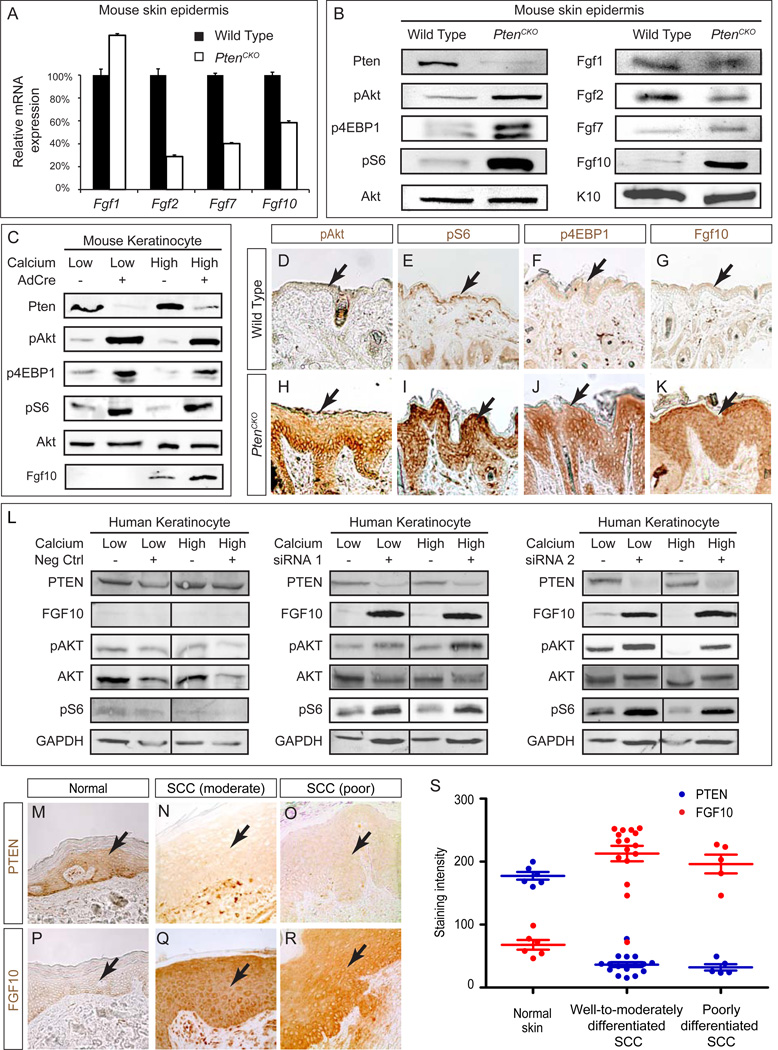
Pten deletion increased Fgf10 protein level in skin epidermis
To gain insight into the role of FGF signaling in SCC, we performed qPCR on the skin epidermal layer removed from PtenCKO mice but found no significant increase in the mRNA levels of Fgfs (Fgf1, 2, 7, and 10) and Fgfrs (Fgfr1, 2, 3 and 4) (Fig. 2A and S1A). Immunoblotting of the same samples confirmed depletion of Pten protein and increased phosphorylation of AKT, 4EBP1 and S6, all downstream effectors of PI3K-mTOR pathway (Fig. 2B). Interestingly, although the protein levels of Fgf1, 2 and 7 remained low, immunoblotting of PtenCKO epidermis revealed a significant increase in Fgf10 protein.
Figure 2. Loss of Pten induces Fgf10 protein but not mRNA expression.
(A) qPCR analysis of Fgf mRNA expressions in the mouse epidermis. (B) Immunoblots of PtenCKO mice facial and cheek epidermis showed a reduction in Pten levels and up-regulation of pAKT, p4EBP1 and pS6. Although there were little changes in Fgf1, 2 and 7 expression, Fgf10 was strongly up regulated in PtenCKO mice. (C) Pten depleted keratinocytes switched from low to high Ca2+ media (basal to suprabasal state) showed increased levels of pAkt, p4EBP1, pS6 and Fgf10. (D-K) Immunohistochemistry confirmed strong pAKT, pS6, p4EBP1 and Fgf10 staining in the PtenCKO facial skin. (L) siRNA mediated PTEN depletion resulted in significant increase of pAKT, pS6 and FGF10 levels in human primary keratinocytes. (M-S) Both well-to-moderately and poorly differentiated human SCC stained weakly for PTEN but strongly for FGF10 in the epidermal layer. Arrows point to epidermis. Data are presented as mean ± s.e.m.
Fgf10 is known to be secreted by intraepithelial γδ T cells in response to epidermal wounding (Werner et al., 1994). However, crossing PtenCKO mouse with TCRδ−/− animals which are unable to produce γδT cells not only failed to reduce Fgf10 levels but even accelerated tumorigenesis (data not shown), suggesting that activation of γδ T cells cannot account for the increase in Fgf10 protein in PtenCKO epidermis. We next harvested primary keratinocytes from neonatal Ptenflox/flox pups and suppressed Pten expression using Cre-expressing adenovirus. Following viral infection, cells were either allowed to remain in a basal state by culturing in low Ca2+ media (basal media) or forced to differentiate into suprabasal cells by culturing in high Ca2+ media (suprabasal media). Similar to results observed in the in vivo model, qPCR of basal or suprabasal state keratinocytes showed no significant change in Fgf10 mRNA expression upon Pten deletion but pAKT, p4EBP1, pS6 protein levels were up regulated on immunoblotting (Fig. 2C and S1B). Importantly, immunoblotting for Fgf10 showed elevated levels in Pten-depleted cells cultured in suprabasal media. Finally, immunohistochemistry of tissue sections showed intense staining of pAKT, pS6, p4EBP1 and Fgf10 in PtenCKO epidermis (Fig. 2D-K). Taken together, these results suggested that loss of Pten results in translational activation of Fgf10 in keratinocytes.
We next examined whether these pathways derived from our mouse model existed in human cells and SCCs. By siRNA mediated knockdown, we first depleted PTEN in primary human keratinocytes. The results presented in Figure 2L shows that, in both basal and suprabasal culture conditions, PTEN deficiency led to an increase in AKT and S6 phosphorylation and elevated FGF10 protein expression. We next analyzed PTEN and FGF10 expression in well-to-moderately differentiated human skin SCC that arose spontaneously (n=9) or developed after undergoing transplantation procedures (n=6) via immunohistochemistry. Normal skin tissue from patients undergoing abdominoplasty was obtained for comparison (n=6). Strikingly, 15 out of 16 of these SCC specimens showed a loss in PTEN and an increase in FGF10 in the epithelial layer when compared to normal skin tissue (P < 0.001) (Fig. 2M-S). This observation was further confirmed in a SCC tissue array, where 42 out of 47 samples displayed elevated levels of FGF10 (data not shown). Finally, we examined 5 poorly differentiated human SCC samples and found that they all stained weakly for PTEN but strongly for FGF10 (Fig. 2O and R). These results thereby emphasize the potential role of PI3K and FGF signaling in promoting human skin SCC.
mTOR signaling in the epidermis regulates Fgf10 expression and tumorigenesis
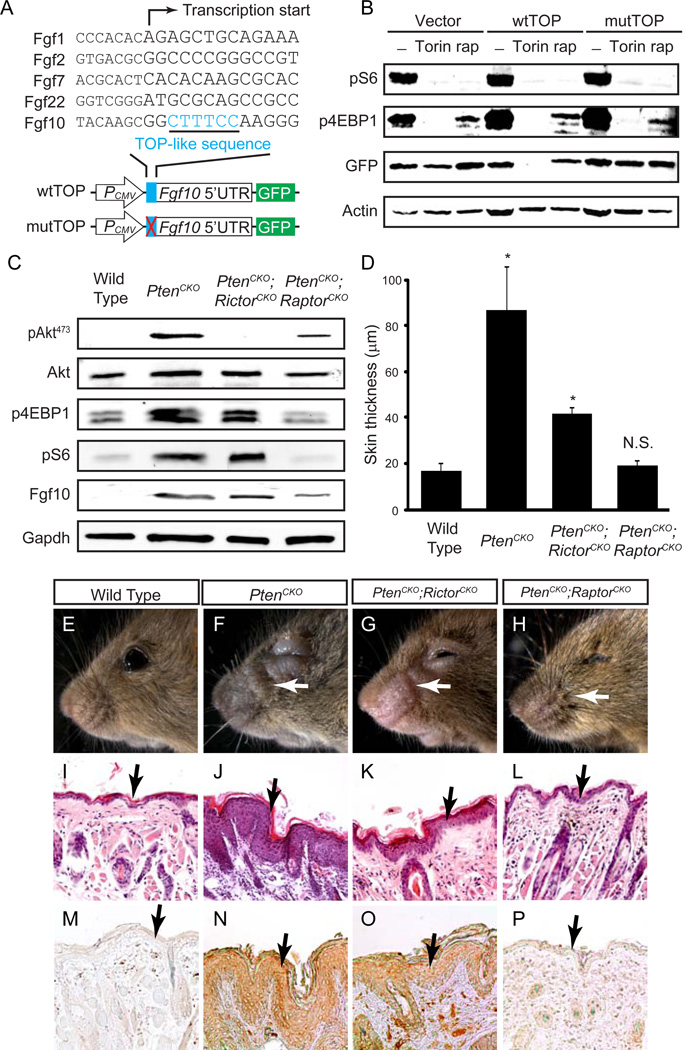
Pten-PI3K signaling is known to promote protein translation via mTOR-mediated phosphorylation of translational suppressor 4EBP1. 4EBP1 phosphorylation activates cap-dependent translation of mRNA sequences containing a TOP-like motif, which is defined as containing at least five pyrimidines near the transcriptional start site (Hsieh et al., 2012; Thoreen et al., 2012). Interestingly, among Fgf mRNAs we detected in keratinocytes, only Fgf10 contains a TOP-like sequence immediately following its transcriptional start site (Fig. 3A), suggesting that translation of Fgf10 may be uniquely regulated by mTOR signaling. It was recently demonstrated that the allosteric inhibitor of mTOR, rapamycin, suppresses phosphorylation of only a subset of mTOR downstream targets, most notably S6K1 and its substrate S6 (Kang et al., 2013). In contrast, the ATP-competitive mTOR inhibitor, Torin, further blocks phosphorylation of T37 and T46 residues of 4E-BP1, sites that are rapamycin-resistant. Since TOP-like mRNA translation is mainly regulated through 4EBPs, but not S6Ks (Kang et al., 2013; Pende et al., 2004), we hypothesized that the Fgf10 TOP-like sequence may be sensitive to Torin but not to rapamycin. To test this idea, we cloned Fgf10 5’UTR into a GFP reporter, carrying either wild type or 6 bp transversions within the TOP-like sequence (Fig. 3A). As expected, Torin treatment of NIH-3T3 cells abolished both S6 and 4EBP1 phosphorylation and prevented expression of the wild type Fgf10 5’UTR-GFP reporter (wtTOP) (Fig. 3B). However, both the vector control and the mutated Fgf10 5’UTR-GFP reporter (mutTOP) were unaffected, demonstrating the critical role of Fgf10 TOP-like sequence in mTOR-induced translation. Although rapamycin treatment eliminated S6 phosphorylation, there was still significant 4EBP1 phosphorylation in transfected NIH-3T3 cells. As a result, neither the wild type nor the mutant GFP reporters exhibit reduced expression. These results support that 4EBPsmediated mTOR signaling controls the translational activity of Fgf10 TOP-like sequence.
Figure 3. mTOR signaling is required for up regulation of Fgf10.
(A) Alignment of RefSeq gene sequences with mouse genome shows that Fgf10 mRNA has a TOP-like sequence motif, defined as containing at least five pyrimidines within four nucleotides of the transcription start site. The Fgf10 5’UTR sequences carrying either wild type TOP-like sequence (wtTOP) or 6 bp transversion mutations (mutTOP) were cloned into GFP reporter vectors. (B) In transfected NIH-3T3 cells, Torin suppresses phosphorylation of S6 and 4EBP1, and expression of GFP in wild type but not mutant Fgf10 reporters. Rapamycin (rap) treatment, however, abolished S6 phosphorylation, but left 4EBP1 phosphorylation partially reduced and GFP expressions unchanged. (C) A reduction in levels of mTORC1 downstream effectors (p4EBP1 and pS6) and Fgf10 was seen in lysates from PtenCKO;RaptorCKO mice. (D-P) Phenotypic comparison of facial skin in wild type (n=9), PtenCKO (n=9), PtenCKO;RictorCKO (n=9) and PtenCKO;RaptorCKO (n=3) mice; Rescue of the hyperkeratosis (L) and loss of Fgf10 (P) were observed in PtenCKO;RaptorCKO mice. One-way ANOVA test: * P < 0.001; N.S., not significant. Data are presented as mean ± s.e.m. White and black arrows point to facial skin (E-H) and epidermis (I-P), respectively.
Torin inhibits activities of both mTORC1 and mTORC2, two distinct complexes of mTOR kinase. To identify which mTOR complexes regulates Fgf10 expression in vivo, we crossed PtenCKO mice with epidermal-specific knockouts of Raptor and Rictor, which are obligatory components of mTORC1 and mTORC2 respectively (Zoncu et al., 2011). Consistent with the known function of mTORC2 as an AKT S473 kinase, Le-Cre;Ptenflox/flox;Rictorflox/flox (PtenCKO;RictorCKO) epidermis completely lost AKTS473 phosphorylation (Fig. 3C). The levels of p4EBP1 and pS6, however, were unaffected by Rictor deletion, while Fgf10 expression was moderately down regulated. In contrast, Le-Cre;Ptenflox/flox;Raptorflox/flox (PtenCKO;RaptorCKO) epidermis exhibited not only attenuated AKTS473 phosphorylation but also significant reduction in p4EBP1, pS6 and Fgf10 levels (Fig. 3C). As a result, epidermal hyperplasia observed in one month old PtenCKO mice was reduced in PtenCKO;RictorCKO littermates and completely prevented in PtenCKO;RaptorCKO animals (Fig. 3D-P). Thus, mTORC1 signaling is critically required for Fgf10 expression and tumorigenesis in Pten mutants.
Autocrine Fgf10-Fgfr2 signaling mediates Pten deletion-induced tumorigenesis
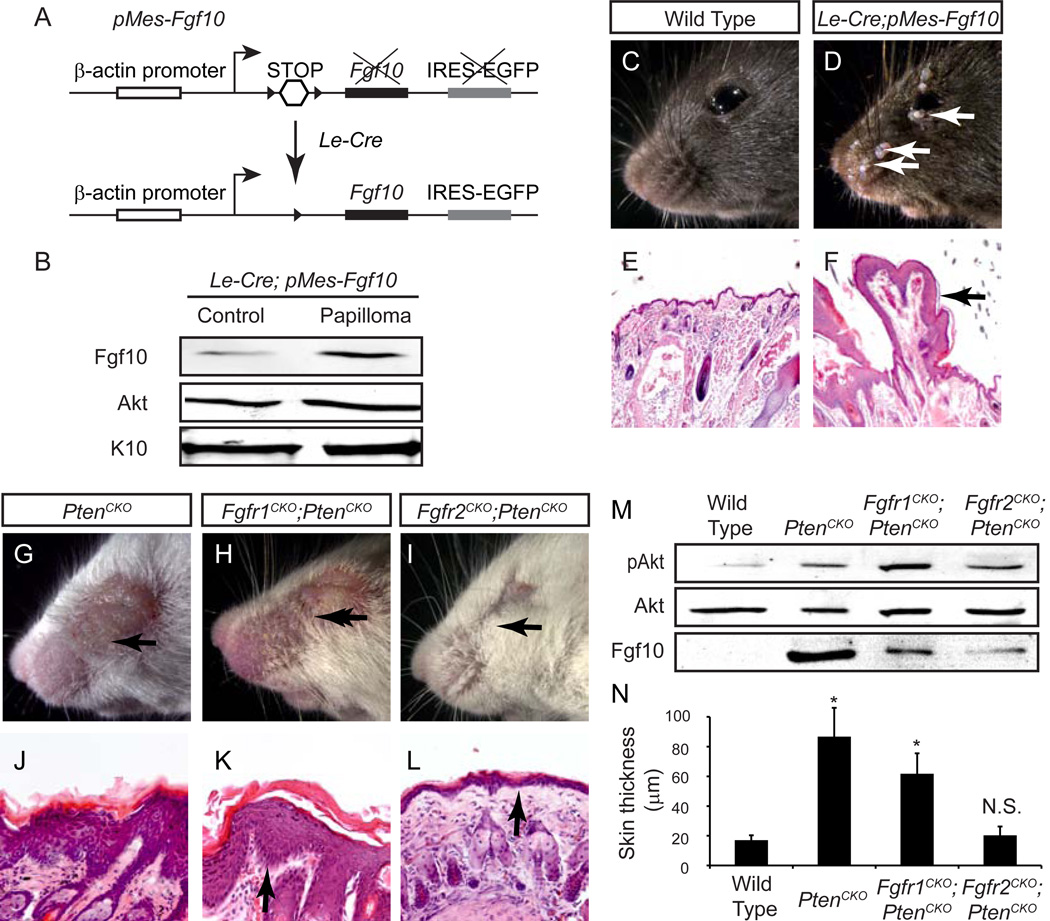
We next explored the functional significance of increased Fgf10 expression in Pten deficient epidermis. We utilized an inducible Fgf10 transgene (pMes-Fgf10) that places a LoxP-flanked transcription STOP cassette between a constitutively active chick β-actin promoter and an Fgf10 cDNA (Fig. 4A) (Song et al., 2013). Preliminary characterization of this transgene indicated that, due to positional variegation, Cre-mediated excision of STOP cassette typically results in a mosaic expression of Fgf10 (data not shown). Nevertheless, all Le-Cre; pMes-Fgf10 mice at 3 weeks of age developed spontaneous papilloma, which appeared as finger-like protrusions arising from epidermis (Fig.4C-F, arrows). Importantly, immunoblotting of tissues lysates indicated that these nodules expressed higher levels of Fgf10 than their adjacent skin control (Fig. 4B). These results demonstrate that overexpression of Fgf10 is sufficient to induce epidermal hyperplasia and papilloma formation in a dose-dependent manner.
Figure 4. Fgf10-Fgfr2 signaling potentiates Pten-deletion induced SCC.
(A) Schematics of pMef-Fgf10 transgene. After crossing with Le-Cre driver, excision of STOP cassette in pMef-Fgf10 transgene permits constitutive expression of Fgf10 in skin epidermis. (B) Fgf10 expression increased in papilloma obtained from Le-Cre;pMef-Fgf10 mice. (C-F) Papillomas appeared in Le-Cre;pMef-Fgf10 cheek and eyelid regions (arrows). (G-L) Epidermal hyperplasia phenotype seen in PtenCKO animals were modestly reduced in PtenCKO;Fgfr1CKO, but abolished in PtenCKO;Fgfr2CKO mice (arrows). (M) Immunoblots showed reduced pAKT and Fgf10 levels in PtenCKO;Fgfr2CKO epidermal lysates. (N) Measurements of epidermal thickness in wild type (n=9), PtenCKO (n=9), PtenCKO;Fgfr1CKO (n=3) and PtenCKO;Fgfr2CKO (n=9) mice are shown as mean ± s.e.m.. One-way ANOVA test: * P < 0.001; N.S., not significant.
Fgf10 is known to signal through primarily Fgfr2 and Fgfr1 to a lesser extent (Zhang et al., 2006). We thus reasoned that if Fgf10 was a key downstream target of Pten-PI3K signaling, the tumorigenic phenotype in PtenCKO mice may be rescued by genetic ablation of Fgfr2. Indeed, epidermal hyperplasia was only slightly reduced in Le-Cre;Ptenflox/flox;Fgfr1flox/flox (PtenCKO;Fgfr1CKO) mice, which still maintained high levels of AKT phosphorylation and Fgf10 expression (Fig. 4G, H, J, K and M, arrows). In contrast, pAKT and Fgf10 in Le-Cre;Ptenflox/flox;Fgfr2flox/flox (PtenCKO;Fgfr2CKO) animals were significantly reduced as compared to PtenCKO mice, suggesting a Fgf10-Fgfr2 autocrine feedback loop (Fig. 4M). Most importantly, PtenCKO;Fgfr2CKO animals displayed a thin layer of epidermis similar to that of wild type mice, and no tumor was ever observed during their lifespan for up to two years (Fig. 4I-N, arrows). Therefore, Pten deficient epidermis tumorigenesis requires Fgf10-Fgfr2 signaling.
DISCUSSION
In this study, we showed that hyperactivation of mTOR in Pten-deficient epidermis translationally stimulated expression of Fgf10 protein. The resulting activation of Fgfr2 in keratinocytes forms a positive autocrine feedback loop that is both necessary and sufficient for development of skin SCC. FGF ligands are generally thought to be regulated transcriptionally to achieve tissue specific expression patterns. In particular, it has been well established that stromal induction of Fgf10 and its binding to Fgfr2 in the epithelia are critical for limb induction, branching morphogenesis and skin development (Beenken and Mohammadi, 2009; Turner and Grose, 2010). However, in contrast to the paracrine function of Fgf10 common in embryonic development, we showed that Pten deletion led to an autocrine Fgf10 signaling in skin SCC. The presence of an mTORC1 regulated TOP-like sequence in Fgf10 mRNA suggests that ectopic Fgf10 expression in skin keratinocytes is induced by constitutive mTOR signaling. Indeed, by exploring the different target specificities of mTORC1 inhibitors Torin and rapamycin, we showed that phosphorylation of 4EBPs but not S6Ks by mTORC1 is critical for translational activation of the Fgf10 TOP-like sequence. Furthermore, genetic disruption of mTORC1 complex not only prevented overexpression of Fgf10 protein but also suppressed tumorigenesis in Pten deficient epidermis. Our results thus revealed a novel translational mechanism of FGF signaling activation by Pten-PI3K-mTOR signaling in skin SCC. To date, FGF signaling deregulation in cancer has been primarily scrutinized at genomic level for genetic mutations or at transcriptomic level for mRNA misexpression (Turner and Grose, 2010). Our findings suggest that proteomic analysis of FGF signaling is a promising venue to identify aberrant FGF signaling in carcinogenesis.
To ensure cutaneous tissue homeostasis, a fine balance of FGF signaling and its consequent mitogenic effects must be maintained. There is compelling evidence that Fgfr2 has tumor suppressive function in normal keratinocytes, as Fgfr2-deficient animals are more prone to develop skin tumors in response to chemical carcinogens (Grose et al., 2007). On the other hand, previous studies have showed that excessive expression of Fgfr2 ligand, Fgf7, could lead to epidermal hyperplasia and gross transformation, and p63-induced Fgfr2 overexpression was essential for survival of autochthonous SCCs (Chikama et al., 2008; Guo et al., 1993; Ramsey et al., 2013). We now present genetic evidence that loss of Fgfr2 signaling completely blocked tumorigenesis in Pten-deficient epidermis, demonstrating that Fgfr2 is important for pathogenesis of skin SCC. These results thus raise the conundrum of how FGF signaling can be both tumor-suppressive and oncogenic in the epidermis. We would like to suggest that these two opposing functions of FGF signaling may be explained by different magnitudes of signaling activity. In this view, FGF signaling at the physiological level is required for proper skin development and homeostasis, lack of which results in abnormal skin barrier, pathological inflammation and deficient tumor surveillance. The Fgfr2 deficiency could be exacerbated by the normal function of Pten, which is expected to further dampen overall mitogenic signaling. This explains the apparent paradox that Fgfr2 deletion induces epidermal hyperplasia only in the presence of wild type Pten, an archetypical tumor suppressor gene. Excessive FGF signaling, however, promotes cell proliferation and survival, potentiates cell migration and invasion, and attracts angiogenesis. These oncogenic effects may be especially potent in Pten-deficient epidermis, where an Fgf10-Fgfr2 autocrine feedback loop greatly amplifies FGF signaling. Therefore, FGF signaling can act as an important potentiating factor in skin SCC.
EXPERIMENTAL PROCEDURES
Mice
Ptenflox, Fgfr1flox, TCRδ−/− and R26R mice were obtained from Jackson Laboratory (Bar Harbor, ME) (Hoch and Soriano, 2006; Itohara et al., 1993; Lesche et al., 2002; Soriano, 1999). Rictorflox and Raptorflox mice were kindly provided by Drs. Markus A. Rüegg and Michael N. Hall (Biozentrum, University of Basel, Basel, Switzerland) and Estela Jacinto (UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ) (Bentzinger et al., 2008). Le-Cre and Fgfr2flox mice were kindly provided by Dr. Ruth Ashery-Padan (Tel Aviv University, Tel Aviv, Israel) and Dr. David Ornitz (Washington University Medical School, St Louis, MO), respectively (Ashery-Padan et al., 2000; Yu et al., 2003). Fgf10 conditional transgenic mouse (pMes-Fgf10) was previously described (Song et al., 2013). All animal experiments were performed according to IACUC regulations.
Histology and immunohistochemistry
Histology and immunohistochemistry were performed as previously described (Pan et al., 2010). Human specimens of normal skin, SCC, and SCC occurring after solid organ transplantation (SOT) were obtained after approval from IRB at Indiana University School of Medicine. For histopathological analysis, 5 µm sections obtained from formalin fixed paraffin embedded skin tissue were used. Image analysis of histology data was performed using ImageJ. The relative pixel intensity in gray scale ranging from 0–255 was obtained by measuring a specified area at three different locations on the same section. Each data point was calculated by taking the average of pixel intensity for three separate images of the same section, and analyzed by one-way ANOVA analysis.
Western Blot
Facial hair was removed using Nair® hair removal cream and skin around the cheek and eyelid area was dissected out in one piece, snap frozen and stored at −80°C until usage. The dissected skin samples were then carefully scraped with a curette to selectively remove only the epidermal layer, which was lysed in RIPA buffer containing protease inhibitors. Equal amounts of the lysates were separated on SDS-PAGE and the blots stained with primary antibodies were visualized using IRDye linked secondary antibody in Odyssey SA scanner (LICOR Biosciences, Lincoln, NE) as previously described (Qu et al., 2011). Antibodies used are specific to Actin (Abcam, Cambridge, MA), GFP (a gift from Dr. Pamela Silver, Harvard Medical School, Boston, MA), Ki67 (BD Pharmingen San Diego, CA), Keratin 10, Keratin 14, phospho-4EBP1 T37/46, phospho-S6S235/236, phospho-AktS473, Pten (all from Cell Signaling Technology, Beverly, MA), phospho-Histone H3 (Upstate, Temecula, CA), Fgf1, Fgf2, Fgf7, and Fgf10 (all from Santa Cruz Biotechnology, Santa Cruz, CA). At least three mice of each genotype were analyzed.
Keratinocyte Culture
Skins removed from neonatal pups were washed in DPBS plus 2% antibiotics, stretched out (dermis side down) on autoclaved filter paper in a 100 mm culture plate and digested overnight at 4°C in 0.25% trypsin. The epidermis was next gently separated from the dermis, finely minced, washed once and filtered through a 70μm cell strainer. Isolated keratinocytes were plated in KSFM media supplemented with growth factors and 0.2 mM CaCl2 (Invitrogen, Carlsbad, CA) and maintained at 370C/5% CO2 with media changed every 2 days. Keratinocyte differentiation from basal to suprabasal stage was induced by increasing the Ca2+ concentration to 1.2 mM in the culture media. At 70% confluency, keratinocytes were infected overnight with Adeno-Cre GFP virus (Gene Transfer Vector Core, University of Iowa, IA) at 100 MOI virus parts per cell. At about 90–100% GFP expression, the cells were lysed in ice cold RIPA buffer supplemented with protease inhibitors and used for immunoblotting experiments.
siRNA transfection
nTERT immortalized primary human keratinocytes were maintained in EpiLife keratinocyte media supplemented with human keratinocyte growth supplement cocktail and 1000 U penicillin-streptomycin (Invitrogen, Carlsbad, CA). At around 60% confluency, cells were transfected with negative control or siRNA against PTEN (ON_TARGET plus siRNA, Dharmacon/ Fisher Scientific, Pittsburgh PA) using Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) as per manufacturer’s instructions. PTEN knockdown was analyzed by immunoblotting 72 hours post infection. siRNA transfection was performed at both basal and suprabasal stage of keratinocyte differentiation.
Fgf10 TOP-like sequence analysis
The entire 5’UTR sequence (675 bp) of Fgf10 gene was amplified by PCR from mouse genomic DNA and inserted into the HindIII site within the pEGFP-N3 vector (Clontech, Mountain View, CA) to generate the wtTOP plasmid. The mutTOP plasmid contains the same 5’UTR sequence, except the six pyrimidines (CTTTCC) within the TOP-like sequence were mutated to GAAAGG. The primers used for cloning are: wtTOP Forward, GTTAAGCTTGGCTTTCCAAGGGACTTGGAG; mutTOP Forward, CGTTAAGCTTGGGAAAGGAGGGACTTGGAGGTGGAGAG; Reverse, ACCAAGCTTAATGTTTGGATCGTCATGGG. For functional analysis, pEGFP-N3, wtTOP or mutTOP plasmids were transfected into NIH-3T3 cells (60% confluency) using Turbofect reagent (Thermo scientific, Waltham, MA). After 24 hrs, cells were treated with mTOR inhibitors Torin 2 (150 nM; R&D systems, Minneapolis, MN) and Rapamycin (100 nM; Cell Signaling Technology, Beverly, MA) for another 24 hrs before lysed for immunoblotting analysis.
Quantitative Real Time PCR
Total RNA extracted from skin and keratinocyte samples was converted to cDNA using SuperScript®III RT kit (Invitrogen, Carlsbad, CA). qPCR analysis was performed in 10 μl reactions with the SYBR GREEN PCR Master Mix and analyzed on a StepOnePlus™ Real- Time PCR instrument (Invitrogen, Carlsbad, CA). Relative standard curves were generated by serial dilutions and all samples were run in triplicates. Primers used are: Fgfr1 (GGACACCGAAGGGCTTTTAT and GGTTTTCTTCCAGCCTTTCC), Fgf2 (GGCTGCTGGCTTCTAAGTCT and TTCCGTGACCGGTAAGTATTG), Fgf7 (GGGAAATGTTCGTTGCCTTA and CCCTGCTGAATGAAACTGGT), Fgf10 (CAATGGCAGGCAAATGTATG and GGAGGAAGTGAGCAGAGGTG), Fgf22 (CGTGTGGACCTTGGTGG and ACACGGACAGAACGGATCTC) and Gapdh (AGGTCGGTGTGAACGGATTTG and TGTAGACCATGTAGTTGAGGTCA).
Supplementary Material
HIGHLIGHTS.
Pten deletion induces Fgf10 expression in skin epidermis.
Fgf10 is regulated at the translational level by mTORC1.
Overexpression of Fgf10 reproduces skin papillomas.
PTEN deficiency in human cutaneous SCC correlates with higher FGF10 levels.
ACKNOWLEDGEMENTS
The authors thank Drs. Ruth Ashery-Padan, Michael N. Hall, David Ornitz and Markus A. Rüegg, Pamela Silver for mice and antibody, Raymond L Konger for advice, and members of the Zhang lab for discussions. The work was supported by grants from NIH (EY017061 and EY018868 to XZ). XZ is supported by Jules and Doris Stein Research to Prevent Blindness Professorship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nature reviews Drug discovery. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell metabolism. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Braun S, auf dem Keller U, Steiling H, Werner S. Fibroblast growth factors in epithelial repair and cytoprotection. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2004;359:753–757. doi: 10.1098/rstb.2004.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikama T, Liu CY, Meij JT, Hayashi Y, Wang IJ, Yang L, Nishida T, Kao WW. Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. The American journal of pathology. 2008;172:638–649. doi: 10.2353/ajpath.2008.070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darido C, Georgy SR, Wilanowski T, Dworkin S, Auden A, Zhao Q, Rank G, Srivastava S, Finlay MJ, Papenfuss AT, et al. Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis. Cancer Cell. 2011;20:635–648. doi: 10.1016/j.ccr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell stem cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Fantl V, Werner S, Chioni AM, Jarosz M, Rudling R, Cross B, Hart IR, Dickson C. The role of fibroblast growth factor receptor 2b in skin homeostasis and cancer development. EMBO J. 2007;26:1268–1278. doi: 10.1038/sj.emboj.7601583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Yu QC, Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993;12:973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch RV, Soriano P. Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development. 2006;133:663–673. doi: 10.1242/dev.02242. [DOI] [PubMed] [Google Scholar]
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Logie A, Dunois-Larde C, Rosty C, Levrel O, Blanche M, Ribeiro A, Gasc JM, Jorcano J, Werner S, Sastre-Garau X, et al. Activating mutations of the tyrosine kinase receptor FGFR3 are associated with benign skin tumors in mice and humans. Hum Mol Genet. 2005;14:1153–1160. doi: 10.1093/hmg/ddi127. [DOI] [PubMed] [Google Scholar]
- Lu ZH, Shvartsman MB, Lee AY, Shao JM, Murray MM, Kladney RD, Fan D, Krajewski S, Chiang GG, Mills GB, Arbeit JM. Mammalian target of rapamycin activator RHEB is frequently overexpressed in human carcinomas and is critical and sufficient for skin epithelial carcinogenesis. Cancer research. 2010;70:3287–3298. doi: 10.1158/0008-5472.CAN-09-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Feng GS, Zhang X. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development. 2010;137:1085–1093. doi: 10.1242/dev.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–310. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Carbe C, Tao C, Powers A, Lawrence R, van Kuppevelt TH, Cardoso WV, Grobe K, Esko JD, Zhang X. Lacrimal Gland Development and Fgf10-Fgfr2b Signaling Are Controlled by 2-O- and 6-O-sulfated Heparan Sulfate. J Biol Chem. 2011;286:14435–14444. doi: 10.1074/jbc.M111.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey MR, Wilson C, Ory B, Rothenberg SM, Faquin W, Mills AA, Ellisen LW. FGFR2 signaling underlies p63 oncogenic function in squamous cell carcinoma. The Journal of clinical investigation. 2013;123:3525–3538. doi: 10.1172/JCI68899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridky TW, Khavari PA. Pathways sufficient to induce epidermal carcinogenesis. Cell Cycle. 2004;3:621–624. [PubMed] [Google Scholar]
- Segrelles C, Lu J, Hammann B, Santos M, Moral M, Cascallana JL, Lara MF, Rho O, Carbajal S, Traag J, et al. Deregulated activity of Akt in epithelial basal cells induces spontaneous tumors and heightened sensitivity to skin carcinogenesis. Cancer research. 2007;67:10879–10888. doi: 10.1158/0008-5472.CAN-07-2564. [DOI] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Song Z, Liu C, Iwata J, Gu S, Suzuki A, Sun C, He W, Shu R, Li L, Chai Y, Chen Y. Mice with Tak1 deficiency in neural crest lineage exhibit cleft palate associated with abnormal tongue development. J Biol Chem. 2013;288:10440–10450. doi: 10.1074/jbc.M112.432286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Itami S, Ohishi M, Hamada K, Inoue T, Komazawa N, Senoo H, Sasaki T, Takeda J, Manabe M, et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer research. 2003;63:674–681. [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature reviews Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Wang Q, Von T, Bronson R, Ruan M, Mu W, Huang A, Maira SM, Zhao JJ. Spatially distinct roles of class Ia PI3K isoforms in the development and maintenance of PTEN hamartoma tumor syndrome. Genes Dev. 2013;27:1568–1580. doi: 10.1101/gad.216069.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Yang J, Meyer M, Muller AK, Bohm F, Grose R, Dauwalder T, Verrey F, Kopf M, Partanen J, Bloch W, et al. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol. 2010;188:935–952. doi: 10.1083/jcb.200910126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.