Abstract
The Src family kinase Lck is crucial for initiation of T cell antigen receptor (TCR) signaling. Lck is tightly controlled to prevent erroneous immune activation, yet allows rapid responses over a range of sensitivities to antigens. Here, using an analog-sensitive Csk we report that Lck is dynamically controlled by a Csk:CD45-controlled equilibrium in T cells. By rapidly inhibiting Csk, we show that changes in this equilibrium are sufficient for activation of the canonical TCR signaling pathways independent of TCR stimulation. The activated signaling pathways show sustained and marked hyperphosphorylation, revealing a feedback circuit that is sensitive to basal signaling activity and is capable of adapting to changes in basal signal transduction machinery. We identify the inhibitory adaptor molecule Dok-1 as a candidate in the adaptive response to alterations in basal signaling activity. Our results also suggest a novel role for Csk in terminating or dampening of TCR signals.
INTRODUCTION
Cell surface receptors, such as the TCR, are studied in the context of ligand activation, and are controlled by a threshold of activation dependent on ligand affinity and avidity. TCR signaling is critical for the development, survival and activation of mature lymphocytes. TCR signal strength greatly influences the repertoire of TCRs on the T cells that populate the immune system. Sufficient activation of TCR signaling is necessary for differentiation of naive T cells into effector and memory T cells during an immune response. Comparatively little work has focused on the basal state of the TCR before ligand binds. Here we uncover an unexpected level of basal signaling of the TCR in the absence of ligand, suggesting the cytoplasmic network is poised to rapidly respond, yet is restrained by a single negative regulatory kinase.
The TCR complex contains no endogenous kinase function, but uses the Src family kinase (SFK) Lck to phosphorylate paired tyrosine residues in cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) in each of the CD3- and ζ-chains of the TCR. The tyrosine kinase C-terminal Src Kinase (Csk) is a critical negative regulator of SFK activity, phosphorylating the conserved C-terminal inhibitory tyrosine in Lck, Y505. Phosphorylation of Lck Y505 results in stabilization of an inactive conformation that prevents Lck access to substrates and catalytic function. In T cells, Csk-mediated phosphorylation of Y505 is functionally opposed by the non-receptor tyrosine phosphatase CD45, which dephosphorylates Y505, poising Lck for its ITAM–phosphorylating function. In contrast to Y505, phosphorylation of the conserved Y394 in the activation loop of the Lck catalytic domain is associated with increased kinase activity, although recent work suggests TCR stimulation may not markedly alter total Y394 phosphorylation (1).
Within the immune system, Csk is crucial for controlling lymphocyte development and preventing aberrant activation of immune cells. Csk is regulated primarily by its subcellular localization and by interactions with other proteins via its SH2 and SH3 domains. In unstimulated T cells, Csk is enriched in plasma membrane lipid raft fractions, the result of putative SH2-mediated interactions with lipid-raft enriched adaptors, including PAG (Phosphoprotein associated with glycosphingolipid-enriched microdomains) (2, 3) and, presumably other proteins. Following TCR stimulation, PAG is rapidly dephosphorylated by an unknown mechanism, releasing Csk into the cytoplasm. Disassociation of active Csk from the plasma membrane favors the action of CD45, promoting the activity of Lck and other SFKs (4). Because PAG-deficient T cells have no obvious phenotype, other yet unknown membrane recruitment mechanisms for Csk are likely to exist.
The regulation of Lck is critical for orchestrating the threshold sensitivity and strength of TCR signaling. However, it remains unclear if in resting T cells the activation state of Lck is ‘fixed’ or is the result of dynamic equilibrium of on-going Csk and CD45 activity. In a fixed state, Lck activation would require specific changes in the localization or catalytic activities of its regulatory proteins, whereas a dynamic equilibrium of Csk and CD45 might continuously alter the phosphorylation status and activity of Lck. Hence, a small imbalance in the activities of either CD45 or Csk would be sufficient to alter Lck activity. Rapid perturbation of Csk function has been hampered due to the prolonged time needed to express exogenous alleles of mutant signaling proteins. No selective small molecule inhibitor of Csk is available, as Csk inhibitors invariably also inhibit SFKs. Furthermore, Csk−/− mice suffer early embryonic lethality, and loss of Csk during early T cell development results in aberrant T cell development (5, 6).
To characterize the role of Csk in regulating basal and induced signaling downstream of the TCR, we established a small-molecule controlled Csk allele, CskAS capable of inhibiting TCR activation, particularly when CskAS is localized to the plasma membrane. Rapid and specific inhibition of membrane-targeted CskAS results in potent and sustained signal transduction and cell activation. This activation is independent of TCR ligation, but utilizes canonical TCR signaling components. These findings reveal a feedback control mechanism that is sensitive to the level of basal signaling by the TCR pathway, and adapts to alterations in the basal signal transduction machinery. We also suggest a new role for Csk in the termination or dampening of the antigen receptor response. Finally, we identify the inhibitory adaptor Dok-1, a protein involved in membrane recruitment of Csk and inhibition of antigen receptor signaling in T cells, as a candidate in the adaptive response to alterations in basal signaling levels.
RESULTS
Generation of the CskAS T266G allele permits rapid and specific chemical inhibition
To achieve selective inhibition of Csk, we mutated the ‘gatekeeper’ residue, thereby enlarging the ATP-binding pocket of Csk. The resulting analog-sensitive allele is referred to asCskAS (Fig. 1A), and can accommodate bulky analogs of the nonselective kinase inhibitor PP1(7). In addition, the amino-terminus of Lck was fused to CskAS to enrich its localization to lipid rafts, and is referred to as membrane-CskAS. Because no Csk-null T cell lines exist, and ablation of Csk in early T cell development results in aberrant maturation of T cells with altered expression of TCR and coreceptors, we expressed CskAS alleles in the human Jurkat T cell line, which has low levels of Csk present at the membrane in resting cells and for which signaling pathways have been well-characterized.
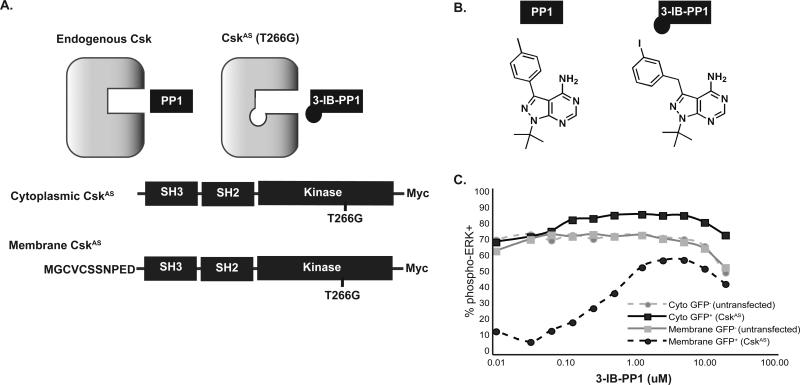
Figure 1. Generation and characterization of the CskAS system.
(A) Mutation of the conserved gatekeeper residue of Csk (T266G) permits access to larger analogs of PP1. The eleven amino-terminal residues of Lck were fused to CskAS to enforce its localization to lipid rafts in the plasma membrane. (B) Structure of 3-IB-PP1. (C) Jurkat T cells were transiently cotransfected with either cytoplasmic- or membrane-CskAS and a GFP cotransfection marker. Cells were serum-starved, then stimulated with anti-TCR for 5 min in the presence of DMSO or 3-IBPP1 at doses shown, and analyzed for phospho-ERK. Data show % of live, GFP− untransfected cells or live GFP+ transfected cells that are phospho-ERK+. Data are representative of 3 independent experiments.
Transfection of either membrane-CskWT or -CskAS into Jurkat T cells efficiently blocked TCR-mediated activation, as assessed by upregulation of the early activation marker CD69 (Fig. S1), but was insufficient to block activation in response to phorbol myristate acetate, which activates protein kinase C downstream of the TCR. A library of PP1 analogs was screened for their selective ability to block the inhibitory effects of membrane-CskAS on T cell activation (Fig. S1). Whereas the nonselective kinase inhibitor PP1 blocked activation of all cell types, bulky PP1 analogs were unable to block TCR-mediated activation of vector-transfected cells. 3-IB-PP1 was identified as a selective inhibitor of CskAS, but not of CskWT (Figs. 1B and S1C, D). Treatment of transfected cells with 3-IB-PP1 blocked the membrane-CskAS inhibitory function, as assessed by phospho-Erk induction, with EC50 values of approximately 0.5 μM (Fig. 1C). However, at comparable levels of expression the cytoplasmic-CskAS allele did not inhibit TCR-induced Erk activation.
Inhibition of CskAS catalytic function activates proximal TCR signaling pathways
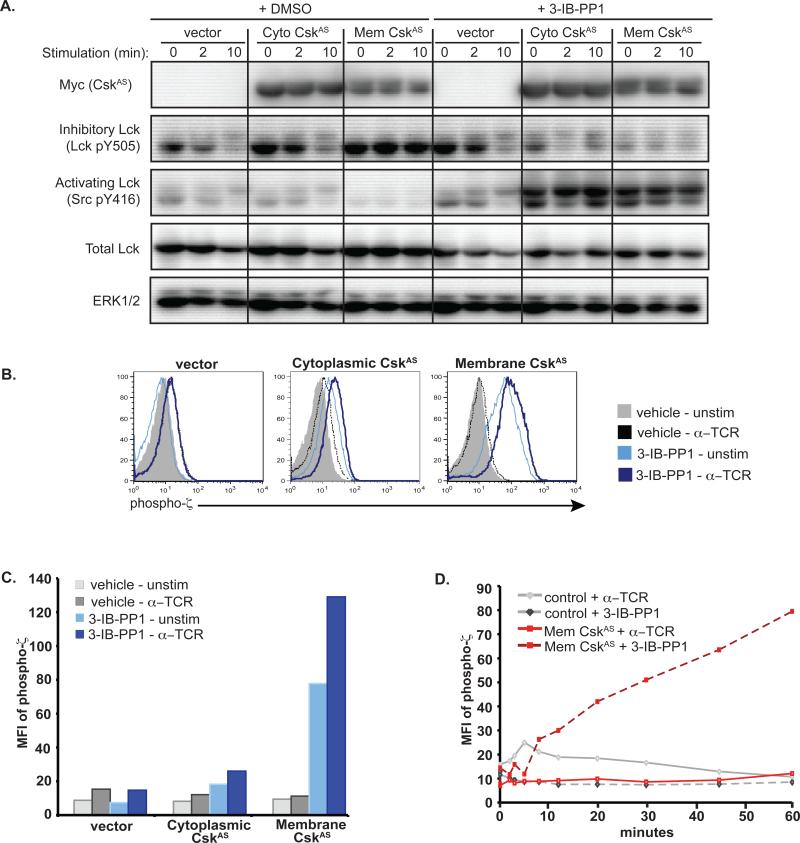
To characterize the effects of 3-IB-PP1 on CskAS activity, we examined the phosphorylation of the activating (Y394) and inhibitory (Y505) tyrosines of Lck following inhibition of CskAS with 3-IB-PP1. In control cells, TCR stimulation resulted in decreased phosphorylation of the inhibitory Y505 residue within 2 minutes, with further decreases seen by 10 minutes (Fig. 2A). In contrast, total phosphorylation of the activating Y394 residue was not substantially changed in response to TCR stimulation, in accord with recent studies(1). However, Lck migrated more slowly due to Erk-mediated phosphorylation of S57 in Lck (8). This pattern of Lck phosphorylation was similar in control cells pretreated with 3-IB-PP1, supporting the insensitivity of endogenous Csk to 3-IB-PP1 pretreatment. Vehicle-treated cytoplasmic-CskAS cells had a moderate increase in inhibitory Lck Y505 phosphorylation in the basal state compared to control cells, but lost Y505 phosphorylation, and phosphorylated Lck S57 in response to TCR stimulation. These data are consistent with the notion that only minimal amounts of CskAS and endogenous Csk are localized to the plasma membrane of resting cells and both are under normal regulatory control mechanisms. In contrast, resting membrane-CskAS cells had markedly increased Lck Y505 phosphorylation and only minimally detectable Lck Y394 phosphorylation, which did not change following TCR stimulation. Thus, as previously described (9), membrane-CskAS potently inhibits Lck.
Figure 2. Effect of CskAS on Lck activity.
(A) Jurkat T cells expressing empty vector, cytoplasmic- or membrane-CskAS were serum-starved, pretreated with DMSO or 3-IB-PP1 for 25 minutes, then lysates were prepared directly or following TCR stimulation. Data are representative of three independent experiments. (B) Vector or CskAS cells were serum-starved, then pretreated with DMSO or 3-IB-PP1 for 20 minutes. Cells were then stimulated with anti-TCR or vehicle for 2 min prior to fixation and staining for ζ-chain phosphorylation. Histograms show ζ-chain phosphorylation in live, transfected cells. (C) Bar graphs of (B), representing the mean fluorescence intensity (MFI) of phospho-ζ-chain in GFP+ transfected cells. (D) Control or membrane-CskAS cells were serum-starved and fixed directly or following anti-TCR stimulation or 3-IB-PP1 treatment. Data represent the MFI of phospho-ζ-chain in GFP+ transfected cells. Data are representative of at least three independent experiments.
The effects of CskAS inhibition by 3-IB-PP1 on Lck activation were striking and unanticipated. Whereas pretreatment of control cells with 3-IB-PP1 did not affect the phosphorylation of Lck, pretreatment of cytoplasmic- or membrane-CskAS cells resulted in markedly reduced Lck Y505 phosphorylation and large increases in Lck Y394 phosphorylation. It is important to emphasize that activation of Lck in response to CskAS inhibition occurred in the absence of TCR stimulation. These data indicate that inhibition of CskAS catalytic activity is sufficient to result in ligand-independent Lck activation, even when only a small amount of CskAS is present at the membrane, as is the case for cytoplasmic-CskAS.
Lck is responsible for phosphorylating ITAMs of the TCR complex and initiating TCR-mediated signaling. TCR stimulation of control cells induced phosphorylation of the ζ- and CD3ε-chains, as did treatment of membrane-CskAS cells with 3-IB-PP1 (Fig. S2). Relative to TCR-stimulated control cells, 3-IB-PP1-treated CskAS cells had greater phosphorylation of the ζ-chain, which correlated with increased recruitment and phosphorylation of the cytoplasmic tyrosine kinase ζ-chain-associated protein kinase 70 (ZAP-70). To quantify the amount of ζ-chain phosphorylation we used a flow cytometric-based approach. Following TCR stimulation, control or cytoplasmic-CskAS cells increased ζ-chain phosphorylation 2-4-fold (Figs. 2B-C). In marked contrast, TCR stimulation of vehicle-treated membrane-CskAS cells did not lead to an increase in ζ-chain phosphorylation. Although Lck Y394 phosphorylation was increased to a similar extent in cytoplasmic- and membrane-CskAS cells pretreated with 3-IB-PP1 (Fig. 2A), the extent of ζ-chain phosphorylation was much greater in cells expressing membrane-CskAS, which increased ζ-chain phosphorylation 5- to 10-fold following 3-IB-PP1 treatment. In both cytoplasmic- and membrane-CskAS cells, ζ-chain phosphorylation could be further increased by TCR stimulation. Control cells responding to TCR stimulation had peak ζ-chain phosphorylation at 5 minutes, returning to basal levels by 45 minutes (Fig. 2D). In contrast, membrane-CskAS cells continued to increase ζ-chain phosphorylation over the course of 1 hour. Thus, phosphorylation of proximal signaling proteins is augmented and sustained in response to CskAS inhibition, relative to TCR stimulation of control cells. This suggests that rather than a return to basal conditions, hyperactivation or an ‘over-shoot’ of Lck activity is observed when CskAS function is inhibited. This effect is amplified in cells expressing the more inhibitory form of CskAS that is targeted to the membrane.
TCR signaling is augmented and prolonged following inhibition of CskAS activity
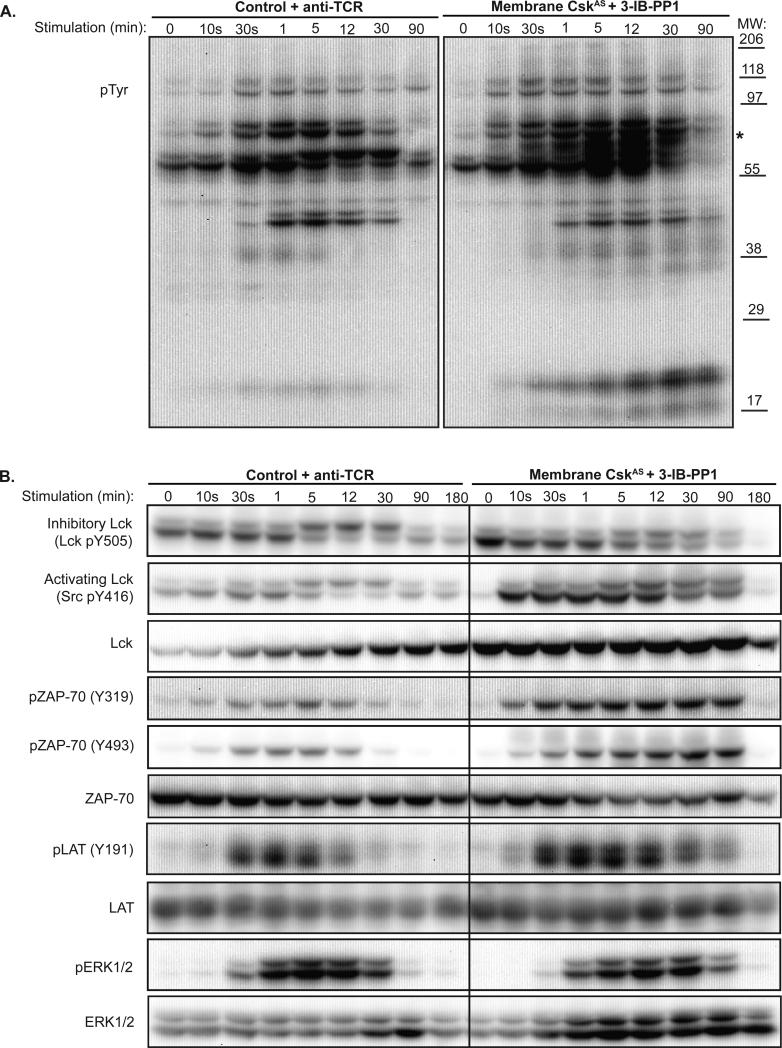
TCR stimulation has been reported to result in sudden dephosphorylation of PAG in T cells, causing the rapid dissociation of Csk to the cytoplasm. Csk reappears in lipid rafts within 5-10 minutes of stimulation (4, 10). The increased and sustained ζ-chain phosphorylation following membrane-CskAS inhibition suggests that normal mechanisms to control TCR-signaling are altered. Following 3-IB-PP1 treatment, membrane-CskAS cells exhibited rapid and prolonged induction of tyrosine phosphorylation of several proteins, including the 21-23 kDa ζ-chain (Fig. 3A). The overall pattern of tyrosine phosphorylation is similar to TCR-stimulated control cells, with the exception of a 65kDa phosphoprotein seen in the 3-IB-PP1 stimulated cells (marked with *). This suggests activation of similar signaling events by inhibition of membrane-CskAS or in response to TCR stimulation in control cells.
Figure 3. Sustained signaling in presence of CskAS inhibition.
Control or membrane-CskAS T cells were serum-starved, then lysed directly or following stimulation. Control cells were stimulated with anti-TCR (left) and membrane-CskAS cells were treated with 3-IB-PP1 (right) for the times indicated. (A) Total tyrosine phosphorylation, and (B) phosphorylation of Lck, ZAP-70, LAT and ERK were assessed by immunoblotting. Data are representative of at least three independent experiments.
We next compared the phosphorylation of specific signaling proteins in response to TCR stimulation of control cells versus inhibition of membrane-CskAS cells (Fig. 3B). ZAP-70 binds to the Lck-phosphorylated ζ-chains, and is phosphorylated by Lck on Y315 and Y319 to maintain an active conformation (11). Y493 is located in the ZAP-70 kinase activation loop, and can be phosphorylated by Lck or ZAP-70 itself. Both Y319 and Y493 exhibited enhanced and sustained phosphorylation following membrane-CskAS inhibition. Active ZAP-70 is necessary for the phosphorylation of two critical adaptor proteins: linker for activation of T cells (LAT) and SH2 domain-containing leukocyte protein of 76KDa (Slp-76). Together, phosphorylated LAT and Slp-76 create a platform for recruitment of numerous other molecules necessary for downstream signaling events that direct transcriptional responses and induce cell proliferation. Similar to ZAP-70, greater and more prolonged LAT phosphorylation was observed in response to 3-IB-PP1, correlating with sustained signaling downstream including ERK phosphorylation. Thus, inhibition of membrane-CskAS was sufficient to rapidly activate and induce sustained signaling events associated with the canonical TCR signaling pathway, but did so in a ligand-independent manner.
Activation of distal signaling events and cellular activation is induced by CskAS inhibition
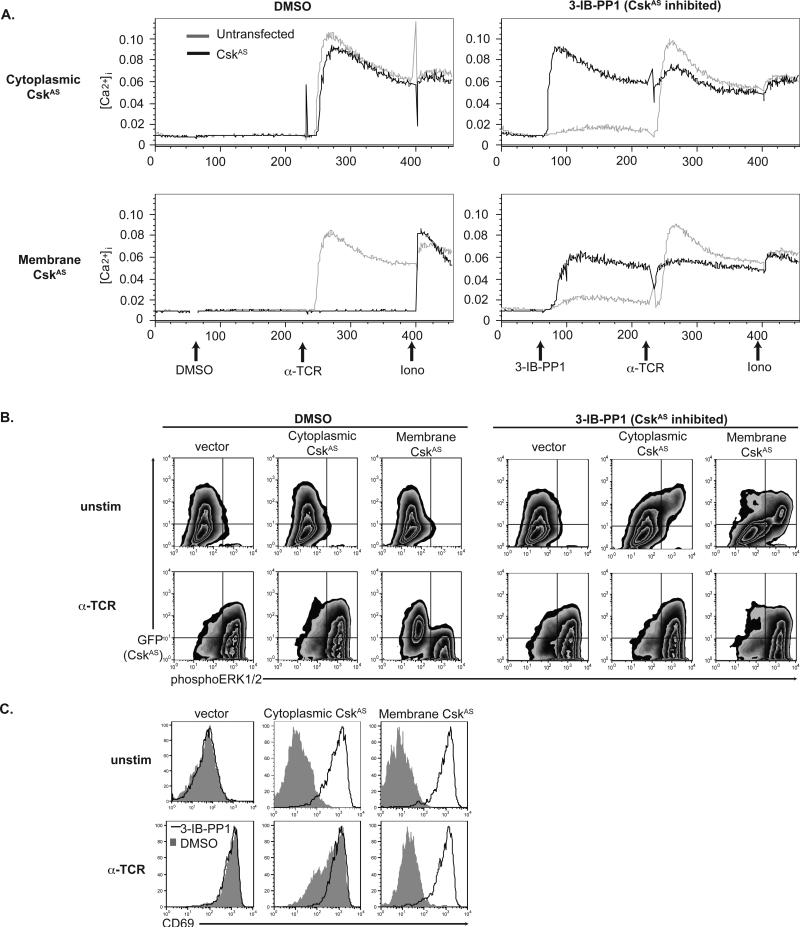
Lck-mediated phosphorylation of TCR ITAMs and ZAP-70 are necessary for inducing cytoplasmic calcium increases ([Ca2+]i) and MAPK activation, which coordinate transcriptional responses, including CD69 and IL-2. To examine if the phosphorylation events described above were sufficient to activate downstream signaling and induce cellular activation, we examined the calcium and phospho-ERK responses as well as CD69 induction. Similar to control cells, cytoplasmic-CskAS cells increased [Ca2+]i in response to TCR stimulation or ionomycin (Fig. 4A). However, membrane-CskAS cells were unable to increase [Ca2+]i in response to TCR stimulation, consistent with their inability to activate Lck or phosphorylate ITAMs. However, cells expressing either the cytoplasmic- or the membrane-CskAS proteins increased [Ca2+]i in response to CskAS inhibition alone, independent of TCR engagement. Similarly, vehicle-treated control cells and those expressing cytoplasmic-CskAS, but not membrane-CskAS proteins, increased phospho-ERK in response to TCR stimulation (Fig. 4B). A small portion of cells expressing a high amount of cytoplasmic-CskAS increased phospho-ERK in response to CskAS inhibition. However, most cells expressing membrane-CskAS phosphorylated ERK in response to 3-IB-PP1. Only those cells expressing the highest amount of membrane-CskAS are unable to phosphorylate ERK or flux calcium (as evident by a moderate reduction in calcium flux) upon 3-IB-PP1 inhibition. Consistent with the role of sustained Ras-MAPK signaling for CD69 transcription, membrane-CskAS cells treated with 3-IB-PP1 alone, but not vehicle nor TCR-stimulated alone, expressed CD69 (Fig. 4C). Cytoplasmic-CskAS cells were able to induce CD69 in response to either CskAS inhibition or TCR stimulation.
Figure 4. Distal TCR signaling and cell activation induced by CskAS inhibition.
(A) Calcium release is triggered by CskAS inhibition alone in T cells expressing cytoplasmic- (top) or membrane- CskAS (bottom). Jurkat T cells transiently cotransfected with CskAS and CD16 constructs. The ratios between Fluo-3 and FuraRed is shown for CD16− untransfected cells or for CD16+ CskAS transfected cells in response to DMSO, 3-IB-PP1, anti-TCR and ionomycin. B) Jurkat T cells expressing membrane-CskAS have impaired ERK phosphorylation that is overcome by inhibition of CskAS alone. Control or CskAS cells were serum-starved, pretreated with DMSO or 3-IB-PP1 for 15 minutes. Cells were then harvested directly (unstim) or after 2 minutes of TCR stimulation. Plots show total live cells, with GFP− untransfected cells in the bottom quadrants and GFP+ CskAS–transfected cells in the upper quadrants. (C) Upregulation of CD69 is impaired in TCR-stimulated membrane-CskAS cells, and is induced in response to CskAS inhibition alone. Transiently transfected Jurkat T cells were treated with either 3-IB-PP1 or DMSO, and were TCR stimulated for 18 hours prior to surface staining for CD69. Data are representative of two (A) or three (B, C) independent experiments.
We verified that these adaptive over-shoot signaling events also occurred in primary T cells by introducing cytoplasmic- and membrane-CskAS into primary mouse T cells. Mouse T cells transfected with vector alone or CskAS had low basal Lck and ERK activation (Fig. S3). TCR stimulation of vector cells demonstrated a moderate increase in Lck Y394 phosphorylation and robust ERK activation. Consistent with the data observed in Jurkat T cells, inhibition of CskAS with 3-IB-PP1 induced Lck activation and ERK phosphorylation.
Thus, we have demonstrated that membrane-CskAS is a potent inhibitor of TCR stimulation. However, blockade of CskAS catalytic function not only releases the Csk-mediated inhibition, but is sufficient to activate T cells independently of TCR stimulation. In the presence of 3-IB-PP1, CskAS cells show an augmented and sustained phosphorylation of signaling proteins, relative to the response to TCR stimulation in control cells. These phosphorylation events are most robust when CskAS has been targeted to the membrane. Rather than a return to the normal pattern and signaling requiring TCR stimulation following CskAS inhibition, a hyperphosphorylation or “adaptive over-shoot” of signaling is observed. Together, these data suggest that ectopic expression of CskAS, particularly when targeted to the membrane, dampens basal signaling in resting cells engaging a compensatory adaptive mechanism to respond to this impaired state. Upon CskAS inhibition, we hypothesize that this mechanism is responsible for the unexpected increases in phosphorylation of signaling proteins. The more prolonged response seen with CskAS inhibition also suggests that Csk may play a role in terminating TCR signaling.
The compensatory adaptive mechanism requires expression of TCR, CD45, and Lck
In resting T cells, the TCR is constitutively internalized and rapidly recycled back to the cell surface. Strong antigenic stimulation induces ζ-chain degradation via Lck-dependent mechanisms. Consistent with the inhibition of basal Lck activity, T cells expressing membrane-CskAS had decreased basal TCR internalization, resulting in elevated levels of surface TCR complexes (Fig. S4). Following CskAS inhibition, TCR surface levels were down-modulated to a level equivalent to TCR-stimulated control cells. We found surface expression of the TCR or other ITAM-containing receptors is required for induction of signaling upon inhibition of CskAS catalytic function, since no response was observed in TCRβ-deficient cells transfected with membrane-CskAS and inhibited with 3-IB-PP1 (Fig. S5). However, increased TCR or ITAM-containing receptor expression is not responsible for the adaptive over-shoot observed when CskAS is inhibited, since cell lines that express either lower surface TCR or a fixed amount of a CD8-ζ chimeric receptor in the absence of TCR are activated in a manner similar to WT Jurkat cells (Figs. S5 and S6).
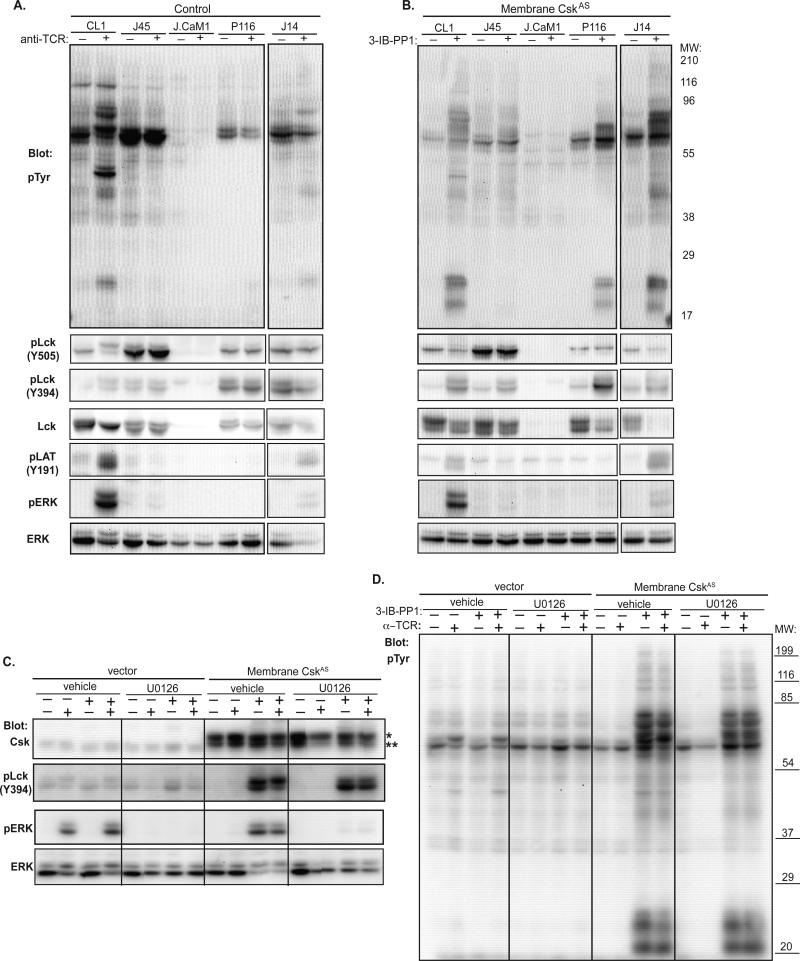
Jurkat mutants deficient for CD45, Lck, ZAP-70 or Slp-76 have revealed the role of these molecules in the initiation of proximal TCR signaling pathways. Signaling downstream of the TCR is blocked in cells deficient in either CD45 (J45) or Lck (J.CaM1) (Fig. 5A). As expected, inhibition of membrane-CskAS when expressed in these cell lines did not induce downstream signaling events (Fig. 5B). Thus, Lck is required for phosphorylation of the ζ-chain and requires CD45 for its activation in response to both TCR stimulation and activation of signaling in response to CskAS inhibition. ZAP-70-deficient P116 cells are also defective in their ability to respond to TCR stimulation, however inhibition of membrane-CskAS was still sufficient to induce phosphorylation of ζ-chain and Lck Y394. However, the ERK-dependent Lck S57 upper band was largely absent following CskAS inhibition, presumably due to the inability of P116 cells to signal downstream of ZAP-70. SLP-76-deficient J14 T cells could activate proximal signaling events in both control and membrane-CskAS transfected cells in response to TCR stimulation or 3-IB-PP1 treatment, respectively; however distal signaling events such as ERK phosphorylation were impaired in both settings. These findings suggest that the compensatory adaptive mechanism for Lck and ζ-chain phosphorylation does not require signaling events dependent upon ZAP-70 and SLP-76, but does require events more receptor-proximal components.
Figure 5. Requirement of TCR signaling components for activation upon CskAS inhibition.
(A) Untransfected or (B) membrane-CskAS transfected Jurkat signaling mutant cells were purified, serum-starved and stimulated with anti-TCR or 3-IB-PP1 for 10 minutes and lysed. Total tyrosine phosphorylation and phosphorylation of Lck, LAT and ERK were assessed by immunoblotting. (C, D) Membrane-CskAS cells were treated for 2 hours with either vehicle or MEK inhibitor U0126. Cells were serum-starved for 30 minutes in the presence of vehicle or U0126, and then stimulated with anti-TCR or 3-IB-PP1 for 5 minutes as noted. Lysates were blotted for total tyrosine phosphorylation, Csk, and phosphorylation of Lck and ERK. The Csk antibody recognizes both endogenous Csk (denoted **) and CskAS (denoted *, larger MW due to Myc tag, linker sequences and membrane targeting motif). Data are representative of at least 3 independent experiments.
Positive and negative feedback loops have been implicated in the regulation of Lck to explain signaling differences related to strength of TCR engagement (8). In this model, strong TCR ligands activate a positive feedback loop involving ERK-mediated phosphorylation of Lck S57, which prevents recruitment of the inhibitory phosphatase SHP-1 to inactivate TCR signaling. Given the importance of Lck activation for the sustained hyperactivation of TCR-dependent signaling in response CskAS inhibition, we examined the requirement for ERK-mediated phosphorylation of Lck in 3-IB-PP1-induced activation. As predicted, inhibition of MEK activation by U0126 prior to 3-IB-PP1 treatment led to a block in ERK activation following CskAS inhibition (Fig. 5C). Additionally, the upper S57-phosphorylated band of Lck was notably reduced in U0126-pretreated cells. However, the level of proximal signaling (phosphorylation of Lck Y394 and ζ-chain) was unaffected by MEK inhibition (Figs. 5C, D), suggesting that the previously reported ERK-mediated phosphorylation feedback mechanism is not required for the augmented signaling following CskAS inhibition. Thus, we conclude that the compensatory adaptive mechanism that occurs in response to CskAS expression requires expression of TCR, CD45 and Lck, but involves molecules(s) upstream of ZAP-70, Slp-76, and ERK.
Dok-1 is phosphorylated and interacts with Csk following CskAS inhibition
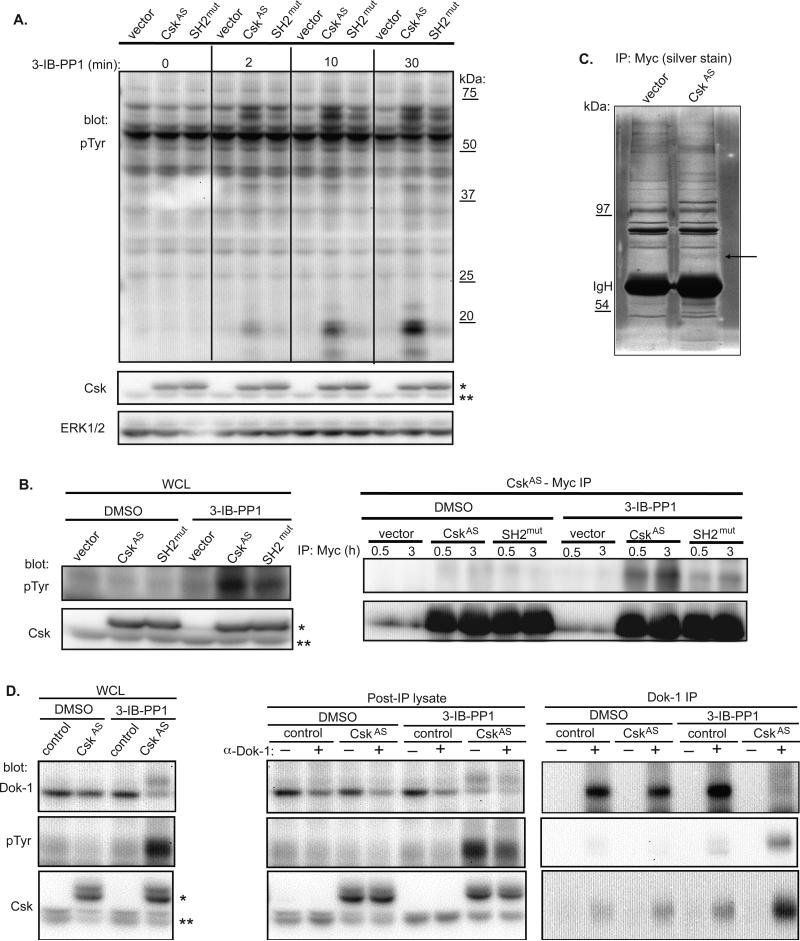
Endogenous Csk is dynamically recruited to the cell membrane due to interactions of its SH2 domain with phosphorylated tyrosines in membrane-anchored adaptor molecules. However, membrane-CskAS is constitutively enriched in lipid rafts of the plasma membrane. Despite this, the SH2 domain may still interact with phospho-proteins important to the regulation of signaling. We postulated that if this were true, expression of membrane-CskAS with an SH2 mutation would alter signaling differently following 3-IB-PP1 treatment. Indeed, mutation of the SH2 domain of CskAS resulted in a clear diminution in ζ-chain phosphorylation as well as other proteins following CskAS inhibition (Fig. 6A). We further observed a tyrosine-phosphorylated band of ~65kDa that was less phosphorylated with the SH2 mutation. We were particularly interested in identifying this 65kDa protein because it's uniquely phosphorylated in cells in which either cytoplasmic- or membrane-CskAS had been inhibited by 3-IB-PP1 (Figs. 3A, 5B). Phosphorylation of this band occurs within 30 seconds of CskAS inhibition, and is detectable for at least 30 minutes. However, this 65kDa phospho-protein is undetectable prior to Csk inhibition or in response to TCR stimulation. Furthermore, immunoprecipitates of membrane-CskAS showed a strong interaction with a tyrosine-phosphorylated protein of 65kDa following CskAS inhibition by 3-IB-PP1, which was reduced when the SH2 domain was mutated (Fig. 6B). We hypothesized that the 65kDa protein may interact with CskAS via its SH2 domain and might play a role in the adaptive “over-shoot” response. Mass spectrometry analysis of CskAS-Myc immunoprecipitations identified various peptides but a single peptide was from the 65kDa band as the adaptor molecule p62dok1 (Downstream of Kinase 1; Dok-1) (Fig. 6C, S7). Interestingly, the Dok-1 peptide identified was tyrosine-phosphorylated and encompassed Y449, a known binding site for the SH2 domain of Csk.
Figure 6. Dok-1 is uniquely phosphorylated upon CskAS inhibition and interacts with Csk.
(A) Jurkat T cells expressing vector, membrane-CskAS or SH2 domain R107K mutant (SH2mut) membrane-CskAS were serum-starved, then lysed directly or following treatment with 3-IB-PP1 for the times indicated. Total tyrosine phosphorylation and levels of Csk and ERK were assessed by immunoblotting. (B) Myc immunoprecipiation of wild-type and SH2mut membrane-CskAS demonstrates p65 interacts with Csk in an SH2-dependent manner. Control or membrane-CskAS cells were treated with 3-IB-PP1 or DMSO for 10 minutes. Lysates were immunoprecipitated for the times indicated with anti-Myc and blotted for pTyr and Csk. (C) Silver stain of Myc immunoprecipitates of control or membrane-CskAS cells. Dok-1 is denoted with arrow. (d) Dok-1 IP of control or membrane-CskAS cells. Cells were treated with 3-IB-PP1 or DMSO for 10 minutes. Lysates were immunoprecipitated with anti-Dok-1 and blotted for Dok-1, pTyr, and Csk. Note: WCL and post-IP lysates are probed with anti-Csk which recognizes both wild type (marked with **) and membrane-CskAS (marked with *), while Dok-1 IP was probed with anti-Myc, which recognizes only membrane-CskAS. Data are representative of at least two (C) or three (A, B) independent experiments.
Dok-1 is a scaffolding protein that has been reported to play a negative regulatory role in receptor signaling and cell activation in several cell types, including T cells (12). Dok-1's inhibitory effect requires its localization to the membrane via binding of its plextrin homology domain to phospholipids (13). Phosphorylation of Dok-1 on Y449 by SFKs creates the Csk-binding site (14) identified by mass spectrometry, and is a likely mechanism for membrane recruitment of Csk following receptor stimulation. Indeed, we found Dok-1 phosphorylation was markedly increased when CskAS was inhibited (Fig. 6D). Immunoprecipitation and partial depletion of Dok-1 from membrane-CskAS cells further confirmed its identity as at least a component of the 65kDa protein band seen in lysates. Importantly, inhibition of CskAS and phosphorylation of Dok-1 induced a CskAS-Dok-1 interaction. We suggest that Dok-1 is phosphorylated by Lck and may serve as an adaptor that normally senses Lck activity and recruits Csk to the plasma membrane, particularly following antigen receptor stimulation. Its hyperphosphorylation by Lck and association with CskAS may play a role in the adaptation and “over-shoot” mechanism that we have observed following membrane CskAS inhibition.
DISCUSSION
We have generated a novel analog-sensitive variant of Csk, whose catalytic function can be rapidly and dynamically controlled, which we used to probe the role of Csk in controlling basal and induced signaling downstream of the TCR. Since Csk is a ubiquitous regulator of SFK activity, these findings may be applicable to other receptor systems dependent on SFK activity. In our studies constitutive association of CskAS with the plasma membrane potently inhibited SFK activity and TCR signaling compared to a cytoplasmic-CskAS, consistent with previous data (9). Inhibition of CskAS led to unanticipated activation of the canonical TCR signaling pathway in the complete absence of ligand binding to the TCR. Upon CskAS inhibition, hyperphosphorylation of proximal signaling molecules indicates that ectopic expression of CskAS may have reset basal signaling tone in the cell. Furthermore, signaling induced by inhibition of CskAS was more sustained than signaling following direct TCR stimulation. These findings suggest a compensatory adaptive mechanism that monitors and controls basal signaling, at least, by SFKs.
These studies suggest that an important function of basal signaling is to monitor and respond to changes in tonic activity of key signaling components. Direct evidence for basal signaling in T cells has been limited, but recent results suggest a role of basal signaling in survival, transcription and effector function. Inducible deletion of surface TCR expression results in a shortened half-life of mature T cells (15). Ablation of TCR signaling perturbs gene expression profiles and sensitivity to foreign antigens (16, 17). Expression of the tandem SH2 domains of ZAP-70 markedly increases ITAM phosphorylation in the absence of TCR stimulation (18). A substantial amount of the ZAP-70 tandem SH2 domains associate with ITAMs in resting cells, suggesting that on-going Lck phosphorylation of ζ-chain is protected from phosphatases by binding of ZAP-70 in the basal state. Our data imply that basal signaling is monitored and regulated by feedback circuitry.
The ability of CskAS inhibition to rapidly activate Lck in the absence of TCR ligands demonstrates the plasticity in the Lck activation state in resting T cells. A recent study focused on the substantial amount of Lck that is phosphorylated on its activation loop in resting T cells (1). Furthermore, a marked subset of Lck molecules contained both pY394 and inhibitory phosphorylation on Y505. We propose, based on these results and our own presented here, that in resting T cells a dynamic equilibrium is imposed on Lck by its interactions with Csk and CD45. These interactions lead to a continuous turnover of Y394 and Y505 phosphorylation of Lck and its activity in a basal state, and allow for rapid and efficient phosphorylation of ITAMs by Lck in response to TCR stimulation. We further propose that this dynamic equilibrium of Lck phosphorylation and activity in resting T cells is monitored and adjusted to signaling perturbations during the inactive basal state. One possible mechanism to prevent increased phosphorylation of ITAMs by Lck in resting cells would be a requirement for ligand-induced structural changes in the TCR for an elevated level of signaling to occur, above the basal level (19-21). In this model, the cytoplasmic portion of TCR ITAM tyrosines may bind to the plasma membrane in resting cells. Upon TCR engagement, allosteric or other changes cause the ITAM-containing tyrosines to dissociate from the membrane and become phosphorylated, driving an increased amount of signaling. An alternative model has suggested allosteric changes in CD3e result in accessibility to a polyproline region that binds Nck (22). This model has been challenged by a recent knock-in of the CD3e proline rich motif (23). Since TCR signaling pathways are activated by inhibiting CskAS activity in the absence of TCR engagement, neither of these models is complete. Our results suggest that either CD3ε and ζ-chains may not associate with the membrane, or the interactions are highly dynamic and transient. We favor a model in which a low level of basal signaling is continuously occurring, but is held in check by the balanced action of numerous phosphatases and other inhibitory mechanisms.
The mechanisms that modulate Csk activity during T cell activation are incompletely understood, however we suggest that Dok proteins, specifically Dok-1, contribute to Csk regulation. During T cell activation, removal of Csk from the immune synapse occurs in response to acute dephosphorylation of Csk-recruiting adaptors, such as PAG. However, this release of Csk is transient and genetic ablation of PAG has no effect on T cell signaling, suggesting Csk is likely to interact with additional proteins at the membrane. We suggest one candidate may be the inhibitory scaffolding molecule Dok-1, since it is rapidly phosphorylated and associates with Csk following 3-IB-PP1 inhibition of CskAS. Dok proteins bind to phospholipids in the plasma membrane and can interact with target proteins through a protein tyrosine-binding domain. Furthermore, several conserved tyrosines can be phosphorylated by Abl and SFKs following SFK activation (14), including Csk-binding site Y449. Indeed, a peptide encompassing this site was found to be tyrosine-phosphorylated and associated with CskAS following 3-IB-PP1 in our studies. In fibroblasts, the interaction of Dok-1 pY449 with Csk's SH2 domain is necessary for Csk membrane recruitment and control of Src activity (24). Dok-1 also directly abrogates MAPK by recruiting Ras-GAPs to the signalosome. In our studies, membrane-CskAS may compete more effectively than endogenous Csk for Dok-1 interaction. Thus, endogenous Csk cannot effectively inhibit or regulate Lck function, explaining the overshoot seen with 3-IB-PP1 inhibition of CskAS function.
T cells express Dok-1 and the related family member Dok-2, which together play a critical role in establishing negative feedback loops in TCR signaling. TCR-stimulated T cells deficient in both Dok-1 and Dok-2 exhibit hyperproliferation and augmented cytokine secretion and have prolonged and hyperphosphorylation of signaling molecules including ζ-chain, ZAP-70, LAT and ERK (25, 26), which is reminiscent of our results following CskAS inhibition. Our attempts to alter Dok-1 expression or function yielded inconsistent results, suggesting that Dok-2 may functionally compensate for Dok-1, as has been previously suggested (26, 27). Future studies will need to clarify the role of Dok proteins as part of a proximal TCR feedback loop to regulate proximal signaling via recruitment of Csk to the plasma membrane. Moreover, together with Csk, Dok-1 may play an important role in regulating the intensity and termination of the TCR response.
In conclusion, we have utilized a novel analog-sensitive variant of Csk to demonstrate that dynamic control of SFK activity downstream of the TCR is necessary for setting basal signal tone and preventing aberrant cellular activation. As Csk regulation of SFKs is ubiquitous, these results will be broadly relevant towards understanding how other SFK-associated pathways are regulated in other cell types. Introduction of the CskAS allele into mice will further allow us to examine the regulatory function of Csk in controlling the basal signaling tone, as well as regulation of stimulated-TCR signaling, in primary cells and during lymphocyte development in the context of endogenous Csk-null T cells. Moreover the CskAS allele holds potential for being able to exogenously control T cell activity independent of ligand-binding.
MATERIALS AND METHODS
Cell Lines, Transfections, and Stimulations
Jurkat T cell line E6-1 was cultured and transfected as described before (28). JCaM1, J45, P116 and J14 T cell lines have been previously described (29-33). Vectors encoding GFP or human CD16 were used as cotransfection controls. Prior to stimulation, cells were serum-starved at 37°C for at least 25 minutes. Stimulations were done in serum-free RPMI at 37°C with a 1:1000 final dilution of C305 (anti-TCR Vβ8). 3-IBPP1 was used at 10 μM unless noted. Prior to lysate preparation, CD16+-transfected cells were purified using Miltenyi human CD16 microbeads. The MAPK inhibitor U0126 was used for 45-120 minutes at 20 μM.
Western blot and FACS analysis
For ERK and ζ-chain phosphorylation flow assays, Jurkat T cells were serum-starved and stimulated as described. The addition of an equivalent volume of Cytofix (BD Pharmagin) stopped the reaction. Cells were pelleted, washed in FACS buffer, and then resuspended in ice-cold 90% methanol. Cells were incubated on ice for 30 min followed by 3 washes in FACS buffer. Cells were stained with anti–phospho-p44/42 MAPK, followed by staining with donkey anti–rabbit Ig-APC or PE (Jackson) or anti-CD3ζ-pY142 used at a 1:5 dilution. For calcium analyses, cells were loaded with the calcium-sensitive dyes Fura-Red and Fluo-3 (Invitrogen) for 30 min at 37°C in RPMI and 5% fetal bovine serum, washed and stained on ice with anti-CD16-Alexa647 (BD Pharmagin) to label transfected cells. After surface staining, cells were resuspended in RPMI and warmed to 37°C for 5 min before stimulation. Basal Ca2+ levels were measured for 1 min prior to addition of vehicle or 10 μM 3-IB-PP1 for 3 min. Cells were then stimulated via the TCR for 3 minutes and treated with 1 μM ionomycin as a positive control for 1 min. Ca2+ increase was measured as the ratio of Fluo-3 to Fura-Red fluorescence and was displayed as a function of time for CD16+ transfected cells and CD16− untransfected cells.
Antibodies
LAT-pY191 (Invitrogen/BIOSOURCE); ZAP-70-pY319 (34), ZAP-70-pY493, Src pY416, p44/42 MAPK pThr202/Tyr204, Myc 9B11 (Cell Signaling); Lck (1F6 from J. B. Bolen); pY (4G10; Upstate Biotechnology); LAT (Abcam); ERK1/2 (Santa Cruz); CD3ε (clone UCHT.1), CD69 (BD Pharmagin); hCD16, Lck pY505, CD3ζ-pY142 (BD Pharmagin); Goat α-Rabbit IgG (H+L)-HRP and Goat α-Mouse IgG (H+L)-HRP (Southern Biotech). The following antibodies have been described previously: 2F3.2 (ZAP-70) (35) and 6B10.2 (CD3ζ) (34).
See supplementary information for details of generation of CskAS allele, 3-IB-PP1, TCR internalization assay, mass spec identification of Dok-1 and additional cell lines and primary T cell cultures.
Supplementary Material
SUMMARY.
Chemical-genetic inhibition of a dominant Csk allele rapidly activates T cells
Acknowledgements
We thank members of the Weiss laboratory for thoughtful input throughout this project. We are very grateful to the staff of the UCSF Sandler-Moore mass spectrometry core facility, especially Drs. Eric Johansen, H. Ewa Witkowska and Steven C. Hall, for their help in these studies. Funding: This work was supported by: HHMI (A.W., K.S.), NIH F32 training grant (J.R.S.), and the Singapore Agency for Science, Technology and Research (Y.X.T.) Mass spectrometry analyses were performed by the UCSF Sandler-Moore Mass Spectrometry Core Facility, which acknowledges support from the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103.
Footnotes
Author contributions: J.R.S. designed, performed and analyzed the experiments and prepared the manuscript; Y.X.T. designed, performed and analyzed the Dok-1 experiments and helped prepare the manuscript; C.Z. and K.S. assisted in the technical design of the analog-sensitive Csk allele and generated the PP1 analog library. A.W. was responsible for the overall supervision of the project and experimental design and helped to prepare the manuscript.
Competing interests: the authors declare that they have no competing interests.
REFERENCES and NOTES
- 1.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 4.Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol Cell Biol. 2007;27:1960–1973. doi: 10.1128/MCB.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmedt C, Tarakhovsky A. Autonomous maturation of alpha/beta T lineage cells in the absence of COOH-terminal Src kinase (Csk). J Exp Med. 2001;193:815–826. doi: 10.1084/jem.193.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 7.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 8.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 9.Cloutier JF, Chow LM, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, Schraven B, Rolstad B, Mustelin T, Tasken K. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 11.Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, Weiss A. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228:41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 12.Mashima R, Hishida Y, Tezuka T, Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev. 2009;232:273–285. doi: 10.1111/j.1600-065X.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 13.Guittard G, Gerard A, Dupuis-Coronas S, Tronchere H, Mortier E, Favre C, Olive D, Zimmermann P, Payrastre B, Nunes JA. Cutting edge: Dok-1 and Dok-2 adaptor molecules are regulated by phosphatidylinositol 5-phosphate production in T cells. J Immunol. 2009;182:3974–3978. doi: 10.4049/jimmunol.0804172. [DOI] [PubMed] [Google Scholar]
- 14.Shah K, Shokat KM. A chemical genetic screen for direct v-Src substrates reveals ordered assembly of a retrograde signaling pathway. Chem Biol. 2002;9:35–47. doi: 10.1016/s1074-5521(02)00086-8. [DOI] [PubMed] [Google Scholar]
- 15.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci U S A. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roose JP, Diehn M, Tomlinson MG, Lin J, Alizadeh AA, Botstein D, Brown PO, Weiss A. T cell receptor-independent basal signaling via Erk and Abl kinases suppresses RAG gene expression. PLoS Biol. 2003;1:E53. doi: 10.1371/journal.pbio.0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 18.Qian D, Mollenauer MN, Weiss A. Dominant-negative zeta-associated protein 70 inhibits T cell antigen receptor signaling. J Exp Med. 1996;183:611–620. doi: 10.1084/jem.183.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7:1023–1026. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 20.Sigalov AB, Zhuravleva AV, Orekhov VY. Binding of intrinsically disordered proteins is not necessarily accompanied by a structural transition to a folded form. Biochimie. 2007;89:419–421. doi: 10.1016/j.biochi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Chou JJ, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 23.Mingueneau M, Sansoni A, Gregoire C, Roncagalli R, Aguado E, Weiss A, Malissen M, Malissen B. The proline-rich sequence of CD3epsilon controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat Immunol. 2008;9:522–532. doi: 10.1038/ni.1608. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Janas JA, Niki M, Pandolfi PP, Van Aelst L. Dok-1 independently attenuates Ras/mitogen-activated protein kinase and Src/c-myc pathways to inhibit platelet-derived growth factor-induced mitogenesis. Mol Cell Biol. 2006;26:2479–2489. doi: 10.1128/MCB.26.7.2479-2489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasuda T, Bundo K, Hino A, Honda K, Inoue A, Shirakata M, Osawa M, Tamura T, Nariuchi H, Oda H, Yamamoto T, Yamanashi Y. Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int Immunol. 2007;19:487–495. doi: 10.1093/intimm/dxm015. [DOI] [PubMed] [Google Scholar]
- 27.Di Cristofano A, Niki M, Zhao M, Karnell FG, Clarkson B, Pear WS, Van Aelst L, Pandolfi PP. p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl). J Exp Med. 2001;194:275–284. doi: 10.1084/jem.194.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phee H, Abraham RT, Weiss A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat Immunol. 2005;6:608–617. doi: 10.1038/ni1199. [DOI] [PubMed] [Google Scholar]
- 29.Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 31.Williams BL, Schreiber KL, Zhang W, Wange RL, Samelson LE, Leibson PJ, Abraham RT. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling nonrecptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 33.Koretzky GA, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci U S A. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Oers NS, von Boehmer H, Weiss A. The pre-T cell receptor (TCR) complex is functionally coupled to the TCR-zeta subunit. J Exp Med. 1995;182:1585–1590. doi: 10.1084/jem.182.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.