Abstract
Background: Hashimoto's thyroiditis (HT) has been found to coexist with differentiated thyroid cancer (DTC) in surgical specimens, but an association between the two conditions has been discounted by the medical literature. Therefore, we performed this study to determine any potential relationship between HT and the risk of developing DTC.
Methods: We collected data for thyrotropin (TSH), thyroxine (T4), thyroid peroxidase antibody (TPO-Ab) titers, surgical pathology, and weight-based levothyroxine (LT4) replacement dose for patients who were referred for thyroid surgery. Patients with HT at final pathology were studied further. To estimate thyroid function, patients with preoperative hypothyroid HT (Hypo-HT) were divided into three equal groups based on their LT4 replacement: LT4-Low (<0.90 μg/kg), LT4-Mid (0.90–1.43 μg/kg), and LT4-High (>1.43 μg/kg). A group of preoperatively euthyroid (Euth-HT) patients but with HT by pathology was also studied. All subjects were also grouped based on their TPO-Ab titer in TPO-high (titer >1:1000) or TPO-low/negative (titer <1:1000 or undetectable) groups. The relationship of HT and DTC was studied extensively.
Results: Of 2811 subjects, 582 had HT on surgical pathology, 365 of whom were Euth-HT preoperatively. DTC was present in 47.9% of the Euth-HT, in 59.7% of LT4-Low, 29.8% of LT4-Mid, and 27.9% of LT4-High groups. The relative risk (RR) for DTC was significantly elevated for the Euth-HT and LT4-Low groups (p<0.001), but not for the LT4-Mid or LT4-High replacement dose groups. TPO-low/negative status conferred an increased RR in the Euth-HT and LT4-Low replacement dose groups (p<0.001 both), while TPO-high status decreased it in Euth-HT group (p<0.05) and made it nonsignificant in the LT4-Low group.
Conclusions: HT pathology increases the risk for DTC only in euthyroid subjects and those with partially functional thyroid glands (LT4-Low) but not in fully hypothyroid HT (LT4-Mid and LT4-High). High TPO-Ab titers appear to protect against DTC in patients with HT.
Introduction
For several years the incidence of differentiated thyroid cancer (DTC) has been increasing at a 6.5% annual rate, making it the fastest growing cancer among Americans (1). Even though enhanced detection has been implicated, this does not fully explain the sharp increase in the disease incidence (1,2). For several years the incidence of Hashimoto's thyroiditis (HT) has also been rising (3).
A link between cancer and inflammation has been recognized for more than a century. As early as 1863, Rudolf Virchow noted leukocytes in neoplastic tissue and suggested that this reflected the origin of cancer at sites of chronic inflammation (4). The transformation of normal tissue to cancer is a complex process, depending on the interaction of environmental triggers, genetic transformations, and immune modulation. A number of inflammatory diseases have been linked to cancers: inflammatory bowel disease to colorectal cancer; chronic esophagitis to esophageal cancer; and primary biliary cirrhosis to hepatocellular carcinoma (5,6). An explanation for the increased incidence of thyroid cancer might be found in the increase of HT if there were to be a link.
A link between HT and DTC has been debatable and, in part, depends on the source of the reports. If the source was surgical pathology, the association of HT and DTC has been commonly reported. If the source was clinical observation, the association has been usually discounted. In 1955, Dailey and colleagues (7) reported for the first time an association between HT and DTC based on surgical pathology data. This was later discounted by Holm and colleagues (8) who clinically followed 829 patients diagnosed with HT for 22 years to find that only 2 developed thyroid cancer. Multiple subsequent studies reported an association between HT and DTC in surgical pathology series, with the frequency of cancer reportedly ranging between 10–58% (9–18) in the general population, and up to 76% in select groups like Japanese and white American women (19). Clearly a controversy still exists. The controversy is that the incidence of thyroid cancer seems to be lower than expected in patients with clinical HT (7) while higher in surgical pathology series (7,9–19).
Could it be that the surgical pathology of HT includes two conditions: one being a destructive, clinically evident hypothyroid form not associated with DTC and the other one a less destructive, nonclinically evident form that associates with DTC? We hypothesized that patients with less destructive (nonclinically evident) HT might be at a higher risk of developing DTC than unaffected patients, or patients affected by destructive (clinically evident) HT. Also, in patients with HT, the presence of high titers of thyroid peroxidase antibodies (TPO-Ab), indicative of a destructive autoimmune response with functional impairment, might be associated with a lower risk of DTC. Finally, patients with prolonged exposure to inflammation (disease duration) might have higher risks for cancer than patients with recent onset of HT.
Here we studied the relationship of HT and thyroid cancer taking into account the clinical state of the autoimmune disease as expressed by the presence of residual thyroid function. We attempted to uncover the possibility of a concealed association between HT and DTC by dissecting histologically diagnosed HT from euthyroid to hypothyroid forms of the disease.
Materials and Methods
Thyroid database
The University of Wisconsin Thyroid Multidisciplinary Clinic is a large referral site for thyroid diseases. To evaluate different aspects of thyroid pathophysiology, databases of clinical features, laboratory data, ultrasound images, cytologic, and pathologic results of patients referred to the clinic for thyroid problems were prospectively developed. We reviewed data collected over 19 years (1994–2013). Patients with cytology suspicious or positive for malignancy on fine-needle aspiration, those with large (>4 cm) benign thyroid nodules, and those with goiter associated with compressive symptoms (such as dysphagia, dyspnea, obstructive sleep apnea, or hoarseness) are usually referred for thyroid surgery. HT per se is not usually an indication for thyroid surgery.
Subjects
Subjects were categorized as having HT if the final surgical pathology report revealed bilateral chronic lymphocytic thyroiditis with germinal center formation, which is the standard for pathologic diagnosis. Patients with focal thyroiditis were excluded. The HT subjects were subdivided in euthyroid (Euth-HT) and hypothyroid (Hypo-HT), based on their preoperative thyroid function. The Euth-HT group included patients whose preoperative TSH and free thyroid hormones were within normal range, were not on thyroid hormone replacement, and had no sign/symptoms of hypothyroidism but had bilateral HT on final surgical pathology report. The Hypo-HT group comprised patients diagnosed with hypothyroidism of HT by the combination of an elevated TSH and low free thyroxine (FT4) levels prior to surgery, which were not a result of previous thyroid surgery or radioactive iodine treatment and had bilateral HT on final surgical pathology report. The remaining subjects were used as a control group and were categorized as non-HT. We excluded subjects on levothyroxine (LT4)-suppressive therapy, when used to prevent growth of a goiter or thyroid nodules, patients with prior exposure to radioactive iodine, prior thyroid surgery, or patients with incomplete records.
For all patients, data were collected for sex, age, preoperative weight, TSH, FT4 levels, TPO-Ab titer, preoperative thyroid hormone replacement dose, surgical indication, and surgical pathology. The weight of the patients was obtained from the anesthesiology records to decrease variability. TSH and FT4 concentrations and TPO-Ab titers were measured at the clinical laboratory of the University of Wisconsin Hospital by chemiluminescent immunoassays. TSH concentration is reported in milli-international unit per liter (mIU/L) and FT4 concentration is reported in nanograms per deciliter (ng/dL). TPO autoantibodies are considered the hallmark for the diagnosis of HT.18 The TPO-Ab titer was considered high, if antibodies were detectable after 1000 times or more dilution of the sample (TPO-High group); otherwise it was considered low or negative if undetectable (TPO-Low/Negative group).
For all patients on LT4 replacement, their dose was unchanged for at least 3 months prior to surgery. Their serum TSH and FT4 levels were recorded at least 2 months after the latest dose titration. The weight-based LT4 dose was calculated by dividing the LT4 dose in micrograms, divided by the patients' preoperative weight in kilograms. Data for LT4 were available for 178 of 192 hypothyroid patients with HT (Hypo-HT). Hypo-HT patients were retrospectively divided in three subgroups (tertiles), according to the LT4 replacement: LT4-Low if the replacement dose was <0.90 μg/kg, LT4-Mid if the replacement dose was between 0.90–1.43 μg/kg, and LT4-High if the replacement dose was >1.43 μg/kg. DTC consisted of papillary (PTC), follicular (FTC), or Hürthle cell thyroid cancer (HCC). For the Hypo-HT patients, data were collected on disease duration as well, either by patient self-reporting or by the first evidence of elevated TSH and low FT4. To eliminate any selection bias, the analysis was repeated after excluding subjects with preoperative diagnosis of thyroid cancer. The collection of patient's data and subsequent analysis was approved by University of Wisconsin Human Subjects Institutional Review Board.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Kruskal-Wallis test with Dunn's posttest was used for comparison of multiple group means, and two-tailed Mann-Whitney test was used when comparing the means of two groups for continuous variables. Two-tailed Fisher's exact test was used for categorical variables and odds ratios (ORs) and relative risks (RRs) were calculated. p Value of <0.05 was considered significant.
Results
Patient characteristics
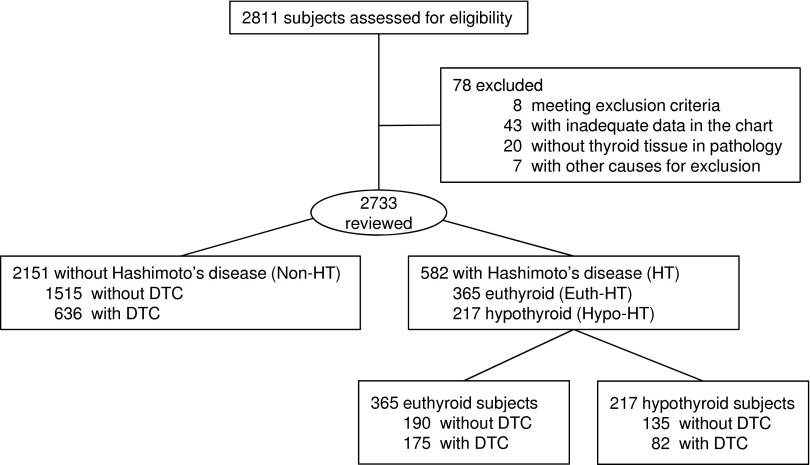
A total of 2811 subjects underwent surgery for thyroid problems between May 1994 and April 2013. Seventy-eight subjects were excluded and data from 2733 subjects were analyzed (Fig. 1). Five hundred ninety-nine were males and 2134 were females. HT was diagnosed postoperatively in 582 patients: 365 were preoperatively euthyroid (Euth-HT group) and 217 (202 of whom had replacement LT4 dose data available) were preoperatively hypothyroid (Hypo-HT group). DTC was diagnosed in 893 subjects: 230 males and 663 females. The age, sex, preoperative TSH, and FT4 concentrations of the total cohort are presented in Table 1.
FIG. 1.
Study flowchart. Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; Hypo-HT, hypothyroid patients with Hashimoto's thyroiditis; DTC, differentiated thyroid cancer (see text for details).
Table 1.
Patients Characteristics
| Hypo-HT | |||||
|---|---|---|---|---|---|
| Non-HT | Euth-HT | LT4-Low | LT4-Mid | LT4-High | |
| n Total | 2151 | 365 | 67 | 67 | 68 |
| n Male | 531 (25) | 46 (13) | 9 (13) | 5 (7) | 5 (7) |
| n Female | 1620 (75) | 319 (87)a | 58 (87)a | 62 (93)a | 63 (93)a |
| Age | 48.8±13.5 | 45.9±15.5a | 49.2±14.1 | 48.2±13.0 | 47.4±13.9 |
| TSH (mIU/L) | 1.5±2.9 | 2.3±1.4a | 3.51±2.61a | 3.46±8.57b | 2.7±3.9b |
| FT4 (ng/dL) | 1.1±0.5 | 1.0±0.2a | 1.0±0.2 | 1.1±0.3b,c | 1.1±0.2b,c |
| Disease duration (months) | __ | __ | 74±91 | 94±87 | 118±105b |
Data are reported as means±standard deviation, or n, followed by the percentage in parenthesis.
p<0.05 vs. Non-HT.
p<0.05 vs. LT4-Low.
p<0.001 vs. Euth-HT.
Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; Hypo-HT, hypothyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose (see text for details); TSH, thyrotropin; FT4, free thyroxine.
Cancer frequency in Euth-HT and Hypo-HT
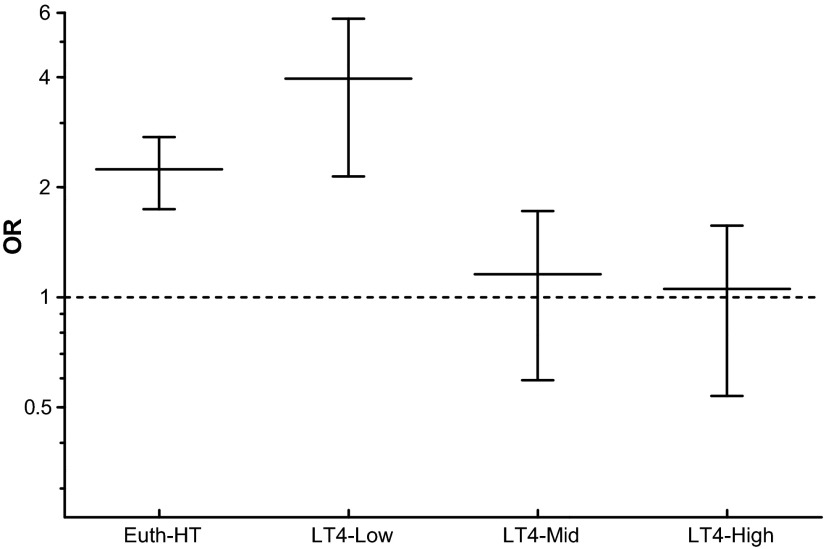
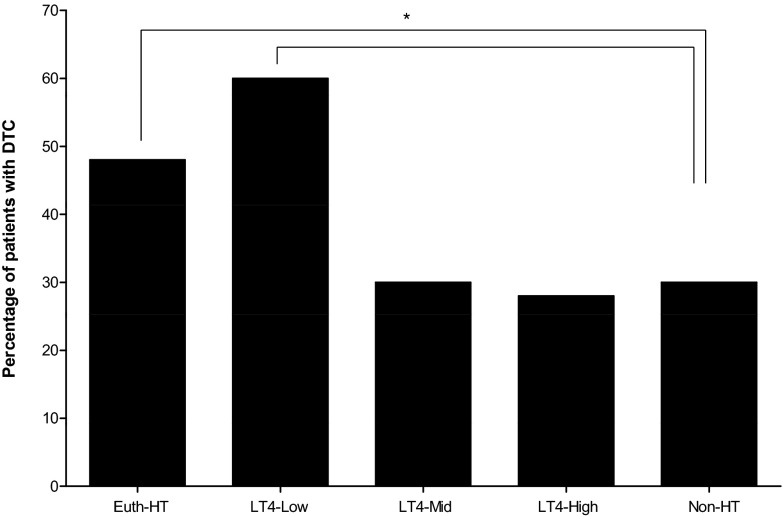
Subjects with HT confirmed by surgical pathology were compared to the Non-HT subjects and the RRs and ORs of having DTC were calculated for Euth-HT and Hypo-HT patients as a whole and for the different LT4 replacement dose subgroups. The Euth-HT group was also compared to the LT4-Low, LT4-Mid, and LT4-High replacement dose subgroups. The RRs are reported in Table 2 and the ORs are depicted in Figure 2. The frequency of DTC in all these groups is depicted in Figure 3. Euth-HT group and LT4-Low subgroup had a significantly higher RR and OR for DTC compared to the control Non-HT group, while the OR and RR were not significantly different for the LT4-Mid and LT4-High dose replacement subgroups. The RR for DTC in the hypothyroid population as a whole (Hypo-HT group) compared to the Non-HT group was 1.04 (1.01–1.07, p=0.0163), while compared to the Euth-HT population the RR was significantly lower at 0.86 (0.76–0.97, p=0.0197). The RR for DTC in the Euth-HT compared to the LT4-Mid was significant at 0.89 (0.83–0.97, p=0.0073). The RR for DTC in the Euth-HT compared to LT4-High was also significant at 0.88 (0.81–0.95, p=0.0022). The RR for DTC in the Euth-HT group compared to the LT4-Low group was not significant at 1.08 (0.99–1.17) p=0.085.
Table 2.
Association of Hashimoto's Thyroiditis and Differentiated Thyroid Cancer
| Non-DTC n (%) | DTC n (%) | Total n (%) | Relative risk (95% CI) | |
|---|---|---|---|---|
| Non-HT | 1515 (70.4) | 636 (29.6) | 2151 (100) | — |
| Euth-HT | 190 (52.1) | 175 (47.9) | 365 (100) | 1.13 (1.09–1.18)a |
| Hypo-HT | ||||
| LT4-Low | 27 (40.3)b | 40 (59.7) | 67 (100) | 1.04 (1.02–1.07)a |
| LT4-Mid | 47 (70.1)b,c | 20 (29.9) | 67 (100) | 1.00 (0.98–1.02) |
| LT4-High | 49 (72.1)b,c | 19 (27.9) | 68 (100) | 1.00 (0.98–1.01) |
Relative risk is calculated compared to the Non-HT group.
Fisher's exact test p<0.001.
Fisher's exact test p<0.05 vs. Euth-HT.
Fisher's exact test p<0.001 vs. LT4-Low.
Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; Hypo-HT, hypothyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High: tertiles of levothyroxine replacement dose; DTC, differentiated thyroid cancer (see text for details); CI, confidence interval.
FIG. 2.
Comparison of the odds ratios for differentiated thyroid cancer (DTC) among the different subgroups of patients affected by Hashimoto's thyroiditis (HT) and the Non-HT population. Euth-HT and LT4-Low subgroups have a significantly increased odds ratio (OR) for DTC, while the LT4-Mid and LT4-High subgroups do not confer such a risk. Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose (see text for details).
FIG. 3.
Frequency of differentiated thyroid cancer (DTC) in the different patient groups. The highest risk for DTC is found in the Euth-HT and the LT4-Low groups. Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose (see text for details). *Relative risk compared to the Non-HT group. Fisher's exact test p<0.001.
TPO-Ab titer and thyroid function
We compared the mean TSH of the Euth-HT patients divided by the TPO-Ab titer with Mann-Whitney test, to assess for the effect of TPO-Ab on thyroid failure. Not surprisingly, we found that the patients with high TPO-Ab titer had a mean TSH of 2.86±1.51, while the mean TSH was 1.85±1.09 in the patients with low or absent TPO-Ab; as expected the difference was statistically significant (p<0.0001).
Cancer frequency and TPO-Ab titer
We subdivided each HT group (Euth-HT and Hypo-HT with subgroups LT4-Low/Mid/High) in two categories based on their TPO-Ab status: TPO-high (TPO-Ab titer >1:1000) and TPO-Low/Negative (TPO-Ab titer <1:1000 or undetectable). The RRs and ORs for DTC in each subdivided group were compared to those in the Non-HT group. We also compared within each HT group the two categories (TPO-High and TPO-Low/Negative). Upon comparing the two categories within the Euth-HT group, the RR for DTC was 1.41 (0.90–2.19), which did not reach statistical significance (p=0.13). For all the hypothyroid HT patients (Hypo-HT), however, the risk was significant (RR for DTC 1.92 [1.31–2.79, p=0.0002]) when TPO-Ab titers were Low/Negative. The risk was not significant within the LT4-Mid replacement dose subgroup—RR 1.23 (0.64–1.28, p=0.68) and the LT4-High subgroup—RR 1.82 (0.71–4.65, p=0.21). In the LT4-Low subgroup, however, the absence or low titers of TPO-Ab was associated with a relative risk for DTC of 1.59 (1.03–2.47) compared to the TPO-High counterpart that trended toward significance (p=0.067). Most strikingly, the association became highly significant when the Euth-HT and LT4-Low subgroups with TPO-Low/Negative titers were compared to Non-HT control group (RRs for DTC 1.06 [1.04–1.09] and 1.01 [1.00–1.02], respectively, and p<0.001 for both). The RRs for DTC are reported in Table 3 and the ORs are depicted in Figure 4.
Table 3.
Differentiated Thyroid Cancer and Thyroid Peroxidase Antibody Status
| Non-DTC n (%) | DTC n (%) | Total n (%) | Relative risk (95% CI) | |
|---|---|---|---|---|
| Non-HT | 1513 (70.3) | 638 (29.7) | 2151 (100) | — |
| Euth-HT | 32 (53.3) | 28 (46.7) | 60 (100) | 1.02 (1.00–1.03)a |
| TPO-High | ||||
| Euth-HT TPO-Low/Neg |
53 (46.1) | 62 (53.9) | 115 (100) | 1.08 (1.05–1.12)b |
| Hypo-HT | ||||
| LT4-Low TPO-High |
14 (53.8) | 12 (46.2) | 26 (100) | 1.01 (1.00–1.02) |
| LT4-Low TPO-Low/Neg |
1 (6.3) | 15 (93.7) | 16 (100) | 1.01 (1.00–1.02)b |
| LT4-Mid TPO-High |
15 (75.0) | 5 (25.0) | 20 (100) | 1.00 (0.99–1.01) |
| LT4-Mid TPO-Low/Neg |
7 (43.8) | 9 (56.2) | 16 (100) | 1.00 (1.00–1.01) |
| LT4-High TPO-High |
18 (85.7) | 3 (14.3) | 21 (100) | 1.00 (0.99–1.00) |
| LT4-High TPO-Low/Neg |
7 (46.7) | 8 (53.3) | 15 (100) | 1.00 (1.00–1.01) |
Relative risk is calculated compared to the Non-HT group by Fisher's exact test.
Fisher's exact test p<0.05.
Fisher's exact test p<0.001.
TPO, thyroid peroxidase antibodies; TPO-High, TPO antibodies titer>1:1000; TPO-Low/Neg, TPO antibodies titer<1:1000 or undetectable (negative); Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; Hypo-HT, hypothyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose; DTC, differentiated thyroid cancer (see text for details); CI, confidence interval.
FIG. 4.
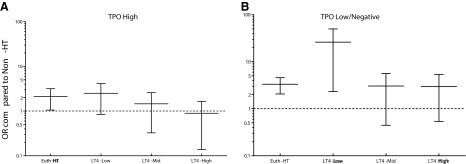
Comparison of odds ratios for DTC among the different subgroups of patients affected by Hashimoto's thyroiditis (HT) and the Non-HT population based on TPO antibody status. Patients affected by HT were split by the TPO-Ab status (see Methods for details) into TPO-High (A) and TPO-Low/Negative (B). We found that the TPO-Low/Negative status conferred an increased risk for DTC in all groups except for the LT4-Mid and LT4-High. On the other hand, the TPO-High group was not significantly different compared to the Non-HT. Non-HT, patients unaffected by Hashimoto's thyroiditis on surgical pathology. TPO-AB, thyroid peroxidase antibodies; TPO-High, TPO-Ab titer>1:1000; TPO-Low/Negative, TPO-Ab titer<1:1000 or undetectable (negative); Euth-HT, euthyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose (see text for details).
Cancer type by group
The absolute number and percentage of patients affected by each different histologic type of thyroid cancer are presented in Table 4 and were not different among all groups. The frequency of PTC, however, was significantly higher in Euth-HT and LT4-Low groups, while lower in LT4-Mid and LT4-High replacement dose groups.
Table 4.
Forms of Differentiated Thyroid Cancer
| PTC n (%) | FTC n (%) | HCC n (%) | |
|---|---|---|---|
| Non-HT | 565 (26.3) | 46 (2.2) | 25 (1.2) |
| Euth-HT | 166 (45.5)a | 8 (2.2) | 1 (0.3) |
| Hypo-HT | |||
| LT4-Low | 38 (56.7)a | 1 (1.5) | 1 (1.5) |
| LT4-Mid | 19 (28.4)b,c | 1 (1.5) | 0 (0.0) |
| LT4-High | 19 (27.9)b,c | 0 (0.0) | 0 (0.0) |
p<0.001 vs. Non-HT.
p<0.05 vs. Euth-HT.
p<0.01 vs. LT4-Low.
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; HCC, Hürthle cell cancer; %, percentile of patients affected by the specific cancer compared to the whole population; Non-HT, subjects without Hashimoto's thyroiditis; Euth-HT, euthyroid patients with Hashimoto's thyroiditis; Hypo-HT, hypothyroid patients with Hashimoto's thyroiditis; LT4, hypothyroid patients on levothyroxine replacement; Low/Mid/High, tertiles of levothyroxine replacement dose (see text for details).
Disease duration
We compared the mean HT disease duration among hypothyroid patients in the different subgroups of LT4 replacement dose and the results are presented in Table 1. We compared the HT disease duration in patients with DTC (102±118 months) and the ones without DTC (92±82 months), using the Mann-Whitney test, and found no statistically significant difference (p=0.59).
Secondary analysis
Because all of our subjects met clinical criteria to undergo thyroid surgery, our results could have been skewed by selection bias. Therefore, we performed a secondary analysis, where all subjects with known preoperative diagnosis of thyroid cancer were excluded. The diagnosis for this secondary analysis was established either by cytology (fine-needle aspiration of a nodule) or by pathology (excisional biopsy of a lymph node or resection of a distant metastatic focus). The study flow sheet is presented in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/thy), the baseline characteristics of our subjects are presented in Supplementary Table S1 and the analysis on cancer risk is presented in Supplementary Tables S2 and S3. The odds ratios for DTC of the HT subgroups as compared to the Non-HT group are depicted in Supplementary Figure S2. The frequency of thyroid cancer by group is depicted in Supplementary Figure S3. In brief, it becomes evident, that independent of the presence of a known diagnosis of DTC preoperatively, Euth-HT and LT4-Low subgroups still have higher risk for thyroid cancer, which is augmented by the absence of high titers of TPO-Ab.
Discussion
Thyroid cancer and thyroid autoimmunity are both on the rise (1,3). Multiple studies have addressed the possibility of a link between both conditions with conflicting results. Several found HT associated with thyroid cancer (7,9–11,14–19), while others did not (8,20).
In an attempt to uncover the possibility of a hidden association between HT and DTC, we divided a histologically diagnosed HT cohort into clinical (hypothyroid) and nonclinical (euthyroid) HT based on the preoperative clinical status. The hypothyroid (clinical) HT cohort was also subdivided in three subgroups according to tertiles of the weight-based LT4 replacement dose. Our main purpose was to separate thyroid functional states in the background of HT and to look at the probability of thyroid cancer development in completely functional glands (euthyroid HT), and thyroid glands partially or completely destroyed (hypothyroid HT) by autoimmune thyroid disease.
Our study revealed a statistically significant association between HT and PTC, only in the euthyroid HT group and the hypothyroid HTs subgroup on low LT4 replacement dose. The thyroid cancer risk trended lower in the hypothyroid HT population on mid and high LT4 replacement dose.
To analyze this finding further, we examined the role of the humoral immune response in the form of TPO-Ab titer on the risk for thyroid cancer. On one hand, we found that an overly active humoral immune response with production of high titers of TPO-Ab led to decreased risk of developing PTC, especially in the severely hypothyroid subgroups, where thyroid function was almost entirely absent. On the other hand, absent or very low TPO-Ab titer was associated with an increased risk of PTC.
Together these observations suggest that the presence of residual thyroid function in patients with HT with negative or low anti-TPO response is placing them at risk for papillary thyroid cancer. On the contrary, patients with advanced autoimmune disease of the thyroid gland as reflected by poor or no thyroid function and high anti-TPO response appeared protected from developing PTC. The unexpected observation that shorter HT duration in the low-dose LT4 replacement group is associated with PTC, while longer duration in the high-dose LT4 replacement group is not, suggests that either chronicity is not a factor in cancer development, or that prolong autoimmunity to the thyroid gland favors tumor control.
Another plausible explanation for that observation could be that the type of immune response in patients who develop mild thyroid failure or remain euthyroid (with low or absent TPO-Ab titers) might be different compared to the population that becomes fully hypothyroid (requiring higher LT4 doses and with high TPO-Ab titers). Furthermore, mild thyroid failure leads to mild increases in TSH levels. Higher but within normal range TSH levels as seen in these patients have been associated with increased risk of DTC (21,22). Stimulation of thyroid cells by TSH activates thyroid hormone synthesis including the activation of thyroid oxidases and the production of hydrogen peroxide, which is used in the organification of iodine (23). In patients with functional HT, stimulation of thyroid cells by TSH could be enhanced compared to the nonfunctional thyroids. Therefore, the exposure to higher concentrations of hydrogen peroxide and free radicals within the follicles could have DNA denaturing/mutagenic effects in thyroid cells and promote carcinogenesis. In patients with thyroid glands utterly destroyed by autoimmunity, the absence of functional thyroid epithelium might not provide the substrate for TSH to act upon, thereby decreasing the risk for thyroid cancer compared to unaffected thyroid glands.
Previous studies have also suggested that inflammation of the thyroid epithelium could be linked to thyroid cancer (15,24,25). In a study by Larson et al. (15) phosphatidylinositol 3-kinase (PI3K) expression was found important for the inflammatory response. PI3K phosphorylates Akt, which in turn suppresses proapoptotic signals thereby promoting carcinogenesis. PI3K/Akt is increased in HT and DTC. Furthermore, Borrello et al. (24), showed that the RET/PTC oncogene, when exogenously expressed in normal thyroid follicular cells, induces expression of a large set of genes involved in inflammation and tumor invasion. Nose et al. (25) demonstrated that stepwise increments in overexpression of COX-2 and iNOS were revealed in epithelial cells in HT, follicular adenoma, and papillary thyroid cancer. The authors concluded that the incremental expression of COX-2 from normal to inflammatory to neoplastic epithelium suggests that this process may have a role in early carcinogenesis.
All these studies suggest a role for inflammation in the development of thyroid cancer. Our study, however, debates whether the immune response in patients who develop thyroid cancer is different from that in patients who develop full-blown autoimmune thyroid failure. The absence or low titers of TPO-Ab in patients with thyroid cancer is further evidence to support the different nature of the immune processes. Hence, another possibility could be that we are dealing with two different processes that appear similar microscopically. In one, the autoimmune response is dominant, in the other cancer seems to control immunity. We cannot rule out the possibility of thyroid cancer itself being capable of regulating the immune response in autoimmune thyroid disease. Cancer immunoediting has been reported for other human cancers already (26). Further studies are needed to assess the types of immunity involved in the different forms of HT, and their effect on thyroid cancer development or vice versa (27).
It is intriguing as well that the observed association is only present with PTC and not with other forms of differentiated or undifferentiated thyroid cancers. This suggests that the inflammatory microenvironment in HT could allow the survival and potentially promote the growth of cells of this tumor type only. Even though no data are available at present, future studies should try to identify the different pathogenic mechanisms by which the immune response in functional HT seem to support papillary thyroid cancer development at the cellular level.
Our study is limited by the use of historical data, even though prospectively collected. Another limitation is the potential for selection bias, since the population studied (patients undergoing thyroid surgery) does not accurately reflect the general HT population. However, we reanalyzed the data excluding all patients with preoperative diagnosis of thyroid cancer. With this analysis we found an even stronger association between euthyroid/functional HT and differentiated thyroid cancer, eliminating any significant effects from this bias. An additional limitation arises from the fact that LT4 therapy is not managed uniformly by all providers, thus leading to TSH concentrations outside the optimal range in many hypothyroid patients. This could have produced some degree of bias towards the difference among the three LT4 replacement subgroups, but did not alter the essence of this analysis, which is that patients with functional HT that undergo thyroid surgery have a higher risk for thyroid cancer, compared to patients without HT. Despite these limitations, we were able to confirm in a large cohort of patients that histologically diagnosed HT is associated with thyroid cancer in the absence of full-blown hypothyroidism due to HT. This risk is of higher magnitude if TPO-Ab are absent or present in very low titers. We were surprised by the fact that the duration of hypothyroidism was shorter in patients at increased risk for thyroid cancer when compared to their fully hypothyroid counterparts. This observation suggests that in patients with euthyroid/functional HT, there is a different immune disorder that does not completely destroy the thyroid gland, or an active participation of cancer in regulating immunity/autoimmunity.
All together, the association of histologically diagnosed HT and thyroid cancer in functional thyroid glands but not in those affected by full-blown autoimmune hypothyroidism, reconciles the opposing views of the association found by some (7,9–11,14–18), but not others (8,20). Furthermore, our study provides an alternative explanation for the increased thyroid cancer risk in patients with higher but within normal TSH and links the observed increase incidence of both diseases over time. In addition, our findings urgently call for a new line of research in thyroid cancer as it relates to inflammation. Finally, our study also supports the concept that autoimmunity is similar to effective tumor immunity (28).
Supplementary Material
Acknowledgments
We thank Dr. Herbert Chen and his group for providing access to the UW surgical database.
Preliminary data were presented in part at the Endocrine Society Annual Meeting, June 2013.
This work was supported in part by National Institutes of Health and Veterans Affairs funding (J.C.J.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM.2013Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980–2008. Thyroid 23:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Schneider D, Mazeh H, Jaume JC, Lubner S.2013Endocrine Cancer. In: Abeloff MD, Armitage JO, Niederhuber JE, Kastan MB, McKenna WG. (eds) Abeloff's Clinical Oncology, 5th edition. Churchill Livingstone Elsevier, Philadelphia, PA, pp. 1112–1142 [Google Scholar]
- 3.Braverman LE.1994Iodine and the thyroid: 33 years of study. Thyroid 4:351–356 [DOI] [PubMed] [Google Scholar]
- 4.Virchow R.1956Standpoints in Scientific Medicine, 1877. Bull Hist Med 30:537–543 [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A.2001Inflammation and cancer: back to Virchow? Lancet 357:539–545 [DOI] [PubMed] [Google Scholar]
- 6.Franks AL, Slansky JE.2012Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res 32:1119–1136 [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey ME, Lindsay S, Skahen R.1955Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 70:291–297 [DOI] [PubMed] [Google Scholar]
- 8.Holm LE, Blomgren H, Lowhagen T.1985Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med 312:601–604 [DOI] [PubMed] [Google Scholar]
- 9.Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, Latteri S, Latteri MA.2005Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg 71:874–878 [PubMed] [Google Scholar]
- 10.Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA.2013Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting hashimotos thyroiditis. J Clin Endocrinol Metab 98:2409–2414 [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg BL, Hensley SD.1989Thyroid cancer with coexistent Hashimoto's thyroiditis. Clinical assessment and management. Arch Surg 124:1045–1047 [DOI] [PubMed] [Google Scholar]
- 12.Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L, Pinchera A, Vitti P.2011Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer 18:429–437 [DOI] [PubMed] [Google Scholar]
- 13.Fiore E, Rago T, Scutari M, Ugolini C, Proietti A, Di Coscio G, Provenzale MA, Berti P, Grasso L, Mariotti S, Pinchera A, Vitti P.2009Papillary thyroid cancer, although strongly associated with lymphocytic infiltration on histology, is only weakly predicted by serum thyroid auto-antibodies in patients with nodular thyroid diseases. J Endocrinol Invest 32:344–351 [DOI] [PubMed] [Google Scholar]
- 14.Jankovic B, Le KT, Hershman JM.2013Clinical Review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 98:474–482 [DOI] [PubMed] [Google Scholar]
- 15.Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, Evers BM.2007Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg 204:764–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott RA, McCall AR, McHenry C, Jarosz H, Armin A, Lawrence AM, Paloyan E.1987The incidence of thyroid carcinoma in Hashimoto's thyroiditis. Am Surg 53:442–445 [PubMed] [Google Scholar]
- 17.Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H.2008Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res 150:49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, Shen Q, Li DS, Wu Y.2012The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer 12:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okayasu I, Fujiwara M, Hara Y, Tanaka Y, Rose NR.1995Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer 76:2312–2318 [DOI] [PubMed] [Google Scholar]
- 20.Rago T, Di Coscio G, Ugolini C, Scutari M, Basolo F, Latrofa F, Romani R, Berti P, Grasso L, Braverman LE, Pinchera A, Vitti P.2007Clinical features of thyroid autoimmunity are associated with thyroiditis on histology and are not predictive of malignancy in 570 patients with indeterminate nodules on cytology who had a thyroidectomy. Clin Endocrinol 67:363–369 [DOI] [PubMed] [Google Scholar]
- 21.Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H.2009Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol 71:434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H.2008Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metabl 93:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilain B, Maenhaut C, Miot F, Van Sande J, Many MC, Dumont JE.2007Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab 92:3764–3773 [DOI] [PubMed] [Google Scholar]
- 24.Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P, Pilotti S, Cassinelli G, Bressan P, Fugazzola L, Mantovani A, Pierotti MA.2005Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA 102:14825–14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nose F, Ichikawa T, Fujiwara M, Okayasu I.2002Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors: significant correlation with inducible nitric oxide synthase. Am J Clin Pathol 117:546–551 [DOI] [PubMed] [Google Scholar]
- 26.Schreiber RD, Old LJ, Smyth MJ.2011Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331:1565–1570 [DOI] [PubMed] [Google Scholar]
- 27.Imam S, Paparodis R, Sharma D, Jaume JC.2014Lynphocytic profiling in thyroid cancer provides clues for failure of tumor immunity. Endocr Relat Cancer 21:505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman HL, Wolchok JD.2006Is tumor immunity the same thing as autoimmunity? Implications for cancer immunotherapy. J Clin Oncol 24:2230–2232 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.