Abstract
Focal therapy of prostate cancer is an evolving treatment strategy that destroys a predefined region of the prostate gland that harbors clinically significant disease. Although long-term oncologic control has yet to be demonstrated, focal therapy is associated with a marked decrease in treatment-related morbidity. Focal laser ablation is an emerging modality that has several advantages, most notably real-time magnetic resonance imaging (MRI) compatibility. This review presents the principles of laser ablation, the role of multiparametric MRI for delineating the site of significant prostate cancer, a summary of published clinical studies, and our initial experience with 23 patients, criteria for selecting candidates for focal prostate ablation, and speculation regarding future directions.
Key words: Laser ablation, Prostate cancer, Focal therapy, Targeted therapy
Prostate cancer is the most common solid organ malignancy and the second most common cause of cancer death among men living in the Western world.1 Widespread prostate-specific antigen (PSA) testing and decreased thresholds for prostate biopsy have led to both a reduction in the proportion of men diagnosed with advanced disease and disease-specific mortality. The consequence of widespread PSA screening has been a dramatic increase in both the detection of low-risk disease and the proportion of men diagnosed with prostate cancer undergoing radical prostatectomy (RP) or radiation therapy (RT).2 In many cases, the complications associated with treating low-risk disease by RP or RT outweigh the benefits.3,4 Although active surveillance (AS) is an appealing alternative for managing low-risk disease, it potentially decreases long-term survival rates.5 Due to the unreliability of disease risk stratification at the time of diagnosis, 14% to 41% of men assigned to AS will cross over to RP or RT due to upgrading or upstaging.6
There is increasing evidence that multiparametric magnetic resonance imaging (mpMRI) localizes the site(s) of clinically significant prostate cancer prior to prostate biopsy.7 These suspicious MRI focal abnormalities can be biopsied directly in the MRI unit or under transrectal ultrasound (TRUS) guidance using software that co-registers and fuses the MRI and ultrasound (US) images.8 In many cases, MRI image-guided biopsy identifies a single clinically significant cancer. Although prostate cancer is typically a multifocal disease, the index, or dominant, lesion is typically predictive of extraprostatic extension and disease progression.9–11 The majority of the secondary tumor sites are composed of small Gleason 6 disease, which represent no immediate threat.12 It is theoretically possible to focally ablate only the index lesion, thereby achieving oncologic control while minimizing treatment-related morbidity by minimizing collateral damage to adjacent structures.
Focal ablation of prostate cancer is an evolving treatment strategy that destroys a predefined region (or target) of the prostate that harbors the clinically significant cancer. A number of energy sources have been investigated for focal ablation of the prostate, including cryotherapy,13 high-intensity focused ultrasound (HIFU),14 photodynamic therapy,15 and laser ablation.16 Although long-term oncologic control has yet to be demonstrated, all of these targeted ablative options are associated with marked decrease in treatment-related complications. One of the advantages of laser technology is that the ablation can be performed with real-time MRI imaging. Because the target lesion are almost always defined by the MRI, laser ablation is currently the most accurate way to deliver ablative energy to the intended target. Other advantages of laser ablation include its homogeneous tissue necrosis, relatively low cost, and wide availability.17 MRI-guided focal ablation allows treatment monitoring using MR thermometry and real-time visualization of the targeted treatment zone.18,19
This review presents the principles of laser ablation, the role of mpMRI for delineating the site of significant prostate cancer, a summary of published clinical studies and the New York University Langone Medical Center (NYULMC)/Sperling Prostate Cancer Center experience on focal laser ablation of prostate cancer, criteria for selecting candidates for focal prostate ablation, and speculation regarding future directions of focal laser ablation for the treatment of localized prostate cancer.
Focal Laser Ablation of the Prostate
Principles of Laser Ablation
Laser ablation refers to the destruction of tissue using a focused beam of electromagnetic radiation emitted from a laser. Other terms for laser ablation include photothermal therapy, laser interstitial therapy, and laser interstitial photocoagulation.
The principle of focal laser ablation therapy is to destroy a tissue target using laser radiation energy. The resulting rapid temperature elevation of the targeted tissue induces protein denaturation, resulting in in vivo tissue destruction. Prostate tissue is well suited for focal laser ablation due to its optical absorption rate without excess vascularity, which allows for finely controlled ablation.20
Effective focal laser ablation for the treatment of prostate cancer requires (1) accurate delivery of the laser energy to the target tissue, (2) sufficient thermal destruction to reliably destroy the target tissue, and (3) minimal thermal destruction to surrounding tissues and neurovascular structures. Each step provides its own set of technical challenges, but ongoing advances in image acquisition and analysis, bioheat transfer modeling, and laser delivery technology make laser ablation of prostate cancer feasible today.
Accurate ablation of the target is accomplished through transperineal or transrectal introduction of a laser fiber into the focal abnormality. Applying real-time, three-dimensional (3D) MRI reconstructions, Stafford and colleagues18 demonstrated that laser applicators were positioned within a mean ± SD of 1.1 ± 0.7 mm of the target site in seven canine prostate models. Accurate laser fiber localization to soft tissue targets is feasible, and real-time MRI during the ablative procedure allows precise estimates of the extent of tissue necrosis.21
Destruction of tissue is mediated by thermal conversion of focused electromagnetic energy, which raises tissue temperature causing coagulative necrosis. Because the heating effect on tissue depends both on the heat energy delivered and the depth of penetration, the extent of tissue destruction is dependent on the wavelength of the laser radiation. The laser originally used for interstitial laser ablation was the infrared emitting 1064 nm Nd:YAG laser. Although the Nd:YAG laser is commonly employed for focal laser ablation, small 1064 nm diode lasers are gaining popularity due to their portability, power, and cost effectiveness.22
van Nimwegen and associates23 described histologic changes to ex vivo canine prostate tissue samples as a function of irradiation time, intensity, and subsequent temperature change. Accordingly, supraphysiologic temperatures (above 42°;C) will result in tissue destruction with longer heating times.23 Spatiotemporal temperature monitoring of the tissue during laser application confirmed a linear increase in temperature with longer radiation time and radiation intensity, as well as exponentially decreasing changes in temperature with tissue depth. These relationships among laser energy, tissue temperature, and tissue viability are critical in dosimetric planning for tissue ablation.
Minimal thermal destruction to surrounding tissues and neurovascular structures is achieved through real-time monitoring during the tissue ablation. This can be achieved by proton-resonance frequency (PRF) shift MR thermometry, which allows near real-time quantification of temperature using changes in the phase of gradient-recalled echo (GRE) images to estimate relative temperature changes (ΔT).24 Peters and colleagues21 demonstrated that PRF shift MR thermometry correlates with temperature measurements of histologically determined areas of tissue necrosis in in vivo canine prostate models. Additionally, Stafford and colleagues found an excellent correlation (r2 = .94) between actual tissue damage seen on 3D T1-weighted MRI and predicted tissue damage using MR thermometry and an Arrhenius damage integral.18 Real-time MR thermometry represents an important tool to optimize ablation of target lesions with minimal thermally induced damage to surrounding tissue and important physiologic structures.
The Role of MRI for Target Identification
Focal ablation is based on the premise that clinically significant prostate cancer can be identified and localized prior to intervention. Ultrasonography, computed tomography (CT) imaging, and T1/T2-weighted MRI lack adequate sensitivity and specificity for detecting clinically significant prostate cancer. As mentioned above, mpMRI, which incorporates diffusion-weighted imaging (DWI) and dynamic contrast enhancement, is emerging as a useful modality to reliably detect and even characterize clinically significant prostate cancer.25,26 A meta-analysis reported that the sensitivity and specificity of DWI for prostate cancer detection are 0.69 and 0.89, respectively, compared to the sensitivity and specificity of T2-weighted imaging alone (0.60 and 0.76, respectively).27
Several studies support the utility of mpMRI for detecting the site of clinically significant prostate cancer.
Bratan and colleagues28 correlated preoperative mpMRI and surgical specimens for 175 men undergoing RP. Focal abnormalities observed on mpMRI were localized using a 27-grid diagram. RP whole mount sections were digitized, and regions of cancer were highlighted. Focal abnormalities on mpMRI were considered true positives if their diameters corresponded between 50% to 150% with a histologic cancer in an overlapping region. mpMRI detected 25%, 48%, and 71% of Gleason 6 diameter tumors < 0.5, 0.5–2, and > 2 cm, respectively; 63%, 85%, and 97% of Gleason 7 diameter tumors < 0.5, 0.5–2, and > 2 cm, respectively; and 80%, 93%, and 100% of Gleason > 7 diameter tumors, respectively. mpMRI was unreliable at detecting small Gleason 6 tumors yet very reliable at detecting the majority of Gleason 7 and virtually all Gleason > 7 cancers.28
Numao and colleagues29 performed mpMRI on 351 consecutive men undergoing prostate biopsy and suspected of having prostate cancer. Of men with a normal or low suspicious mpMRI result, only 20% had significant prostate cancers, whereas 46% and 74% of mpMRIs graded 4 or 5 exhibited significant prostate cancer, respectively. This study provides compelling evidence that mpMRI is a useful tool for identifying those cases harboring clinically significant prostate cancer. There were limitations to the study, however, including ascertainment bias because cancer was based on biopsy alone, the utilization of various biopsy protocols, and the use of mpMRI guidance in only a small subset of biopsy procedure cases.29
Haffner and colleagues30 were the first to demonstrate the utility of mpMRI for directing prostate biopsies. A standard 12-core TRUS random guided biopsy was performed in all 555 cases. Of the 351 cases with a positive mpMRI result, additional tissue cores were directed into the focal abnormalities using visual estimation, or cognitive co-registration. Visual estimation identified virtually all of the clinically significant cancers while failing to detect many of the clinically insignificant cancers
Software now exists for performing coregistered MRI targeted biopsy. Sonn and colleagues31 have recently summarized the experience with mpMRI/US coregistration biopsy. The MRI-guided prostate biopsy appears to improve sampling efficiency, increasing the detection of clinically significant cancers with fewer cores and reducing the detection of insignificant cancers.32 In a comparative study of computer versus visual estimation coregistration biopsy, Wysock and associates33 reported a trend showing increased detection of all cancers and Gleason > 6 cancers with the computer coregistration.
These studies collectively demonstrate a high specificity of mpMRI for identifying significant prostate cancer. In addition, a negative mpMRI has exceedingly high negative predictive value for the absence of significant prostate cancer.25 On the basis of these observations, at NYULMC we obtain an mpMRI prior to performing even routine prostate biopsy. All focal abnormalities suspicious for prostate cancer are target biopsied using the Eigen Artemis (Grass Valley, CA) computer coregistration system. Today, we continue to perform a computer generated 12-core TRUS-guided biopsy, although we recognize it may be superfluous. Men with a single biopsy proven mpMRI-guided prostate cancer are deemed candidates for focal prostate cancer ablation.
Focal Laser Ablation of the Prostate: Clinical Trials
To date, all published investigations of focal laser ablation for prostate cancer are small nonrandomized studies with only short-term oncological follow-up (Tables 1 and 2).
Table 1.
Operative Parameters
| Study | Anesthesia | Real-Time Imaging | Wavelength (Laser Source)a | Number of Fibers | Energy (Joules), Power (Watt), Time (Sec) | Cancer Volume (cm3) | Location | Thermal Damage Monitoring | Visible Dimension of Thermal Necrosis |
|---|---|---|---|---|---|---|---|---|---|
| Amin Z et al34 | Sedation and local anesthesia | Transrectal ultrasound | 805 nm (Diomed diode) | 3 | 3000 J, 2 W, 500 s | Unknown | Peripheral zone | No | Unknown |
| Atri M et al35 | General | Transrectal CEUS | 830 nm (Indigo diode) | 2 | 2880 J, 2–15W (temperature control at 100°;C), 720 sec | 0.25 | Mid-posterior region | Yes | 176 |
| Lindner U et al35 | General | Transrectal CEUS | Unknown | 1 or 2 | Unknown | Mean 0.25 (range, 0.1–0.99) | Unknown | Yes | 300–4000 |
| Raz O et al37 | General | MRI-guidance and CEUS | 980 nm (Visualase diode laser) | ≥2 | Unknown | Unknown | Medial mid-prostate; peripheral zone | Yes | Unknown |
| Lindner U et al19 | General | CEUS | Unknown | 2 or 3 | 3260–5900 J | Not reported | Not reported | Yes | 2500–4500 |
| Woodrum et al39 | General | MRI-guidance | Unknown | 2 or 3 | Unknown | 2.56 | Right of urethral anasto-mosis at the bladder base anterior to the rectum | Yes | Unknown |
| Lindner G et al38 | General | Robotic MRI-guidance | Unknown | Unknown | Unknown | 0.79; 0.24 | Both cases irregularly shaped lesions, obliquely angled | Yes | 8700–9300 |
| Oto A et al16 | Sedation and local anesthesia | MRI-guidance | 980 nm (Visualase diode laser) | 1 | 2967 J, 11.5 W, 258 s | Max diameter 7 mm (range, 4–12) | All in peripheral zone | Yes | Unknown |
aDiomed, Andover, MA; Indigo® manufactured by Ethicon Endo-Surgery, Cincinnati, OH; Visualase, Houston, TX. CEUS, contrast-enhanced ultrasound; MRI, magnetic resonance imaging; US, ultrasound.
Table 2.
Clinical Outcomes
| Study | Number of patients (n) | Age (y) | Preoperative PSA (ng/mL) | Gleason Score on Biopsy | Potency Deteriorationa | Continence Deteriorationa | Mean Postoperative PSA (ng/mL) | Follow-Up | Adverse Events | Results or Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Amin Z et al34 | 1 | 65 | 53 | Unknown | None | None | 40 | CT and biopsy | Mild dysuria relieved a few days later | Cancer cells on 3-mo biopsy; 2nd session confirmed presence of necrosis |
| Atri M et al35 | 1 | Unknown | Unknown | 6 | None | None | Not reported | Gd-enhanced MRI | None | Coagulated lesion on CEUS corresponds well with lesion seen on Gd-enhanced MRI on 7 d |
| Lindner U et al36 | 12 | 51–62; Mean 56.5 | 5.7 ± 1.1 | 6 | None (IIEF-5) | None (IPSS) | Not reported | MRI and prostate biopsy | Perineal discomfort (3) Mild hematuria (2) Hematospermia (2) Fatigue (1) | Based on multicore total prostate biopsy at 6 mo, 67% of patients were free of tumor in the targeted area and 50% were free of disease |
| Raz O et al37 | 2 | 72; 74 | 4.79; 2.74 | 6; 6 | Not reported | Not reported | Not reported | MRI | None | MR scans performed 2 wk post-treatment showed no evidence of complications with preservation of rectum and neurovascular bundles |
| Lindner U et al19 | 4 | 64; 73; 68; 61 | 2.9; 14.8; 4.9; 3.5 | 6; 7; 7; 6 | Not reported | Not reported | Not reported | MRI | Not reported | Strong correlation between MRI findings and vital stain histopathology images |
| Woodrum et al39 | 1 | 59 | 2.0 | 7 | None | None | 0.42 | MRI | None | Dynamic contrast-enhanced MRI after procedure showed no definitive abnormal enhancement |
| Lindner G et al38 | 2 | 50; 65 | 5.84; 10.65 | Unknown | None (IIEF-5) | None (IPSS) | Not reported | MRI | Not described | Ablation zone treatment area encompassing the tumor on MRI |
| Oto A et al16 | 9 | 52–77; mean 61 | Mean 5.5 ± 2.6 | Eight patients 6, one patient 7 | None (SHIM) | None (IPSS) | 5.7 ± 4.4 (1 mo); 4.7 ± 3.6 (3 mo); 5.5 ± 4.0 (6 mo) None significant. | MRI and biopsy | Abrasion on perineum (1) Transient paresthesia of 1/4 glans penis (1, self-resolved) | At 6 mo, MRI-guided biopsy of ablation zone revealed benign prostate in 7 patients (78%) and Gleason grade 6 cancer in 2 (22%) |
EUS, contrast-enhanced ultrasound; CEUS, contrast-enhanced ultrasound; CT, computed tomography; Gd, gadolinium; IIEF, International Index of Erectile Function; IPSS, International Prostate Symptom Score; MRI, magnetic resonance imaging; PSA, prostate-specific antigen; SHIM, Sexual Health Inventory in Men.
aDetermined by questionnaire.
In 1993, Amin and colleagues34 reported the first case of focal laser ablation for prostate cancer. Two attempts at focal laser ablation were required to achieve local disease control following failed external beam RT. The ablated area on follow-up CT was nonenhancing and avascular. Biopsies from the treated region confirmed the presence of only necrosis. There were no significant treatment-related complications during the procedure.
Atri and colleagues35 demonstrated the first application of contrast-enhanced ultrasound (CEUS)-guided focal laser ablation with thermal monitoring. The coagulated lesion measured by intraoperative CEUS corresponded with the gadolinium (Gd)-enhanced MRI lesion at 7 days, suggesting that intraoperative CEUS provides a measure of the treatment effect.
Lindner and colleagues36 reported the first phase I study (NCT00448695) assessing the feasibility and safety of CEUS-guided focal laser ablation. Eligibility criteria include no prior diagnosis of prostate cancer; low risk prostate cancer (T1c or T2a, PSA < 10 ng/mL; Gleason score ≤ 6; only 1 of 12 cores exhibiting < 30% cancer following TRUS-guided biopsy); and the location of the positive tissue core corresponding with a focal abnormality on mpMRI.
The focal ablation was performed under general anesthesia in the dorsal lithotomy position. A high-resolution 3D US was acquired using an automated system. The planned treatment volume defined by the mpMRI was transferred to the 3D US-derived image. The laser fibers were directed into the predefined treatment zone using a modified brachytherapy template and inhouse treatment planning software. Photothermal therapy was delivered via the Indigo® Optima laser (Ethicon Endo-Surgery, Cincinnati, OH). Intra-prostatic temperatures were monitored during the procedure with fluoroptic temperature to ensure that temperatures over 55°;C were achieved at the borders of the target. Additional temperature probes were positioned near critical structures to ensure temperatures at these sites did not exceed 42°;C. Photothermal effect was monitored with CEUS.
Of the 12 men undergoing focal laser ablation, 2 patients complained of perineal discomfort, 2 developed mild hematuria, 2 had hematospermia, and 1 complained of fatigue. The median treatment volume based on post-treatment MRI was 2.2 cm3. Repeat 10-core TRUS-guided biopsies along with two additional cores directed into the targeted area were obtained at 3 and 6 months following ablation. Based on multicore total prostate biopsy at 6 months, 67% of patients were free of tumor in the targeted area, and 50% were totally free of disease. Two patients had tumor on the contralateral untreated side. Of the four patients with residual disease in the targeted areas, two had minimal disease, and the other two had two cores > 50% Gleason 6 disease. Validated questionnaires revealed no decrease in mean urinary and sexual function scores.36
The same group of researchers demonstrated that focal laser ablation creates confluent ablation with no evidence of viable cells in treated regions by assessing whole-mount histology of four patients who underwent focal laser ablation followed by RP. MRI-calculated ablated volume correlated well with histopathology (range, 0.96–1.29; r = .89), suggesting postablation MRI is a useful tool for assessing extent of tissue ablation.19
In 2010, Raz and colleagues37 reported an initial experience with real-time, MRI-guided, focal laser ablation. The advantages of MRI guidance are numerous: improved visualization of the target; real-time guidance of the laser fiber into the target, real-time monitoring and control of the zone of ablation and surrounding tissue; and the immediate confirmation of the extent of treatment. In this case, immediate post-treatment contrast-enhanced MRI confirmed devascularization of the target.37
The first case of robotic MR-guided focal laser ablation was described by Lindner and colleagues38 in 2011. The robotic laser fiber insertion device allows for both precise transperineal placement and oblique angulation of the fibers, resulting in a reduction in targeting time and more accurate targeting of the cancer. Post-treatment Gd-enhanced MRI scans showed the ablation treatment area encompassing the tumor in two cases.38
Oto and colleagues16 published the results of a phase I trial (NCT01192438) investigating the feasibility and safety of MRI-guided focal laser ablation. Eligibility criteria included low-risk prostate cancer characteristics (clinical stage T1c-T2a prostate cancer, PSA level < 10 ng/mL, and Gleason score of ≤ 7); a maximum of three cores containing cancer following a 12-core, TRUS-guided random biopsy; no single biopsy core with more than 50% tumor involvement; and a suspicious lesion visible on MRI corresponding to the biopsy sites of cancer.
Under conscious sedation, the patients were placed supine in the bore of a 1.5 T MR unit. All MR images were obtained using a combination of cardiac array and endorectal coils. A modified brachy-template along with saline filled fiducials was secured against the perineum. An open-ended 14-ga, 14-cm catheter with an MRI-compatible titanium obturator was inserted transperineally into the target lesion. After confirming the location of the obturator by T2-weighted imaging, an optical fiber with a 1-cm diffusing tip and 980-nm diode laser surrounded by a 1.65-mm cooling catheter manufactured by Visualase, Inc. (Houston, TX) was attached to a flow circuit of room temperature sterile saline and inserted into the target lesion. Temperature was monitored by temperature-dependent PRF shift from phase-sensitive images every 5 seconds. The duration of the entire procedure ranged from 2.5 to 4 hours, and the mean duration of the ablation was 4.3 minutes (range, 1.5–7.5 min).
At 6 months, two to three tissue cores were directed into the treated area under MRI guidance. Benign prostate was revealed in seven patients (78%), and Gleason grade 6 cancer was detected in two (22%) (2.5 mm and 1 mm, respectively). Quality-of-life questionnaires were administered at baseline and at designated times postablation. At 6 months, there was no significant change from baseline in the mean International Prostate Symptom Score (IPSS) or mean sexual function scores. MRI-guided biopsy of the ablation zone revealed benign prostate in seven patients (78%) and Gleason grade 6 cancer in two patients (22%). On retrospective review of the ablation images, the lesion site was not completely covered by the ablation zone for the two patients with residual cancer at follow-up biopsy.16
The oncologic efficacy of focal laser ablation is currently under investigation in phase II clinical trials at the University of Chicago (NCT01792024), University of Toronto (NCT01094665), and National Cancer Institute (NCT01377753).
The NYULMC/Sperling Prostate Cancer Center
Between April 2013 and April 2014, 21 men underwent focal laser ablation as part of a collaboration between the NYULMC Smilow Comprehensive Prostate Cancer Center and the Sperling Prostate Cancer Center. All candidates for this collaborative focal laser ablation of the prostate study signed informed consent to participate in a longitudinal outcomes study.
The selection criteria for focal laser ablation included a 10-year life expectancy, between one to two focal abnormalities on mpMRI consistent with prostate cancer, no Gleason pattern 4 disease on random TRUS-guided biopsies of the normal appearing prostate on mpMRI, focal abnormality on MRI < 15 mm, and no Gleason score over 7. In most cases, preservation of potency was a very high priority. Candidates are extensively counseled regarding the very limited short-term and lack of long-term oncologic outcomes data with focal prostate cancer laser ablation.
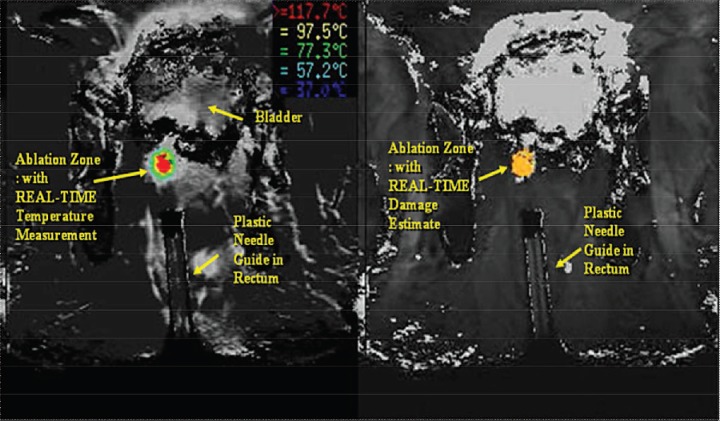
The entire ablation procedure is performed in the MR unit in the prone position. Valium, 10 mg by mouth, is administered immediately prior to the lidocaine periprostatic nerve block, which is administered with US guidance. The needle sleeve biopsy guide is then placed in the rectum. The localization device is attached to the biopsy guide and preliminary sequences (T2 axial and sagittal sequences) are acquired to document optimal positioning (Figure 1). Focal abnormalities are localized using software and needle track pathways, determined using coordinates from the calibrated localization device. The device base is adjusted and a confirmation MRI scan is conducted to confirm needle-guide trajectory to target. A 14-ga cooling guide with MR-compatible trochar is placed into the target lesion. The trochar is then removed, and the laser applicator is inserted through the cooling guide into the lesion. MRI scans are performed to confirm the location of the laser applicator.
Figure 1.
Fiber placement T2-weighted axial sequence.
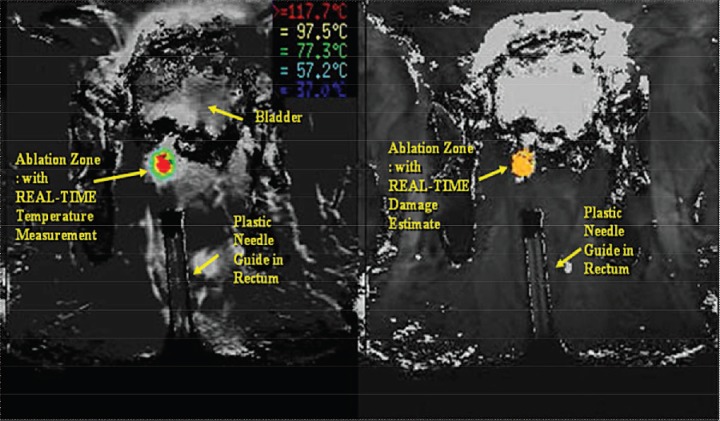
A planning imaging plane is chosen, usually T1, and loaded into the thermometry tracking system. Temperature-sensitive fast spoiled gradient-recalled echo images are acquired repeatedly on the MRI and transferred in real time to the thermometry workstation for analysis to ensure minimal thermal destruction to surrounding tissues and neurovascular structures (Figure 2). After establishment of baseline, we utilize a 980-nm diode-based laser activated at a reduced power level at 3 W, insufficient to cause thermal injury. This intermediary step is performed to verify proper placement of the applicator and proper operation of thermal imaging.
Figure 2.
Temperature-sensitive fast spoiled gradient-recalled echo images.
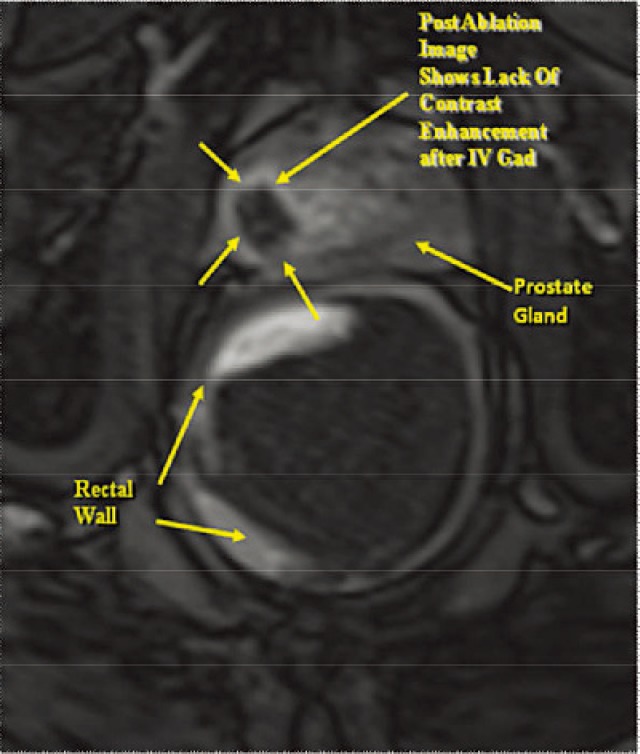
Subsequent to this test pulse, laser treatment is initiated utilizing a 400-μm laser silica fiber with a 10-mm diffused tip firing at 8 W. The 980-nm laser is continued for approximately 30 to 60 seconds after cessation of laser irradiation in order to fully visualize cooling and return to baseline. The diode laser has a high tissue absorption coefficient of 0.482 cm−1 and is thus capable of producing rapid, localized tissue heating creating an ablation zone with very distinct boundaries, allowing for very fine control of the ablation zone. A 1.65-mm diameter cooling catheter is used, and room temperature sterile saline solution is pumped through it using tubing to prevent charring of the laser fiber tip. During each laser treatment, temperatures of the rectal wall and urethral structures closest to the area of prostate being treated are monitored. If temperature reaches a safety threshold above 50°;C in any of the carefully placed safety zones (three are typically used), the laser will shut off automatically. The laser is activated for an average of 3 to 4 minutes per ablation zone. Multiple overlapping ablations are performed in order to assure complete coverage of the target. Depending on the site of the focal abnormality, the ablation zone is typically about 150% of the target volume. Postablation, intravenous Gd is administered and typically demonstrates the absence of enhancement to the tumor focus treated by laser ablation (Figure 3).
Figure 3.
Postablation gadolinium-enhanced T1-weighted axial sequence.
The American Urological Symptom Score (AUASS), IPSS, UCLA Prostate Cancer Index, and Sexual Health Inventory for Men (SHIM) questionnaires are administered preoperatively and at 2 weeks and 3 months postprocedure. Periprocedure complications are captured via questionnaire at the 2-week follow-up visit. At 3 months postablation, mpMRI is performed. Between 2 to 4 tissue cores of the ablation zone are obtained in the MRI unit or using the Eigen Artemis computer coregistration system. At 1 year, tissue samples are obtained from the ablation zone along with a computer generated random 12-core TRUS-guided biopsy.
Of the 23 patients who have undergone focal laser ablation, 13 have undergone a targeted biopsy of the ablation zone. Of these 13 cases, 12 (92.3%) showed no cancer in the ablated zone, and 1 showed residual Gleason 7 (3 + 4) disease. One patient was noted on the 3-month mpMRI to have a focal abnormality that was not recognized previously. The new focal abnormality was target biopsied and had Gleason 6 disease. Both the residual and unrecognized focal abnormalities were subsequently reablated.
To date, there has been no significant change in the mean AUASS, IPSS, or SHIM between 3 to 6 months and baseline (Table 3). Incontinence has not been reported by any patient in the perioperative (2-week) or postoperative (3–6 month) setting. The mean preoperative PSA value was 5.40 (Table 3). Among the 12 patients in whom the 6-month PSA was available, the mean PSA value decreased from 5.10 at baseline to 3.69 at 6-month follow-up (P = .089).
Table 3.
Preliminary Outcomes Following Focal Laser Ablation: The NYULMC/Sperling Outcomes
| Outcome | Baseline | Postablation (2 wk) | Postablation (3–6 mo) |
|---|---|---|---|
| AUASS (mean) | 5.7 | 7.41 | 4.0 |
| SHIM (mean) | 18.7 | 18.2 | 17.8 |
| Incontinence (%) | 0 | 0 | 0 |
| PSA (mean) | 5.40 | – | 3.69 |
| Residual cancer (%) | – | – | 9.1 |
AUASS, American Urological Association Symptom Score; NYULMC, New York University Langone Medical Center; SHIM, Sexual Health Inventory in Men; PSA, prostate-specific antigen.
Conclusions
Today, there is no consensus on choosing an appropriate candidate for focal ablation of prostate cancer. Some believe there is insufficient evidence to justify offering anyone this treatment option. Recognizing the spectrum of prostate cancer and the limitations of curative intervention (RP and RT) and AS, we believe there are acceptable candidates, provided they are properly counseled about limitations of the procedure and the limited short-term and total lack of long-term oncologic outcomes.
One of the major problems arising from the lack of specificity of PSA screening and random biopsy of the prostate is the detection of minute foci of prostate cancer which, if untreated, will cause no harm. These men should be offered AS and not focal ablation. Ideally, the goal should be not to diagnose these insignificant cancers. One strategy to minimize overdetection is to eliminate random biopsies in men with normal or minimally suspicious mpMRIs.
By definition, focal laser ablation mandates visualizing a focal abnormality on mpMRI that is biopsy-proven cancer. By mandating the presence of an image-able target, men with microscopic disease do not qualify as candidates for focal laser ablation. At NYULMC, we obtain an mpMRI prior to prostate biopsy in all cases and use 3D MRI/US coregistration software to target biopsy all focal abnormalities seen on mpMRI. This approach greatly enhances assessment of size, location, and aggressiveness of the cancer, thereby enhancing our ability to optimize treatment selection.
Our experience, along with the literature, suggests that even with first-generation makeshift technology, we can successfully ablate focal prostate cancer in many cases. If the initial postablation biopsy shows residual cancer, reablation is feasible. At present, the very high negative predictive value of mpMRI for clinically significant disease, together with the absence of Gleason pattern 4 disease on random biopsies of the nontarget areas, provides reassurance that significant disease has not been unrecognized in the nontargeted areas. Nevertheless, candidates selecting focal laser ablation today must recognize the need for AS of both the treated and nontreated areas of the prostate due to the lack of long-term oncologic data. The optimal postablation surveillance regimen has yet to be determined and will be heavily influenced by short- and intermediate-term oncologic outcomes.
The literature and our experience provide compelling evidence that focal laser ablation can be offered with the assurance of preserving quality of life. For men with low- and intermediate-risk cancer in a single biopsy-proven mpMRI cancer target, focal ablation is a very reasonable option, provided that there is a high priority for preserving sexual function, and if the patient is willing to accept uncertainties regarding oncological control both in the present and future.
There is clearly an unmet need for focal ablation of prostate cancer. The data are preliminary but very encouraging. Selecting candidates and performing this procedure is challenging and demands the collaboration of a urologist who understands the disease and an interventional radiologist who is highly skilled at interpreting mpMRI and performing the ablation. It is imperative to acquire meticulous and reliable outcomes data in order to ultimately define appropriate candidates, optimal technique, extent of the ablation, and post-treatment assessment of residual and recurrent disease.
Future Trends
It is readily apparent that mastering the technical caveats of the procedure is a challenge. It is unclear whether teams of urologists and radiologists will invest the time and effort to safely and effectively offer this procedure.
Urologists are highly skilled in performing US imaging of the prostate. It is likely that urologists will embrace 3D MRI/US coregistration prostate biopsy as a tool to improve risk-stratified prostate cancer. Further development of software platforms will likely enable urologists to destroy MRI targets using 3D MRI/US coregistration under local anesthesia in a surgicenter or office-based operating room. HIFU, cryotherapy, photodynamic therapy, and laser are all US compatible. We believe that using 3D MRI/US coregistration platforms combined with US tissue monitoring technologies represents the future of focal ablation of prostate cancer. Owing to the many attractive features of laser as a source for tissue ablation, it is likely that focal laser ablation will be included in the armamentarium of urologists who embrace focal ablation of prostate cancer.
Main Points.
There is currently no consensus on choosing an appropriate candidate for focal ablation of prostate cancer, but the authors believe there are acceptable candidates, provided they are properly counseled about limitations of the procedure and the limited short-term and total lack of long-term oncologic outcomes.
One of the major problems arising from the lack of specificity of prostate-specific antigen screening and random biopsy of the prostate is the detection of minute foci of prostate cancer which, if untreated, will cause no harm. These men should be offered active surveillance (AS) and not focal ablation. By definition, focal laser ablation mandates visualizing a focal abnormality on multiparametric magnetic resonance imaging (mpMRI) that is biopsy-proven cancer.
In the authors’ practice, an mpMRI is obtained prior to prostate biopsy in all cases and they use 3D MRI/ultrasound coregistration software to target biopsy all focal abnormalities seen on mpMRI, which greatly enhances assessment of size, location, and aggressiveness of the cancer.
The current literature and the authors suggest that even with first-generation makeshift technology, urologists can successfully ablate focal prostate cancer in many cases. If the initial postablation biopsy shows residual cancer, re-ablation is feasible.
Candidates selecting focal laser ablation today must recognize the need for AS of both the treated and nontreated areas of the prostate due to the lack of long-term oncologic data. The optimal postablation surveillance regimen has yet to be determined and will be heavily influenced by short- and intermediate-term oncologic outcomes.
The literature and our experience provide compelling evidence that focal laser ablation can be offered with the assurance of preserving quality of life. The data are preliminary but very encouraging.
References
- 1.Shibata A, Ma J, Whittemore AS. Prostate cancer incidence and mortality in the United States and the United Kingdom. J Natl Cancer Inst. 1998;90:1230–1231. doi: 10.1093/jnci/90.16.1230. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–1055. doi: 10.1016/j.eururo.2013.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 4.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 7.Hoeks CM, Barentsz JO, Hambrock T, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 8.Moore CM, Robertson NL, Arsanious N, et al. Imageguided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125–140. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi M, Stamey TA, McNeal JE, Nolley R. Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol. 2003;170:459–463. doi: 10.1097/01.ju.0000070928.49986.04. [DOI] [PubMed] [Google Scholar]
- 10.Stamey TA, McNeal JE, Yemoto CM, et al. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donin NM, Laze J, Zhou M, et al. Gleason 6 prostate tumors diagnosed in the PSA era do not demonstrate the capacity for metastatic spread at the time of radical prostatectomy. Urology. 2013;82:148–152. doi: 10.1016/j.urology.2013.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Bahn D, de Castro Abreu AL, Gill IS, et al. Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol. 2012;62:55–63. doi: 10.1016/j.eururo.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed HU, Moore C, Emberton M. Minimallyinvasive technologies in uro-oncology: the role of cryotherapy, HIFU and photodynamic therapy in whole gland and focal therapy of localised prostate cancer. Surg Oncol. 2009;18:219–232. doi: 10.1016/j.suronc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Arumainayagam N, Moore CM, Ahmed HU, Emberton M. Photodynamic therapy for focal ablation of the prostate. World J Urol. 2010;28:571–576. doi: 10.1007/s00345-010-0554-2. [DOI] [PubMed] [Google Scholar]
- 16.Oto A, Sethi I, Karczmar G, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–940. doi: 10.1148/radiol.13121652. [DOI] [PubMed] [Google Scholar]
- 17.Colin P, Mordon S, Nevoux P, et al. Focal laser ablation of prostate cancer: definition, needs, and future. Adv Urol. 2012;2012:589160. doi: 10.1155/2012/589160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford RJ, Shetty A, Elliott AM, et al. Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol. 2010;184:1514–1520. doi: 10.1016/j.juro.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner U, Lawrentschuk N, Weersink RA, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol. 2010;57:1111–1114. doi: 10.1016/j.eururo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol. 2010;24:791–797. doi: 10.1089/end.2009.0440. [DOI] [PubMed] [Google Scholar]
- 21.Peters RD, Chan E, Trachtenberg J, et al. Magnetic resonance thermometry for predicting thermal damage: an application of interstitial laser coagulation in an in vivo canine prostate model. Magn Reson Med. 2000;44:873–883. doi: 10.1002/1522-2594(200012)44:6<873::aid-mrm8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Huang GT, Wang TH, Sheu JC, et al. Lowpower laserthermia for the treatment of small hepatocellular carcinoma. Eur J Cancer. 1991;27:1622–1627. doi: 10.1016/0277-5379(91)90429-h. [DOI] [PubMed] [Google Scholar]
- 23.van Nimwegen, L’Eplattenier HF, Rem AI, et al. Nd:YAG surgical laser effects in canine prostate tissue: temperature and damage distribution. Phys Med Biol. 2009;54:29–44. doi: 10.1088/0031-9155/54/1/003. [DOI] [PubMed] [Google Scholar]
- 24.Rieke V, Vigen KK, Sommer G, et al. Referenceless PRF shift thermometry. Magn Reson Med. 2004;51:1223–1231. doi: 10.1002/mrm.20090. [DOI] [PubMed] [Google Scholar]
- 25.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol. 2013;190:1721–1727. doi: 10.1016/j.juro.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamada T, Sone T, Jo Y, et al. Diffusion-weighted MRI and its role in prostate cancer. NMR Biomed. 2014;27:25–38. doi: 10.1002/nbm.2956. [DOI] [PubMed] [Google Scholar]
- 27.Tan CH, Wei W, Johnson V, Kundra V. Diffusionweighted MRI in the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol. 2012;199:822–829. doi: 10.2214/AJR.11.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratan F, Niaf E, Melodelima C, et al. Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol. 2013;23:2019–2029. doi: 10.1007/s00330-013-2795-0. [DOI] [PubMed] [Google Scholar]
- 29.Numao N, Yoshida S, Komai Y, et al. Usefulness of prebiopsy multiparametric magnetic resonance imaging and clinical variables to reduce initial prostate biopsy in men with suspected clinically localized prostate cancer. J Urol. 2013;190:502–508. doi: 10.1016/j.juro.2013.02.3197. [DOI] [PubMed] [Google Scholar]
- 30.Haffner J, Lemaitre L, Puech P, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 31.Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy [published online ahead of print Nov. 13, 2013] Urol Oncol. doi: 10.1016/j.urolonc.2013.08.006. doi: 10.1016/j.urolonc.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjurlin MA, Taneja SS. Standards for prostate biopsy. Curr Opin Urol. 2014;24:155–161. doi: 10.1097/MOU.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wysock JS, Rosenkrantz AB, Huang WC, et al. A Prospective, Blinded Comparison of Magnetic Resonance (MR) Imaging-Ultrasound Fusion and Visual Estimation in the Performance of MR-targeted Prostate Biopsy: The PROFUS Trial [published online ahead of print Nov. 8, 2013] Eur Urol. doi: 10.1016/j.eururo.2013.10.048. doi: 10.1016/j.eururol.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Amin Z, Lees WR, Bown SG. Technical note: interstitial laser photocoagulation for the treatment of prostatic cancer. Br J Radiol. 1993;66:1044–1047. doi: 10.1259/0007-1285-66-791-1044. [DOI] [PubMed] [Google Scholar]
- 35.Atri M, Gertner MR, Haider MA, et al. Contrast-enhanced ultrasonography for real-time monitoring of interstitial laser thermal therapy in the focal treatment of prostate cancer. Can Urol Assoc J. 2009;3:125–130. doi: 10.5489/cuaj.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindner U, Weersink RA, Haider MA, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 37.Raz O, Haider MA, Davidson ST, et al. Real-time magnetic resonance imaging-guided focal laser therapy in patients with low-risk prostate cancer. Eur Urol. 2010;58:173–177. doi: 10.1016/j.eururo.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Lindner G, Horland R, Wagner I, et al. De novo formation and ultra-structural characterization of a fiber-producing human hair follicle equivalent in vitro. J Biotechnol. 2011;152:108–112. doi: 10.1016/j.jbiotec.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Woodrum DA, Mynderse LA, Gorney KR, et al. 3.0 T MR-guided laser ablation of a prostate cancer recurrence in the postsurgical prostate bed. J Vasc Interv Radiol. 2011;22:929–934. doi: 10.1016/j.jvir.2011.02.039. [DOI] [PubMed] [Google Scholar]