Abstract
Proton beam therapy for prostate cancer has become a source of controversy in the urologic community, and the rapid dissemination and marketing of this technology has led to many patients inquiring about this therapy. Yet the complexity of the technology, the cost, and the conflicting messages in the literature have left many urologists ill equipped to counsel their patients regarding this option. This article reviews the basic science of the proton beam, examines the reasons for both the hype and the controversy surrounding this therapy, and, most importantly, examines the literature so that every urologist is able to comfortably discuss this option with inquiring patients.
Key words: Prostate cancer, Proton beam therapy, External beam radiation therapy, Intensity modulated radiation therapy
Proton beam therapy (PBT) has become a source of controversy in the urologic community. It is not uncommon to hear mixed messages regarding the issue, from zealous advocates to cost-conscious skeptics, leaving many urologists unsure what to tell their patients with prostate cancer. What is clear, however, is that the technology is disseminating across the nation, and as our patients turn to the internet to learn more about their diagnosis, they are going to encounter increasingly more information about PBT, both scientific and promotional in nature. Hence, it is necessary for every urologist to understand the basics of PBT to help guide our patients through treatment options. This article reviews and compares the basic science of conventional external beam radiation therapy (EBRT) with PBT, examines the reasons for both the hype and the controversy surrounding this therapy, and, most importantly, examines the literature so that all urologists are adequately equipped to counsel their patients on this subject.
External Beam Radiation Therapy: How It Works
EBRT refers to the external delivery of any type of radiation, and can use several different types of energy including photons, electrons, and heavy particles (carbon ions, neutrons, and protons). Additionally, there has been an evolution in the methods to deliver this energy (typically with photons), progressing from two-dimensional radiation therapy to three-dimensional conformal radiation therapy (3DCRT), and, more recently, intensity-modulated radiation therapy (IMRT).
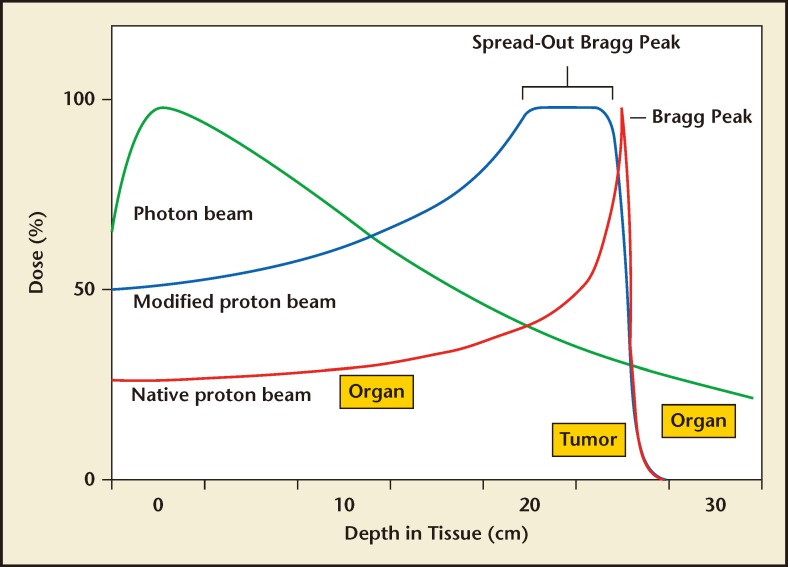
The most common beam used for EBRT is composed of photons or radiation. A photon has no mass and no charge; therefore, when released by a linear accelerator, it can travel easily through a target material, and typically does not stop within the patient. There is an initial build-up of energy as the photons interact with and excite electrons in the target material, which enhances the radiation effect. The peak dose occurs within a few centimeters from the entrance surface, and then the dose slowly attenuates as lower-energy photons are absorbed or scattered, and fewer photons are available to travel to deeper depths. Clinically, this initial build-up allows for a skin-sparing effect; however, the shallow depth of maximum dose portends that the largest dose is still being deposited at a superficial level. This means that to effectively radiate deeper tissue such as the prostate, normal tissues that are in line with the beam in front of the target must be radiated at a higher dose than the target itself (Figure 1). Although EBRT has been used to treat prostate cancer for decades, these properties make it easy to understand why photon beam therapy is not an ideal form of radiation to treat organs that are located at a great depth within the body.
Figure 1.
Dose distribution curves of photon and proton beams. The spread-out Bragg peak is demonstrated here to cover the entire target with the maximal dosage.
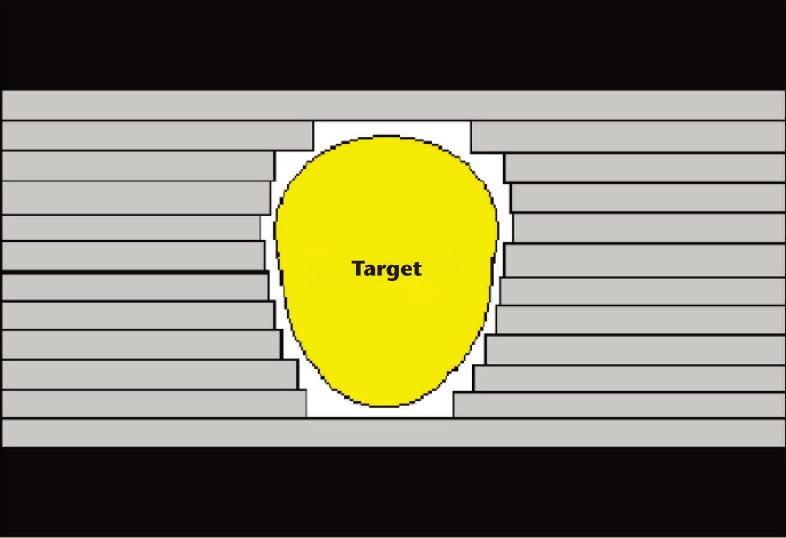
One way to compensate for the depth dose characteristics of photons is to use multiple beams that converge at the target from different directions, thus delivering the maximal dose within the target, while spreading a moderate dose bath over the surrounding normal tissues. Additionally, there have been major improvements in the treatment planning and delivery techniques for EBRT in the past few decades. Historically, with conventional EBRT, the location of the prostate was estimated by the location of the pubis and femoral heads, and then later inferred by using a Foley catheter and rectal tube that were visible on a plain radiograph. The incorporation of computed tomography scans for treatment planning led to the development of 3D-CRT. The target and surrounding normal organs are reconstructed in three dimensions for optimal design of beam angle and dose coverage. The availability of multileaf collimators allows shaping of the beam to conform it to the target, and lessens the dose to surrounding tissue, as shown in Figure 2. This technology allowed significant dose escalation to the prostate, which has been shown in multiple randomized trials to reduce progression.1,2 The next breakthrough in radiation therapy was IMRT, which is currently the most advanced method for delivering photon-based EBRT. IMRT employs computer algorithms that can inversely plan prescribed radiation doses to certain targets. Dose distribution is optimized by modifying the intensity of the beams across the designated volume by dividing a beam into several smaller beamlets, each of varying intensity to conform to a target of irregular shape and allowing better sparing of the surrounding normal organs. IMRT has thus been associated with fewer gastrointestinal (GI) side effects,3 which has allowed further dose escalation with very similar toxicity rates as 3D-CRT.4
Figure 2.
Schematic of multileaf collimator. Individually positioned tungsten leaflets conform the shape of the beam to the target as used in three-dimensional conformal radiation therapy.
Advances in image guidance such as on-board imaging units (imaging equipment attached to the linear accelerator) have helped to deliver the radiation more accurately to the prostate. These have dramatically decreased the uncertainty in target localization and improved the accuracy of radiotherapy to the prostate. However, despite all of these advances, the physical parameters of a photon beam will always include both entrance and exit doses and, ultimately, a significant volume of normal tissue receiving low to moderate doses.
Protons: A Different Mechanism
Protons have completely different dose distribution properties and have the potential to avoid most of the extra-target radiation that is inherent to photons. Unlike a photon, a proton is a heavy particle (roughly 1800 times the mass of an electron) with an elementary charge, which confers certain dosimetric advantages. Heavy particles, as opposed to photons, will stop within a target. A proton beam is produced by a cyclotron or synchrotron that separates the proton from a hydrogen molecule and accelerates it. The large mass and energy imparted by the acceleration system gives protons a specific momentum that carries them into a body. After traveling a specified distance, the velocity is slowed by interactions due to their mass and charge and then stopped very abruptly at a very specific depth. This is the point at which it will interact most with surrounding electrons, delivering its energy and causing ionization of molecules and radiation damage within the DNA of the target cell. This unique property allows protons to be targeted so that they have their most damaging effects in the tumor itself, with less radiation delivered in front of the target, and no dose delivered beyond it. This peak of energy delivery is commonly referred to as the Bragg peak (Figure 1). The Bragg peak is very narrow and must be spread out using multiple proton energies to ensure that the peak encompasses the entire target. This spread-out Bragg peak (SOBP) is applied to the prostate to maximize the radiation damage to the cancerous organ while still delivering less radiation to surrounding tissues. Additionally, protons also have a mildly greater radiobiologic effect than photons that confers more damage to cancer cells.5
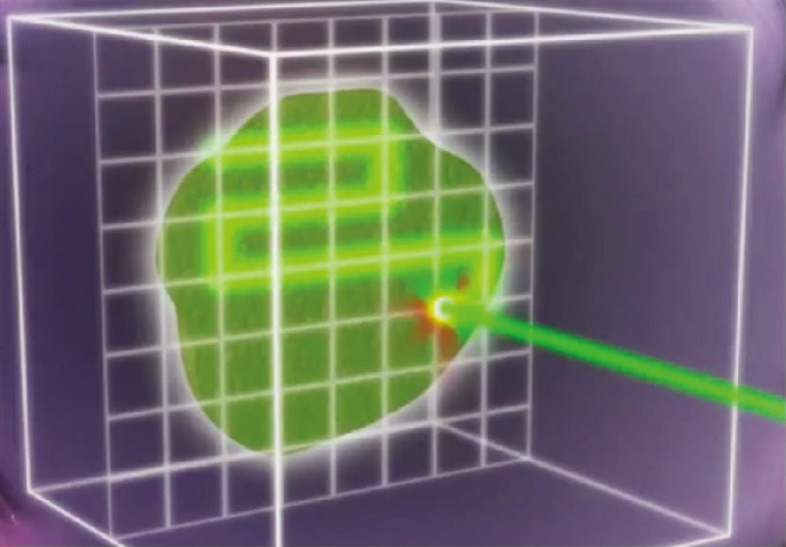
Protons can also be administered by two methods. The older method includes large beams of passively scattered protons that are shaped with the use of high-density blocks or apertures to shape the large beam as it exits the nozzle. Compensators are employed within the beam to alter the beam profile to better conform the SOBP to the actual tumor. A second, newer method employs a very narrow, pencil thin beam to paint the dose on the target, and no blocks or compensators are needed. The pencil beam is swept in a raster pattern back and forth across a target guided out of the nozzle by magnets (Figure 3). This allows the delivery of intensity-modulated proton beam therapy (IMPT), with a greater ability to conform the dose to an irregularly shaped target. Although not widely available, many new facilities are being planned with pencil beam-only systems.
Figure 3.
Pencil beam scanning. The pencil beam is swept in a raster pattern back and forth across a target. Reproduced with permission from Mayo Clinic News Network.
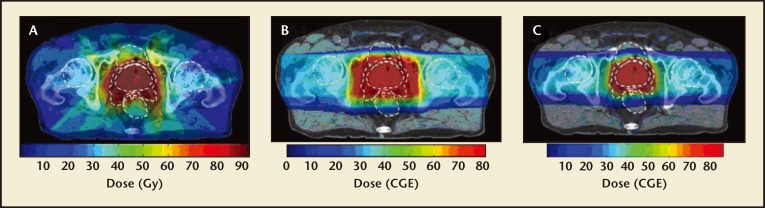
Dosimetric studies have been performed to compare passively scattered PBT and IMRT in their ability to spare nearby organs. In one recent study, Vargas and colleagues6 compared PBT and IMRT dosimetric plans developed for the same cohort of patients for prostate cancer treatment. Compared with IMRT, PBT reduced the mean rectal and bladder dose by 59% and 35%, respectively. Similarly, another study comparing 3D-CRT, IMRT, and PBT found that both photon techniques resulted in increased mean doses to organs at risk in the low- and medium-dose levels.7 IMPT can further reduce the low-to-medium dose to nontarget tissues and achieve a small improvement in planning target volume dose heterogeneity.8 Figure 4 demonstrates dosimetry plans with the typical reduced dosage PBT and IMPT to surrounding tissues compared with IMRT.
Figure 4.
Dosimetry plans of various techniques. Radiation dosage across various tissues compared among (A) IMRT, (B) PBT, and (C) IMPT. IMPT, intensity-modulated proton beam therapy; IMRT, intensity-modulated radiation therapy; PBT, proton beam therapy. Reproduced with permission from Trofimov A et al, Int J Radiat Oncol Biol Phys. 2007;69:444–453.
Theoretical Causes for Concern
There are some potential disadvantages to PBT for prostate cancer. There is no widely used method for confirming the proton range or that the SOBP is encompassing the prostate in vivo. However, the algorithms used to calculate the doses appear accurate enough that this is not generally a significant concern if sufficient compensation is used. Positron emission tomography scanning and similar technologies are being developed that may allow for in vivo determination of proton dose and range. Another possible disadvantage is the increased sensitivity of PBT to target motion because of the steep dose depletion beyond the SOBP.9 The prostate is not completely fixed and varies in position depending on the fullness of the bladder and rectum. However, fiducial markers with pretreatment localization and placement of rectal balloons to fix the prostate in location have been successfully employed to compensate for this issue.
Cost
The construction and maintenance of a proton-beam center involves significant cost, which is a source of great controversy and one of the most frequent objections raised by critics. For example, the cost of the proton beam facility currently under construction at our own institution was estimated at approximately $180 million. Multiple studies have been performed to calculate cost effectiveness with conflicting conclusions. These studies are limited by the uncertainties in estimating both cost and benefit. The Institute of Clinical and Economic Review (Boston, MA) estimated the lifetime costs and quality-adjusted life expectancy for IMRT to be $45,591 and 13.81 years, and $72,789 and 13.7 years for PBT.10 Another study used a Markov model to assess the cost difference per quality-adjusted life-year (QALY) of 91.8 GyE of PBT versus 81 Gy of IMRT. The authors assumed that the higher dose would lead to a 10% 5-year freedom from biochemical failure advantage (93% vs 83%) with similar toxicity. Despite the optimistic assumption, the incremental cost effectiveness ratio for PBT over IMRT was calculated to be $63,578 per QALY for a 70-year-old man and $55,726 per QALY for a 60-year-old man. However, adding to the uncertainty of this study is the fact that 91.8 Gy has not been used clinically. The authors concluded that using the commonly accepted, although arbitrary standard of $50,000 per QALY, PBT did not appear to be cost effective.11 In contrast, a separate study using different assumptions (assuming a 20% reduction in cancer recurrence and cancer-specific mortality and a 0.6 relative risk of adverse events, compared with conventional radiation) and cost modeling, estimated a cost of €26,800 (approximately $35,500) per QALY, which would fall well within that standard.12
Proponents of PBT counter that as the proton technology matures and efficiency in delivery increases, costs will become more manageable, as is true with most technologies. Furthermore, cyclotrons have a much longer lifespan than a typical linear accelerator. PBT may also become more affordable if the total dose can be delivered over fewer fractions (a process called hypofractionation). An open phase 3 trial (GU002-10/NCT01230866: Low Risk Prostate Cancer [standard fractionation vs hypofractionation]) is currently comparing 44 PBT fractions with 5 PBT fractions. This study would create a pathway to dramatically decreasing the cost of PBT if the 5-treatment arm is found to achieve similar or better treatment outcome than the 44-treatment arm.
Effectiveness and Toxicity: Reviewing the Literature
Although PBT has generated much enthusiasm, its utility can be best confirmed by clinical trials. Unfortunately, there are no randomized trials completed that directly compare 3D-CRT or IMRT with PBT, and thus we must rely mainly on single-arm studies.
Loma Linda University Medical Center (Loma Linda, CA) was the first center to open a hospital-based proton facility in 1990, and reported the first large single-arm experience.13 The authors analyzed 1255 patients, 731 of whom received 3D-CRT plus a boost with PBT and 524 of whom received PBT only. Included were patients with low-, intermediate-, and high-risk prostate cancer. Using American Society for Therapeutic Radiology Oncology consensus criteria, the 5- and 8-year biochemical failure free (BCFF) survival was 75% and 73%, respectively. More importantly, there were very low rates of Radiation Therapy Oncology Group grade 3 or higher morbidity: 1% genitourinary (GU) and 0.2% GI. To put these numbers in perspective, patients treated with IMRT at our institution had 6% GU and 1% GI grade 3 or higher morbidity.14
The Proton Research Oncology Group 9509 tested the hypothesis that increasing radiation doses delivered to men with early-stage prostate cancer would improve clinical outcomes.15 However, it achieved more than this stated goal as it included PBT boost after photon therapy. Men with T1b-T2b prostate cancer and PSA ≤ 15 ng/mL were included. All 393 men received 50.4 Gy delivered with photons in 1.8-Gy fractions to the prostate and seminal vesicles. Patients were randomized to receive a PBT boost of either 19.8 GyE or 28.8 GyE in 1.8-GyE fractions. No patient received androgen suppression therapy with radiation therapy. At 10 years, the Phoenix Consensus Definition biochemical failure (BF) rates were 32% versus 17% for conventional- and high-dose radiation, respectively (P < .001). Two percent of patients in both arms experienced late grade ≥ 3 GU toxicity, and 1% of patients in the high-dose arm experienced late grade ≥ 3 GI toxicity. In contrast to the dose escalation studies performed with 3D-CRT, this PBT dose escalation was achieved without an increase in grade ≥ 3 late urinary or rectal morbidity, as shown in Table 1.
Table 1.
Biochemical control and Toxicity Rates in Randomized controlled Trials of Dose Escalation
| Study | Boost Modality | Planning Technique | LD Arm, Gy | HD Arm, Gy | HD 5-y control, % | ≥ Grade 3 GI Toxicity, % |
≥ Grade 3 GU Toxicity, % |
||
|---|---|---|---|---|---|---|---|---|---|
| LD | HD | LD | HD | ||||||
| Kuban DA et al27 | Photons | 2D, 3D | 70.0 | 78.0 | 85.0a | 1.0 | 7.0 | 5.0 | 3.0 |
| Peeters ST et al28 | Photons | 3D, IMRT | 68.0 | 78.0 | 64.0b | 4.0 | 5.0 | 12.0 | 13.0 |
| Dearnaley DP et al2 | Photons | 3D | 64.0 | 74.0 | 71.0c | 6.0 | 10.0 | 2.0 | 4.0 |
| Beckendorf V et al29 | Photons | 3D | 70.0 | 80.0 | 72.0b | 2.0 | 6.0 | 3.0 | 2.0 |
| Zietman AL et al30 | Protons | 3D | 70.2 | 79.2 | 80.4b | 0.0 | 1.0 | 2.0 | 2.0 |
aAs determined by Phoenix criteria. bAs determined by American Society for Therapeutic Radiology Oncology criteria. c Determined by a rise in prostate-specific antigen to > 50% than the nadir and > 2 ng/mL.
2D, two-dimensional; 3D, three-dimensional; GI, gastrointestinal; GU, genitourinary; HD, high dose; IMRT, intensity-modulated radiation therapy; LD, low dose.
Coen and colleagues16 performed a comparative retrospective analysis using a case-matched methodology. The 8-year BF rates, using the Phoenix definition, were 7.7% and 16.1% for PBT and brachytherapy, respectively. This difference was not significant when stratified by risk group (P = .42).16
In a multi-institutional prospective phase II PBT trial focusing on rectal toxicity, no patients experienced acute or late grade 3 rectal toxicity, and only 1% of patients had late grade 3 urinary toxicity using the National Cancer Institute (NCI) Common Toxicity Criteria.17 The 3-year Phoenix BCFF survival rate was 94% in a cohort of low- and intermediate-risk prostate cancer patients, only 28% of whom received any hormonal therapy.
Mendenhall and colleagues18 published early outcomes of three prospective trials of PBT for low-, intermediate-, and high-risk prostate cancer. In this study, each risk group underwent a separately prescribed treatment plan, with only the high-risk group receiving androgen deprivation. The 2-year progression-free survival for the entire cohort is 99%, whereas the grade 3 GU and GI toxicities were similar at 1.8% (all of which were transient) and 0.5%, respectively. Although not directly comparable, these toxicity results do appear more favorable than results commonly reported with 3D-CRT or IMRT.
Recently, two well-publicized retrospective analyses called these potential benefits of PBT into question and received a tremendous amount of publicity. The first was based on the NCI’s surveillance, epidemiology and end results (SEER) database.19 The authors compared morbidity and disease control between IMRT, 3D-CRT, and PBT, using propensity-score matching. They found that patients who underwent IMRT compared with PBT were significantly less likely to undergo a GI-related procedure or have a new GI diagnosis compared with those who underwent PBT, and hence concluded that IMRT was associated with less GI morbidity. However, among the several flaws of this study, the most fundamental was that outcome surrogates (ie, claims for colonoscopy) were used to measure toxicity rates. This would be an imprecise surrogate for any population, but is particularly so in PBT patients, many of whom specifically seek out this advanced technology and travel to a specialized center. This is the same population that might also be more vigilant about colonoscopy screening recommendations, and therefore would receive more GI procedures and diagnoses. Furthermore, the rate of GI diagnoses and procedures were nearly statistically different at baseline. Therefore, this study should not be used to relay any important morbidity information to inquiring patients.
In the second study, Yu and colleagues20 identified 27,647 men, 553 (2%) of whom received PBT and 27,094 (98%) of whom received IMRT, within the Medicare database. Compared with patients treated with IMRT, patients receiving PBT were younger, healthier, and from more affluent areas. The median Medicare reimbursement was $32,428 for PBT and $18,575 for IMRT. Although PBT was associated with a statistically significant reduction in GU toxicity at 6 months (5.9% vs 9.5%; P = .03), by 12 months there was no difference between the two (18.8% vs 17.5%; P = .66). There was similarly no significant difference in GI or other toxicity at 12 months post-treatment. However, inherent to this analysis was the attempt to make valid conclusions about endpoints that this database was not designed to measure. Many toxicity parameters that were included are generally unrelated to radiotherapy, including GU infection, upper tract dysfunction, and systemic derangements. Common radiotherapy toxicities such as irritative bladder, rectal voiding side effects, and rectal bleeding that did not result in transfusion, were not considered in this analysis. Including toxicity codes that were unrelated to radiotherapy while excluding the more common side effects would make the outcomes appear more similar than different.21
The few studies on quality of life have shown some advantages for PBT compared with conventional treatments with regard to acute side effects, but these have been modest. A multi-institutional study prospectively collected quality of life (QoL) data to compare PBT with 3D-CRT and IMRT using validated instruments (Prostate Cancer Symptom Indices scale and the Expanded Prostate Cancer Index Composite) to assess patient-reported bowel and urinary toxicity.22 PBT patients reported minimal acute bowel morbidity, whereas the other two groups reported a significant initial decrease in bowel QoL. By 24 months, however, all three cohorts reported small but clinically meaningful decrements in bowel QoL. None of the three cohorts reported a decline in urinary QoL at 24 months.
The risk of developing a secondary malignancy after radiation therapy is also of concern, as population-based studies have demonstrated an increased risk of several cancers, most notably rectal and bladder cancer, in patients who undergo EBRT for prostate cancer.23 PBT results in a significantly lower radiation dose to the surrounding normal tissues, thus reducing the risk of secondary malignancies. However, there is some increased scatter of neutrons that arises from the heavier proton particle that may lead to an increased risk of secondary malignancies. Although large, population-based studies are not currently available, the current literature appears to favor PBT. A recent study that used risk modeling to compare IMRT and PBT based mostly on the dosage exposure of organs at risk estimated that PBT reduced the risk of secondary neoplasms by 26% to 39%.24 In a matched retrospective cohort study, Chung and colleagues25 compared the risk of secondary malignancy in 1450 patients treated with PBT from 1974 to 2001 at the Harvard Cyclotron Laboratory (Cambridge, MA) with patients treated with photon therapy in the SEER cancer registry. Patients were matched by age at radiation treatment, year of treatment, cancer histology, and site of treatment with a minimum of 1 year of follow-up. They found that second malignancies occurred in only 6.4% of proton patients compared with 12.8% of photon patients.25 In an update, the authors adjusted for sex, age at treatment, primary site, and year of diagnosis. They found that the adjusted hazard ratio of a secondary cancer developing for a patient treated with proton radiation in comparison with photon radiation was 0.52 (P = .009).26
In summary, PBT has significant theoretical dosimetric advantages over photon EBRT. The available literature mainly consists of prospective phase II trials as well as retrospective studies, showing favorable treatment outcomes for localized prostate cancer using PBT. Although there are no randomized controlled trials for review at this time, there is a current phase III trial open (Proton Therapy vs IMRT for Low or Low-Intermediate Risk Prostate Cancer; NCT01617161) that will help clarify the clinical value of PBT relative to IMRT. However, this will only be possible if patients accept randomization, which is sometimes difficult for those who travel long distances, specifically with the desire to receive PBT.
The Next Frontier
Currently, most proton facilities deliver PBT with the older passively scattered beam technology; thus, the results reported from all of the single-arm and dose escalation trials have been using the older technology. However, pencil beam scanning as described above is now available and likely will replace scattered beam as a preferred option of PBT. Pencil beam PBT will allow intensity modulation of the proton beam analogous to IMRT with photons, with better conformation of the prescribed dose to the target while avoiding surrounding structures. As this very new technology is currently operational in very few centers, no clinical studies employing pencil beam PBT have been reported at this time. However, the goal is to improve the therapeutic ratio by increasing the target dose to achieve greater disease control, all while reducing the dose to surrounding organs resulting in fewer side effects and secondary malignancies.
Conclusions
The unique dose distribution properties of protons give us the ability to increase radiation doses to the target while reducing the exposure of surrounding tissues and organs. Theoretically, this should lead to superior disease control while reducing toxicity and second malignancies, therefore explaining the enthusiasm it has generated in the radiation oncology community. The significant cost of PBT remains a barrier to its widespread use, but its cost effectiveness requires more study as the results to date conflict. Hypofractionation has the potential to dramatically decrease the cost if shorter PBT regimens prove to be equally effective to conventional fractionation. Most early, single-arm clinical studies have shown favorable cancer control with exceptionally low toxicity rates, but, to date, there are no completed phase III trials comparing photon RT with PBT. However, such a trial recently opened that we hope will better define the value of PBT in the treatment of prostate cancer.
Main Points.
Proton beam therapy (PBT) has become a source of controversy in the urologic community; it is clear that technology is disseminating, and, as our patients turn to the internet to learn more about their diagnosis, they are going to encounter increasingly more information about PBT, both scientific and promotional in nature.
Advances in conventional external beam radiation therapy (EBRT) and intensity modulated radiation therapy (IMRT) have dramatically decreased the uncertainty in target localization and improved the accuracy of radiotherapy to the prostate; despite this, there are significant drawbacks.
PBT has significant theoretical dosimetric advantages over photon EBRT. The available literature mainly consists of prospective phase II trials as well as retrospective studies, showing favorable treatment outcomes for localized prostate cancer using PBT.
The unique dose distribution properties of protons give us the ability to increase radiation doses to the target while reducing the exposure of surrounding tissues and organs, which, in theory, should lead to superior disease control while reducing toxicity and second malignancies.
The significant cost of PBT remains a barrier to its widespread use, but its cost effectiveness requires more study as the results to date conflict.
Most early, single-arm, clinical studies have shown favorable cancer control with exceptionally low toxicity rates, but, to date, there are no completed phase III trials comparing photon RT with PBT.
Pencil beam scanning is now available and likely will replace scattered beam as a preferred option of PBT.
References
- 1.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 2.Dearnaley DP, Sydes MR, Graham JD, et al. Escalateddose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Fuks Z, Leibel SA. Intensity-modulated radiation therapy for prostate cancer. Semin Radiat Oncol. 2002;12:229–237. doi: 10.1053/srao.2002.00000. [DOI] [PubMed] [Google Scholar]
- 4.Wong WW, Vora SA, Schild SE, et al. Radiation dose escalation for localized prostate cancer: intensitymodulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009;115:5596–5606. doi: 10.1002/cncr.24558. [DOI] [PubMed] [Google Scholar]
- 5.Abeloff MD, et al. Abeloff ’s Clinical Oncology. 4th ed. Philadelphia: Churchill Livingstone Elsevier;; 2008. [Google Scholar]
- 6.Vargas C, Fryer A, Mahajan C, et al. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–751. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 7.Mock U, Bogner J, Georg D, et al. Comparative treatment planning on localized prostate carcinoma conformal photon-versus proton-based radiotherapy. Strahlenther Onkol. 2005;181:448–455. doi: 10.1007/s00066-005-1317-7. [DOI] [PubMed] [Google Scholar]
- 8.Cella L, Lomax A, Miralbell R. Potential role of intensity modulated proton beams in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:217–223. doi: 10.1016/s0360-3016(00)01368-7. [DOI] [PubMed] [Google Scholar]
- 9.Yoon M, Shin D, Kwak J, et al. Characteristics of movement-induced dose reduction in target volume: a comparison between photon and proton beam treatment. Med Dosim. 2009;34:191–201. doi: 10.1016/j.meddos.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Kagan AR, Schulz RJ. Proton-beam therapy for prostate cancer. Cancer J. 2010;16:405–409. doi: 10.1097/PPO.0b013e3181f8c25d. [DOI] [PubMed] [Google Scholar]
- 11.Konski A, Speier W, Hanlon A, et al. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 12.Lundkvist J, Ekman M, Ericsson SR, et al. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44:850–861. doi: 10.1080/02841860500341157. [DOI] [PubMed] [Google Scholar]
- 13.Slater JD, Rossi CJ Jr, Yonemoto LT, et al. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004:59. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Vora SA, Wong WW, Schild SE, et al. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coen JJ, Zietman AL, Rossi CJ, et al. Comparison of high-dose proton radiotherapy and brachytherapy in localized prostate cancer: a case-matched analysis. Int J Radiat Oncol Biol Phys. 2012;82:e25–e31. doi: 10.1016/j.ijrobp.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Nihei K, Ogino T, Onozawa M, et al. Multi-institutional Phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys. 2011;81:390–396. doi: 10.1016/j.ijrobp.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Mendenhall NP, Li Z, Hoppe BS, et al. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–221. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu JB, Soulos PR, Herrin J, et al. Proton versus intensity-modulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:25–32. doi: 10.1093/jnci/djs463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schild SE, Keole SR, Foote RL. Re: Proton vs intensitymodulated radiotherapy for prostate cancer: patterns of care and early toxicity. J Natl Cancer Inst. 2013;105:748. doi: 10.1093/jnci/djt074. [DOI] [PubMed] [Google Scholar]
- 22.Gray PJ, Paly JJ, Yeap BY, et al. Patient-reported outcomes after 3-dimensional conformal, intensity-modulated, or proton beam radiotherapy for localized prostate cancer. Cancer. 2003;119:1729–1735. doi: 10.1002/cncr.27956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107:991–998. doi: 10.1002/cncr.22083. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, Lee AK, Newhauser WD. Risk of secondary malignant neoplasms from proton therapy and intensity-modulated x-ray therapy for early-stage prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:616–622. doi: 10.1016/j.ijrobp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung CS, Keating N, Yock T, Tarbell N. Comparative analysis of second malignancy risk in patients treated with proton therapy versus conventional photon therapy. Int J Radiat Oncol Biol Phys. 2008;72(suppl):S8. [Google Scholar]
- 26.Chung CS, Yock TI, Nelson K, et al. Incidence of second malignancies among patients treated with proton versus photon radiation. Int J Radiat Oncol Biol Phys. 2013;87:46–52. doi: 10.1016/j.ijrobp.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 27.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 29.Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80:1056–1063. doi: 10.1016/j.ijrobp.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 30.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]