Abstract
Polymicrobial bacterial infections are commonly found in cases of Fournier gangrene (FG), although fungal growth may occur occasionally. Solitary fungal organisms causing FG have rarely been reported. The authors describe a case of an elderly man with a history of diabetes who presented with a necrotizing scrotal and perineal soft tissue infection. He underwent emergent surgical debridement with findings of diffuse urethral stricture disease and urinary extravasation requiring suprapubic tube placement. Candida albicans was found to be the single causative organism on culture, and the patient recovered well following antifungal treatment. Fungal infections should be considered as rare causes of necrotizing fasciitis and antifungal treatment considered in at-risk immunodeficient individuals.
Key words: Fournier gangrene, Fournier’s Gangrene Severity Index, Candida albicans
Fournier gangrene (FG) is a rare, rapidly progressive, necrotizing infection of the perineum and genital area that was first described in 1883 by Jean Alfred Fournier in five young male patients.1 The infectious flora causing necrotizing fasciitis are typically polymicrobial, involving aerobic and anaerobic bacteria derived from gastrointestinal, genitourinary, and cutaneous sources.2,3 Certain predisposing conditions increase the risk of developing FG, including diabetes, chronic kidney disease, immunosuppression, local trauma, urethral stricture, or genitourinary infections.4–6
It is essential to diagnose FG early and treat it emergently because the infection can quickly progress, with mortality rates of 7.5% to 50% cited in various series.7,8 Aggressive management involves hemodynamic stabilization, broad spectrum antibiotics to empirically cover all potential organisms, and wide surgical debridement.3–5 Early surgical debridement with excision of all nonviable tissue is the most important component of treatment. Multiple surgical debridements are often required, as the areas of cutaneous involvement may not indicate the full extent of subcutaneous disease.5
Rapid initiation of broad spectrum antibiotic coverage is also necessary to stabilize the presenting patient with FG before and after surgical management. The infection is generally caused by three or more microorganisms, most commonly Escherichia coli, Proteus, Enterococcus, and anaerobes.4 Fungal etiologies of necrotizing infections are rare but have been increasingly reported in the literature.9–12 Candida species are commonly part of the normal flora in the gastrointestinal and genitourinary tracts of humans but may cause acute disease in the setting of compromised host immunity. This report describes a case of primary C albicans necrotizing fasciitis of the genitalia and reviews the literature regarding fungal FG to determine possible predisposing factors.
Case Report
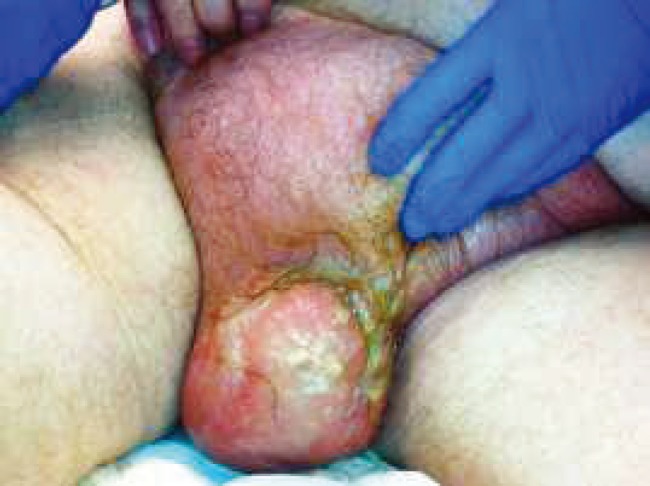
A 73-year-old man with a history of benign prostatic hyperplasia, diabetes mellitus, hypertension, and urinary incontinence presented with complaints of scrotal discomfort and swelling. On review, he endorsed symptoms of low-grade fevers, genital pain, dysuria, nausea, and weakness which began 1 week prior to presentation. He was initially seen at an outside hospital and was given antibiotics and underwent scrotal ultrasound before transfer to our institution. When he arrived, he was afebrile but had significant leukocytosis (22.8 K/uL). Physical examination identified erythema, edema, and induration of the scrotum with tenderness extending onto the suprapubic area without associated crepitus (Figure 1). The outside ultrasound revealed bilateral hydroceles with diffusely thickened skin and no abscesses. He was started on vancomycin and piperacillin/tazobactam for broad spectrum antibiotic coverage. Within 24 hours of admission, the scrotal skin became dusky, and purulent drainage was noted. He remained hemodynamically stable but was taken emergently to the operating room for scrotal exploration with debridement.
Figure 1.
Preoperative image of patient’s scrotum and suprapubic region with buried penis.
On operative evaluation, approximately one-third of the anterior scrotal skin was found to be necrotic and incision into scrotal skin revealed extravasated urine mixed with purulent drainage. The abscess extended beyond the posterior scrotum into the retroperitoneal space posterior to the bladder. Cystoscopy revealed a completely obliterated urethra with numerous wide-caliber circumferential strictures in the pendulous urethra; the proximal urethra and bladder were unable to be visualized. A suprapubic tube was then placed through a separate open incision for bladder drainage. Scrotal abscess and deep tissue gram stains showed many polymorphonuclear leukocytes and few yeast. Final scrotal abscess and deep tissue cultures grew C albicans as the sole organism. The final urine culture also grew more than 100,000 colonies/mL of C albicans, but blood cultures were negative. Antibiotics were discontinued, and the patient was given one dose of caspofungin before transitioning to fluconazole to complete a 20-day course. With continued local wound care, his wound healed by secondary intention over subsequent weeks.
Discussion
FG is a severe and life-threatening infection with a persistently high mortality rate despite modern medical and surgical therapy. Other series have shown a variable mortality rate between 7.5% to 50%.7,8 Common causes of death in patients with FG include severe sepsis, coagulopathy, acute kidney injury, diabetic ketoacidosis, and multisystem organ failure. The Fournier’s Gangrene Severity Index (FGSI) has been validated as a sensitive and specific predictor of mortality in patients with FG.7,13 The FGSI is calculated based on physiologic parameters at hospital admission including temperature, heart rate, respiratory rate, serum sodium, potassium, creatinine, bicarbonate, hematocrit, and leukocyte count. Based on their series, Laor and colleagues7 proposed that an FGSI score of 9 or more indicated a 75% probability of mortality, whereas a score of 9 or less indicated a 78% survival probability. Our patient had an FGSI score of 9 at admission, indicating that his infection was severe enough to be on the cusp of a dramatically elevated mortality rate.
Although polymicrobial bacterial infection tends to be the etiology of FG, fungi may be seen and have been reported with increasing frequency along with increasingly resistant bacterial causes. Relatively few reports in the literature, however, acknowledge solitary fungal infections as leading to FG. Candida species were identified as the only growth from tissue cultures in few reported cases of FG.9–11 Single cases of FG caused by the low-virulence organism Lactobacillus gasseri and cutaneous Rhizopus microsporus have been reported as well.12,14 Many of the patients described have predisposing immunodeficiencies that may make them more susceptible to fungal growth, colonization, or even infection. In the case presented by Jensen and colleagues,11 the patient had a history of type 1 diabetes and a prior renal transplant requiring chronic immunosuppression. Likewise, the case of FG caused by C glabrata described by Loulergue and associates10 occurred in a patient with a history of diabetes, which may lead to compromised immunity. In the case of R microsporus infection, the patient also had a history of acute myelogenous leukemia and diffuse large B-cell lymphoma previously treated with chemotherapy and an autologous bone marrow transplant.14 Our patient’s only risk factor for immunodeficiency was a longstanding history of diabetes, which was kept under modest control (hemoglobin A1c, 6.9%) with oral medications.
Urinary tract infection was commonly identified in those patients who developed FG with fungal etiologies.9,10,12 In one case, intra-operative findings demonstrated a perforation of the posterior bulbous urethra, suggesting that urinary extravasation contributed to the development of FG.9 Patients with chronic urinary retention of various etiologies are often colonized with fungal species, specifically Candida. Our patient was found to have diffuse urethral stricture disease and was likely in overflow incontinence prior to presentation. He had gross findings of extravasated urine on operative exploration that facilitated the spread of urine colonized with C albicans to the perineum and genitalia. Urinary extravasation is not uncommonly noted in cases of FG, although estimates of the true prevalence are unknown.
The most recent guidelines for treatment of FG published by the European Association of Urology recommend full surgical debridement and treatment with broadspectrum antibiotics on presentation with subsequent tailoring per cultures.15 Other reviews recommend combination antibiotic therapy, including a penicillin for streptococcal species, third-generation cephalosporin (with or without an aminoglycoside) for gram-negative organisms, and metronidazole for anaerobes.5 Owing to the increase in fungal necrotizing infections, some have suggested empiric antifungal treatment with fluconazole if the patient is at risk for fungal infections, such as those who are immunocompromised.8,9 However, others have highlighted that a large number of non-albicans Candida species are resistant to azole therapy but not caspofungin.11 A patient with FG due to C glabrata was successfully treated with caspofungin, suggesting that caspofungin may be a good choice for empiric antifungal therapy as well. Alternatively, use of amphotericin B is advocated in some series if initial stains show the presence of fungus or if fungus is grown in culture.4,5 The best empiric antifungal for FG remains to be determined, but one should be considered, especially in patients at risk for fungal infections. Antimicrobial selection can be further tailored based on intraoperative gram stain results, which should always be performed and may indicate if fungi are present.
In our case, urinary retention and stricture disease with suspected chronic Candida colonization contributed flora that caused FG after urinary extravasation. There were several early indicators of a fungal infection, including baseline immunodeficiency and a lack of crepitus upon examination, suggesting a paucity of gas-forming bacteria. Additionally, the intraoperative findings included a retroperitoneal abscess, which would be unusual for classic FG and suggested possible underlying urinary extravasation secondary to urethral stricture disease. Although this case represents a single report, other reports in the literature suggest an increasing frequency of fungal infections in necrotizing fasciitis. With increasing reports or series, more data will be available to determine risk factors and appropriate treatment regimens. We suggest that patients presenting with FG and chronic retention, prior fungal infections, stricture disease with suspected urinary extravasation, or compromised immunity be treated with an empiric antifungal agent in addition to broad spectrum antibiotics with urgent debridement.
Conclusions
Fungal etiologies of necrotizing infections are rare but becoming increasingly recognized. Consideration should be made to adding empiric antifungal agents in the treatment algorithm of atrisk patients such as immunodeficient individuals.
Main Points.
Fournier gangrene (FG) is a severe and life-threatening infection with a persistently high mortality rate, ranging from 7.5% to 50%.
A 73-year-old diabetic man presented with complaints of scrotal discomfort, swelling, and necrosis, consistent with FG.
The cultures from this evaluation grew Candida albicans as the sole organism present. The patient was treated with one dose of caspofungin and then fluconazole to complete a 20-day course, which resolved the patient’s symptoms.
Although polymicrobial bacterial infection tends to be the etiology of FG, fungi may be seen and have been reported with increasing frequency along with increasingly resistant bacterial causes.
In this case, urinary retention and stricture disease with suspected chronic Candida species colonization contributed flora that caused FG after urinary extravasation. There were several early indicators of a fungal infection.
The authors suggest that patients presenting with FG and chronic retention, prior fungal infections, stricture disease with suspected urinary extravasation, or compromised immunity be treated with an empiric antifungal agent in addition to broad-spectrum antibiotics with urgent debridement.
References
- 1.Fournier JA. Jean-Alfred Fournier 1832–1914. Gangrène foudroyante de la verge (overwhelming gangrene). Sem Med 1883. Dis Col Rectum. 1988;31:984–988. doi: 10.1007/BF02554904. [DOI] [PubMed] [Google Scholar]
- 2.Yanar H, Taviloglu K, Ertekin C, et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg. 2006;30:1750–1754. doi: 10.1007/s00268-005-0777-3. [DOI] [PubMed] [Google Scholar]
- 3.Corman JM, Moody JA, Aronson WJ. Fournier’s gangrene in a modern surgical setting: improved survival with aggressive management. BJU Int. 1999;84:85–88. doi: 10.1046/j.1464-410x.1999.00140.x. [DOI] [PubMed] [Google Scholar]
- 4.Shyam DC, Rapsang AG. Fournier’s gangrene. Surgeon. 2013;11:222–232. doi: 10.1016/j.surge.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Thwaini A, Khan A, Malik A, et al. Fournier’s gangrene and its emergency management. Postgrad Med J. 2006;82:516–519. doi: 10.1136/pgmj.2005.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nisbet AA, Thompson IM. Impact of diabetes mellitus on the presentation and outcomes of Fournier’s gangrene. Urology. 2002;60:775–779. doi: 10.1016/s0090-4295(02)01951-9. [DOI] [PubMed] [Google Scholar]
- 7.Laor E, Palmer LS, Tolia BM, et al. Outcome prediction in patients with Fournier’s gangrene. J Urol. 1995;154:89–92. [PubMed] [Google Scholar]
- 8.Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urology. 2013;81:752–758. doi: 10.1016/j.urology.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Johnin K, Nakatoh M, Kadowaki T, et al. Fournier’s gangrene caused by Candida species as the primary organism. Urology. 2000;56:153. doi: 10.1016/s0090-4295(00)00527-6. [DOI] [PubMed] [Google Scholar]
- 10.Loulergue P, Mahe V, Bougnoux ME, et al. Fournier’s gangrene due to Candida glabrata. Med Mycol. 2008;46:171–173. doi: 10.1080/13693780701636567. [DOI] [PubMed] [Google Scholar]
- 11.Jensen P, Zachariae C, Gronhoj Larsen F. Necrotizing soft tissue infection of the glans penis due to atypical Candida species complicated with Fournier’s gangrene. Acta Derm Venereol. 2010;90:431–432. doi: 10.2340/00015555-0847. [DOI] [PubMed] [Google Scholar]
- 12.Tleyjeh IM, Routh J, Qutub MO, et al. Lactobacillus gasseri causing Fournier’s gangrene. Scand J Infect Dis. 2004;36:501–503. doi: 10.1080/00365540410015916. [DOI] [PubMed] [Google Scholar]
- 13.Corcoran AT, Smaldone MC, Gibbons EP, et al. Validation of the Fournier’s gangrene severity index in a large contemporary series. J Urol. 2008;180:944–948. doi: 10.1016/j.juro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Durand CM, Alonso CD, Subhawong AP, et al. Rapidly progressive cutaneous Rhizopus microsporus infection presenting as Fournier’s gangrene in a patient with acute myelogenous leukemia. Transpl Infect Dis. 2011;13:392–396. doi: 10.1111/j.1399-3062.2011.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabe M, Bishop M, Bjerklund-Johansen T, et al. Guidelines on urological infections. European Association of Urology. 2010. [Accessed April 28, 2014]. Available at http://www.uroweb.org/gls/pdf/Urological%20Infections%202010.pdf.