Abstract
Broncho-Vaxom (OM85-BV) is an extract mixture from 8 strains of Gram+ and Gram− bacteria and plays an important role in anti-infection immune response by regulating macrophage activity and cytokine productions. However, the mechanism by which OM85-BV enhances the cytokine expression is still obscure. In this study, we evaluated the effects of OM85-BV on the productions of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) in RAW264.7 murine macrophages. Exposure of RAW264.7 cells to 100 μg/mL OM85-BV upregulated the expression of IL-1β, IL-6, and TNF-α at the mRNA and protein levels in a time- and dose-dependent manner. In addition, OM85-BV induced extracellular signal-regulated kinase (ERK) 1/2 and nuclear factor-kappa B (NF-κB) phosphorylation. Pretreatment with U0126 or Bay11-7082, respectively, could decrease IL-1β, IL-6, and TNF-α productions induced by OM85-BV. Application of Toll-like receptor (TLR) 4 or TLR2 small-interfering RNA (siRNA) into RAW264.7 cells could inhibit the productions of cytokines and ERK1/2 and NF-κB phosphorylation induced by OM85-BV. Consistent with this, downregulating either myeloid differentiation factor 88 (MyD88) or TRIF-related adaptor molecule (TRAM) gene with MyD88-siRNA or TRAM-siRNA separately could reduce the productions of cytokines and ERK1/2 and NF-κB phosphorylation induced by OM85-BV. Our study demonstrated that the productions of IL-1β, IL-6, and TNF-α induced by OM85-BV in RAW264.7 cells were through TLR4 and TLR2 signaling pathway-mediated activation of ERK1/2 and NF-κB.
Introduction
Broncho-Vaxom (OM85-BV) is a low-endotoxin immunemodulator derived from 8 of the most common bacteria strains in upper respiratory tract infection (URI), including Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozeanae, Staphylococcus aureus, Streptococcus viridans, Streptococcus pyogenes, and Neisseria catarrhails. OM85-BV has been widely used to prevent recurrent URI in both adults and children (Orcel and others 1994; Jara-Pérez and Berber 2000; Gutiérrez-Tarango and Berber 2001; Schaad and others 2002). A recent meta-analysis indicated that OM85-BV reduced morbidity rate by ∼26.2% (Schaad 2010). Solèr and others (2007) found that OM85-BV could significantly decrease the frequency of acute exacerbations in patients with a history of chronic bronchitis and mild COPD. In addition, Razi and others (2010) demonstrated that oral administration of OM-85 BV reduced the rate and duration of wheezing attacks in preschool children with acute respiratory tract illnesses. These researches suggested that OM85-BV might be beneficial for the prevention and treatment of respiratory tract infection.
OM85-BV regulates the acquired immune response by affecting the activity of lymphocytes and the synthesis of immunoglobulin (Rozy and Chorostowska-Wynimko 2008). OM85-BV is a polyclonal B cell activator and induces B cell proliferation (Bessler and others 1997). Also, OM85-BV increased the ratio of CD4+/CD8+ T cells in bronchoalveolar lavage fluid of patients with nonobstructive chronic bronchitis (Emmerich and others 1992). Navarro and others (2011) found that OM85-BV could recruit the regulatory T cells (CD4+CD25+foxp3+) to the airways so as to prevent asthma. Our previous study showed that the oral administration of OM85-BV attenuated the reductions of the proportions of CD4+T cells and CD4+/CD8+ T cells in blood of immunosuppressive patients with autoimmune nephrosis (Zhang and others 2012). OM85-BV encouraged the preferential development of the Th1-mediated immunity characterized by amplified interferon (IFN)-γ and decreased interleukin (IL)-4 production in immature rats (Bowman and Holt 2001). Moreover, in human studies, an increased content of serum IgG, IgA, and IgM levels was observed upon OM85-BV treatment (Puigdollers and others 1980; Maestroni and Losa 1984).
As a typical bacterial immunostimulator, OM85-BV has been reported to affect innate immunity by regulating the activity of macrophages and the productions of proinflammatory cytokines. Cytokines secreted by macrophages also contribute to the anti-infection immune response. OM85-BV has been shown to enhance the productions of tumor necrosis factor-α (TNF-α), IL-1β, IL-6, IL-8, and IFN-γ in vitro (Broug-Holub and others 1995; Keul and others 1996; Huber and others 2005). These cytokines protect the respiratory tract against infections by the induction of the coordinated expression of leukocytes and vascular adhesion molecules as well as the establishment of a chemotactic gradient, which is essential for the recruitment of inflammatory cells to the site of infection (Broug-Holub and Kraal 1997). However, the mechanism by which OM85-BV enhances the productions of the cytokines is still obscure. Alyanakian and others (2006) reported that OM85-BV stimulated the production of transforming growth factor (TGF)-β by dendritic cells in a MyD88-dependent fashion. But it remains controversial which toll-like receptor (TLR) is necessary for the productions of cytokines induced by OM85-BV (Huber and others 2005; Alyanakian and others 2006; Navarro and others 2011). Thus, this study was undertaken to evaluate the effect of OM85-BV on the IL-1β, IL-6, and TNF-α productions in RAW264.7 murine macrophages and the role of OM85-BV in TLR-mediated signaling pathway.
Materials and Methods
Drug
OM85-BV was purchased from OM Laboratories, Switzerland. The lyophilizate was dissolved in phosphate-buffered saline at room temperature and used at a concentration of 100 μg/mL.
Cell culture
The RAW264.7 murine macrophage cells were purchased from the Cell Repository, Chinese Academy of Sciences. Cells were cultured in DMEM (Hyclone) with 10% FBS (Hyclone) and incubated in a humidified atmosphere containing 5% CO2 at 37°C. Before OM85-BV (100 μg/mL) treatment, the cells were plated in 6-well culture plates with serum-free medium for 24 h.
Small-interfering RNA transfection
Small-interfering RNAs (siRNAs) that target murine TLR4, TLR2, MyD88, and Toll/IL-1 receptor domain-containing adaptor inducing interferon-β (TRIF)-related adaptor molecule (TRAM) were designed and synthesized by Guangzhou Ribo Bio Co., Ltd. RAW264.7 cells cultured in a 6-well plate were transfected with either 150 nM siRNA or control siRNA by using Lipofectamine 2000 (Invitrogen, Inc.), according to the manufacturer's instructions. Western blot was performed to evaluate the efficiency of transfection at 48 h after transfection. In some experiments, the cells were stimulated for 6 h with 100 μg/mL OM85-BV after transfection for 48 h. The sequences of primers used for siRNA were listed in Table 1.
Table 1.
The Sequences of Primers Used for siRNA
| Sense (5′→3′) | Antisense (5′→3′) | |
|---|---|---|
| TLR4-siRNA1 | CAAUUCUGUUGCUUGUAUA dTdT | UAUACAAGCAACAGAAUUG TdTd |
| TLR4-siRNA2 | CCAUGAACUGACUCUAAGA dTdT | UCUUAGAGUCAGUUCAUGG TdTd |
| TLR4-siRNA3 | CCCAAUUGACUUCAUUCAAGA | UUGAAUGAAGUCAAUUGGGUU |
| TLR2-siRNA1 | GGAGUCUCUGUCAUGUGAU dTdT | dTdT CCUCAGAGACAGUACACUA |
| TLR2-siRNA2 | GUCCAGCAGAAUCAAUACA dTdT | dTdT CAGGUCGUCUUAGUUAUGU |
| TLR2-siRNA3 | GCAGGCGACAACCACUUUG dTdT | dTdT CGUCCGCUGUUGGUGAAAC |
| MyD88-siRNA1 | CAACCUGGGUCAAGUGUAA dTdT | UUACACUUGACCCAGGUUG TdTd |
| MyD88-siRNA2 | UGUUAGACCGUGAGGAUAU dTdT | AUAUCCUCACGGUCUAACA TdTd |
| MyD88-siRNA3 | GACUGAUUCCUAUUAAAUA dTdT | UAUUUAAUAGGAAUCAGUC TdTd |
| TRAM-siRNA1 | UCUUGUUACUGACUGAGAA dTdT | UUCUCAGUCAGUAACAAGA TdTd |
| TRAM-siRNA2 | GGCGCUGCAAACCAUCAAU dTdT | AUUGAUGGUUUGCAGCGCC TdTd |
| TRAM-siRNA3 | GCAAACCAUCAAUGCCUUA dTdT | UAAGGCAUUGAUGGUUUGC TdTd |
Reverse transcription quantitative real-time polymerase chain reaction
Total RNA was extracted by Trizol and 1 μg RNA was reversely transcripted into cDNA according to the manufacturer's instruction. IL-1β, TNF-α, IL-6, IFN-β, TLR2, TLR4, MyD88, and TRAM were amplified by using SYBR Green master mix (Finnzyme, New England Biolabs) and β-actin served as an internal control. Relative mRNA levels were analyzed by the 2−ΔΔCt method. The sequences of primers used for quantitative reverse transcription-polymerase chain reaction (RT-PCR) were listed in Table 2.
Table 2.
The Sequences of Primers Used for Quantitative Reverse Transcription-Polymerase Chain Reaction
| Name | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| IL-1β | TTTGAAGTTGACGGACCCC | GTGCTGCTGCGAGATTTGA |
| IL-6 | CCACGGCCTTCCCTACTTC | CTCATTTCCACGATTTCCCAG |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| TLR4 | AGTTTAGAGAATCTGGTGGCTGTG | TTCCCTGAAAGGCTTGGTCT |
| TLR2 | CTGAGAATGATGTGGGCGTG | ATGGGAATCCTGCTCACTGTAG |
| MyD88 | CCAGCGAGCTAATTGAGAAAAG | ATAGTGATGAACCGCAGGATAC |
| TRAM | GCAGCACAAGTACAACTCCGTC | TGCCTCTCAAATACAGACTCCC |
| IFN-β | GAGTTACACTGCCTTTGCCATC | CAAGTGGAGAGCAGTTGAGGAC |
| β-actin | CTGAGAGGGAAATCGTGCGT | CCACAGGATTCCATACCCAAGA |
Western blot
RAW264.7 cells were lysed by RIPA lysis buffer with cocktail protease and phosphatase inhibitors at 4°C for 1 h. Cytoplasmic and nuclear proteins were obtained by Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime) according to the manufacturer's instruction. Protein concentration was determined with BCA Assay Kit (Beyotime). Sixty micrograms of protein was separated on 10% SDS-PAGE, and then transferred to positively charged nylon membranes. After transfer, the membranes were blocked with 5% skimmed milk in TBS with 0.1% Tween-20 for 1 h at 37°C, and then blotted at 4°C overnight with TLR4, TLR2, MyD88, TRAM (Abgent), phosphorylated extracellular signal-regulated kinase (ERK) 1/2, AKT, P38, P65 (ser536), nuclear factor-κB inhibitory protein (IκB) and ERK1/2, AKT, P38, P65, and IκB primary antibodies (Cell Signaling Technology). Specific bands were detected with an ECL Assay Kit (Bipec Biopharma). Image J software was used to quantify the density of bands.
ELISA assay
Concentrations of IL-1β, IL-6, and TNF-α in culture supernatants were quantified by ELISA Assay Kit (eBioscience) according to the manufacturer's instruction. Briefly, ELISA plate was coated with 100 μL/well of capture antibody and incubated overnight at 4°C. The samples were added to the wells at room temperature for 2 h. Follow washing, the secondary antibody (1:100) was added and incubated at room temperature for 1 h. About 100 μL/well of Avidin-HRP (1:100) was added into the wells for a 30-min incubation, and 3,3′,5,5′-tetramethylbenzidine (TMB) was used to detect. The optical density (OD) value was detected by an ELISA Reader (Thermo Fisher Scientific, Inc.)
Immunocytochemistry
RAW264.7 cells grown on cover slips were incubated with anti-TLR4 and anti-TLR2 antibodies (Abgent) at 4°C overnight, followed by incubation with FITC-conjugated goat anti-rabbit IgG at room temperature for 1 h. Ultimately, slides were counterstained with DAPI to dye the cell nuclei and analyzed by confocal laser scanning microscopy.
Statistical analyses
All data were analyzed by LED t-test or a one-way analysis of variance with SPSS 13.0. Data were expressed as means±SDs and represented at least 3 independent experiments. P<0.05 was considered statistically significant.
Results
OM85-BV increased the expression of IL-1β, IL-6, and TNF-α in time- and dose-dependent manners
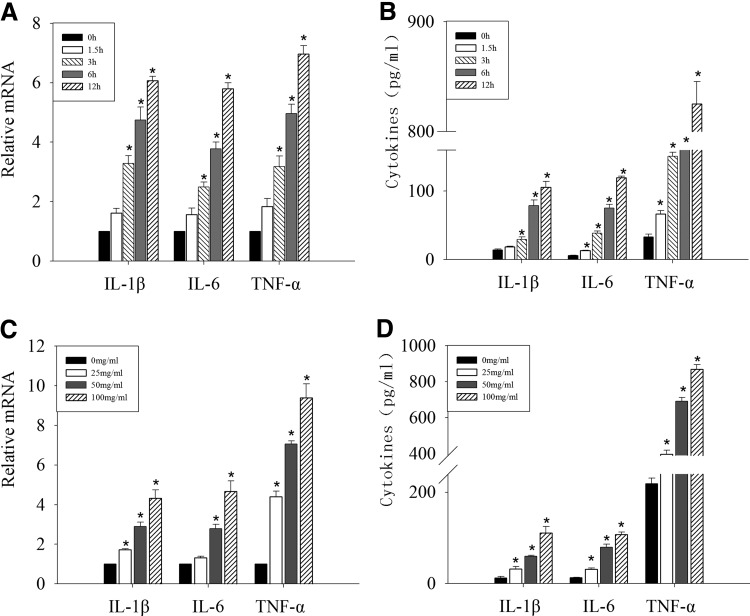
First, we tested the expression of IL-1β, IL-6, and TNF-α after OM85-BV treatment. RAW264.7 murine macrophages were stimulated with 100 μg/mL OM85-BV for 1.5, 3, 6, and 12 h. IL-1β, IL-6, and TNF-α mRNA expression significantly increased over time (Fig. 1A). Also, we assessed cytokine secretion in culture media. Consistent with the mRNA expression, the amounts of IL-1β, IL-6, and TNF-α in the supernatant increased in a time-dependent manner (Fig. 1B). Then, after exposure to 25, 50, or 100 μg/mL OM85-BV, respectively, for 12 h, the levels of IL-1β, IL-6, and TNF-α mRNA markedly enhanced compared with the basal level in macrophages (Fig. 1C). In line with the mRNA expression, OM85-BV boosted IL-1β, IL-6, and TNF-α productions in a dose-dependent manner (Fig. 1D). Thus, our results indicated that OM85-BV could upregulate the productions of IL-1β, IL-6, and TNF-α in time- and dose-dependent manners.
FIG. 1.
OM85-BV-induced IL-1β, IL-6, and TNF-α expression was time and dose dependent. RAW264.7 murine macrophages were treated with 100 μg/mL OM85-BV for 0, 1.5, 3, 6, and 12 h, respectively. The expression of IL-1β, IL-6, and TNF-α mRNA was analyzed by RT-PCR (A) and the levels of cytokines in supernatants were detected by ELISA (B). RAW264.7 cells were exposed with 0, 25, 50, or 100 μg/mL OM85-BV for 6 h. The expression of IL-1β, IL-6, and TNF-α mRNA was analyzed by RT-PCR (C) and the levels of cytokines in supernatants were detected by ELISA (D). *P<0.05 compared with control group. IL, interleukin; RT-PCR, reverse transcription-polymerase chain reaction; TNF-α, tumor necrosis factor-α.
OM85-BV induced cytokine release in a TLR4- and TLR2-dependent manner
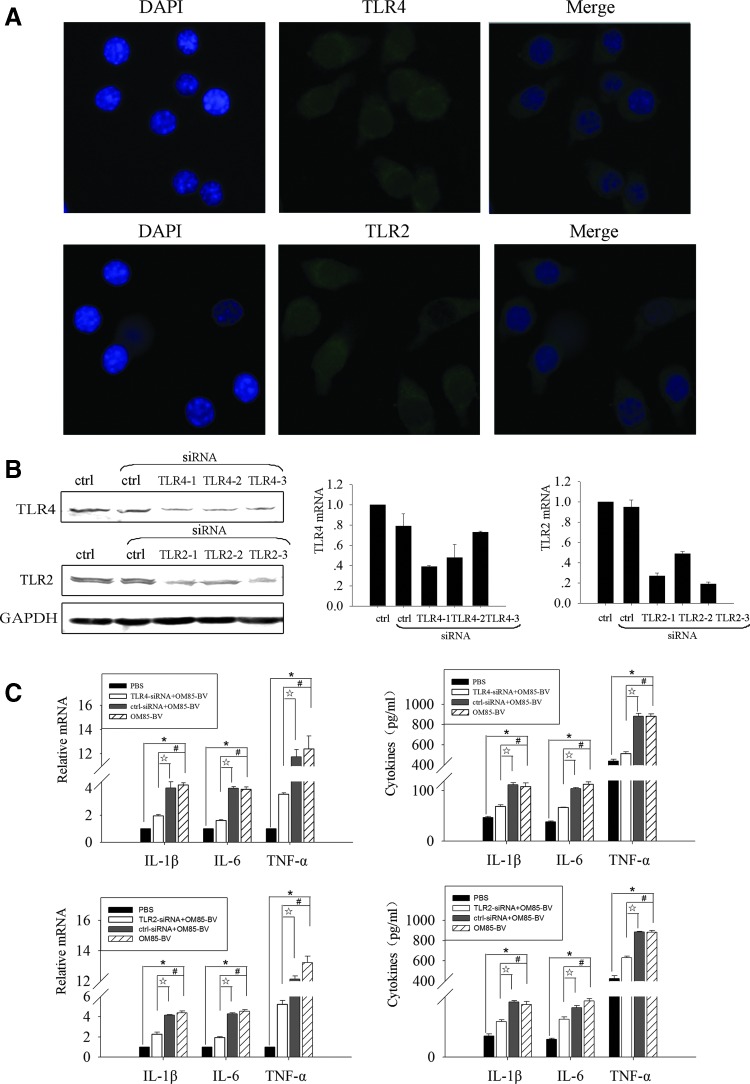
OM85-BV is composed of 8 Gram+ and Gram− strains. To determine whether these components could activate TLRs and thus potentially contribute to the immunomodulatory effect, its capacity to stimulate cytokine productions in a TLR-dependent way was examined. TLR4 is a representative member of the TLR family, recognizing the lipopolysaccharide (LPS) of Gram− bacteria. Moreover, TLR2 is involved in the recognition of a wide range of pathogen-associated molecular patterns (PAMPs) derived from fungi, bacteria, parasites, and viruses (Kawai and Akira 2010). Specifically, TLR2 generally forms heterodimers with TLR1 or TLR6, and the TLR2-TLR1 heterodimer recognizes triacylated lipopeptides from Gram− bacteria, whereas the TLR2-TLR6 heterodimer recognizes diacylated lipopeptides from Gram+ bacteria (Akira and others 2006). So we hypothesized that OM85-BV might upregulate cytokines in a TLR4- and TLR2-dependent way. To test this hypothesis, we first detected the expression of TLR4 and TLR2 in RAW264.7 cells. Immunocytochemistry staining showed that TLR4 and TLR2 were expressed on the surface of RAW264.7 cells (Fig. 2A). Then, siRNA-mediated knockdown assay was used to evaluate the role of TLR4 and TLR2 in cytokine productions induced by OM85-BV. We transfected the RAW264.7 cells with 3 different TLR4- or TLR2-siRNA sequences to inhibit the expression of TLR4 or TLR2. The results showed that the expression of TLR4 mRNA decreased by 71%, 52%, and 27% individually; however, the expression of TLR2 mRNA decreased by 73%, 51%, and 81% (Fig. 2B). So we chose the first TLR4-siRNA and the third TLR2-siRNA sequence for the following experiments. Pretreatment of RAW264.7 cells with TLR4- or TLR2-siRNA partly abrogated the productions of cytokines induced by OM85-BV at both mRNA and protein levels (Fig. 2C). Thus, these data suggested that the productions of cytokines induced by OM85-BV were partially TLR4 and TLR2 dependant.
FIG. 2.
OM85-BV induced cytokine release via TLR4 and TLR2 signaling pathway. (A) Immunocytochemistry staining of TLR4 and TLR2 in RAW264.7 cells. Magnification×400. (B) RAW264.7 cells were transfected with 3 different TLR4- or TLR2-siRNA sequences for 48 h. Western blot and RT-PCR were used to detect the efficiency of interference. (C) RAW264.7 cells were transfected with TLR4- or TLR2-siRNA for 48 h, and then cells were stimulated with 100 μg/mL OM85-BV for 6 h. The expression of IL-1β, IL-6, and TNF-α mRNA was analyzed by RT-PCR and the levels of cytokines in supernatant were detected by ELISA. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. siRNA, small-interfering RNA; TLR, toll-like receptor.
OM85-BV induced cytokine release in a MyD88/TRAM-dependent manner
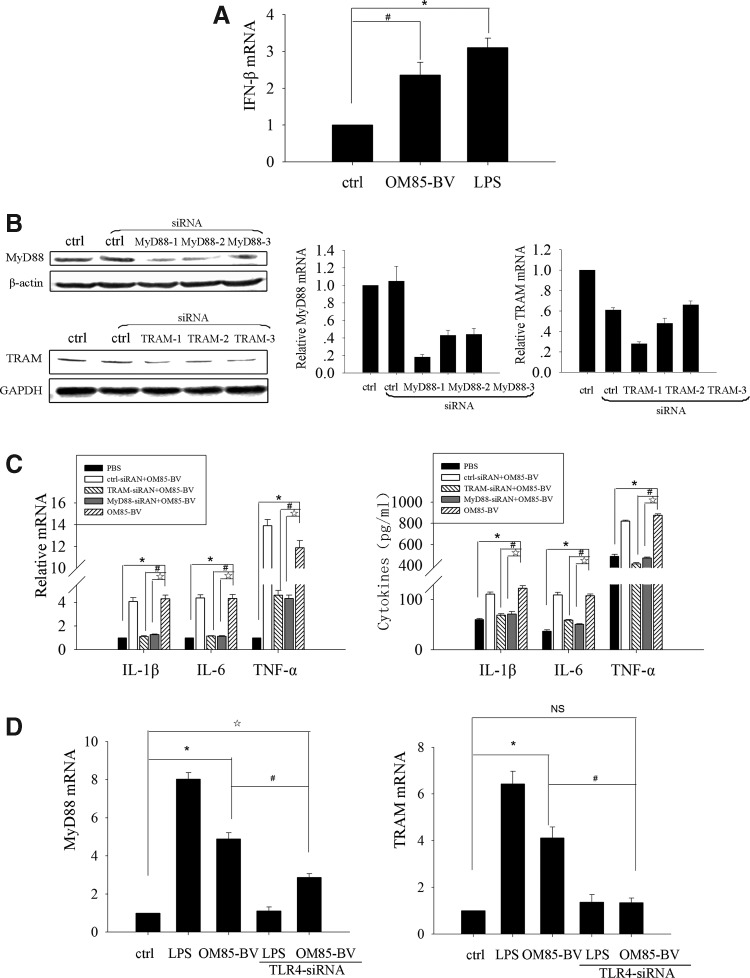
TLR signaling pathways can be largely classified as either MyD88-dependent pathways, which recruits myeloid differentiation factor 88 (MyD88) paired with MyD88-like protein (Mal, also called TIRAP) and induces the productions of inflammatory cytokines, or TRIF-dependent pathways, which recruits TRIF paired with TRAM and drives the induction of type I interferon as well as inflammatory cytokines (Sheedy and O'Neill 2007; Kawai and Akira 2010). MyD88 is a common adaptor that is universally used by all TLRs except for TLR3, whereas TRIF mediates the MyD88-independent pathway from TLR3 and TLR4. Yamamoto and others (2003) further confirmed that TRAM was specifically involved in the TLR4-mediated MyD88-independent signaling pathway, but not shared by TLR3. Our results disclosed that OM85-BV-induced cytokine release was TLR4 and TLR2 dependant, and further we explored whether the productions of cytokines induced by OM85-BV were MyD88 or TRAM dependent. First, we found that OM85-BV, as well as LPS, could induce IFN-β mRNA increase (Fig. 3A). Then, RAW264.7 cells were transfected with 3 different MyD88-siRNA or TRAM-siRNA sequences, and the interference efficiency was determined by western blot and RT-PCR. We found that both the first MyD88-siRNA and TRAM-siRNA sequence had the similar interference efficiency, separately 82% and 72% (Fig. 3B). In the light of this, we chose the first MyD88-siRNA and TRAM-siRNA to do the following experiments. Our data showed that silencing either MyD88 or TRAM gene could partially inhibit IL-1β, IL-6, and TNF-α productions induced by OM85-BV (Fig. 3B). In addition, to further illustrate the link between TLR4 and MyD88/TRAM, the expression of MyD88 and TRAM mRNA at the transcription level was observed when the cells were transfected with TLR4-siRNA. We found that MyD88 mRNA partly decreased but TRAM mRNA entirely reduced (Fig. 3C). Therefore, these data indicated that both MyD88 and TRAM of the TLR signaling pathway were involved in OM85-BV-induced cytokine productions.
FIG. 3.
MyD88/TRAM played a role in OM85-BV-induced cytokine productions. (A) RAW264.7 murine macrophages were treated with 100 μg/mL OM85-BV or 10 ng/mL LPS for 6 h and the expression of IFN-β mRNA was analyzed by RT-PCR. n ≥3, *P<0.05 and #P<0.05. (B) RAW264.7 cells were transfected with 3 different MyD88-siRNA or TRAM-siRNA sequences for 48 h. Western blot and RT-PCR were used to detect the efficiency of interference. (C) RAW264.7 cells were transfected, respectively, with MyD88-siRNA or TRAM-siRNA for 48 h, and then cells were stimulated with 100 μg/mL OM85-BV for 6 h. The expression of IL-1β, IL-6, and TNF-α mRNA was analyzed by RT-PCR and the levels of cytokines in supernatant were detected by ELISA. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. (D) RAW264.7 cells were transfected with TLR4-siRNA for 48 h, followed by the stimulation of 100 μg/mL OM85-BV or 10 ng/mL LPS for 6 h. The expression of MyD88 and TRAM mRNA was analyzed by RT-PCR. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. IFN, interferon; LPS, lipopolysaccharide; MyD88, myeloid differentiation factor 88; NS, not significant; TRAM, TRIF-related adaptor molecule.
Activation of ERK1/2 contributed to OM85-BV-induced cytokine productions
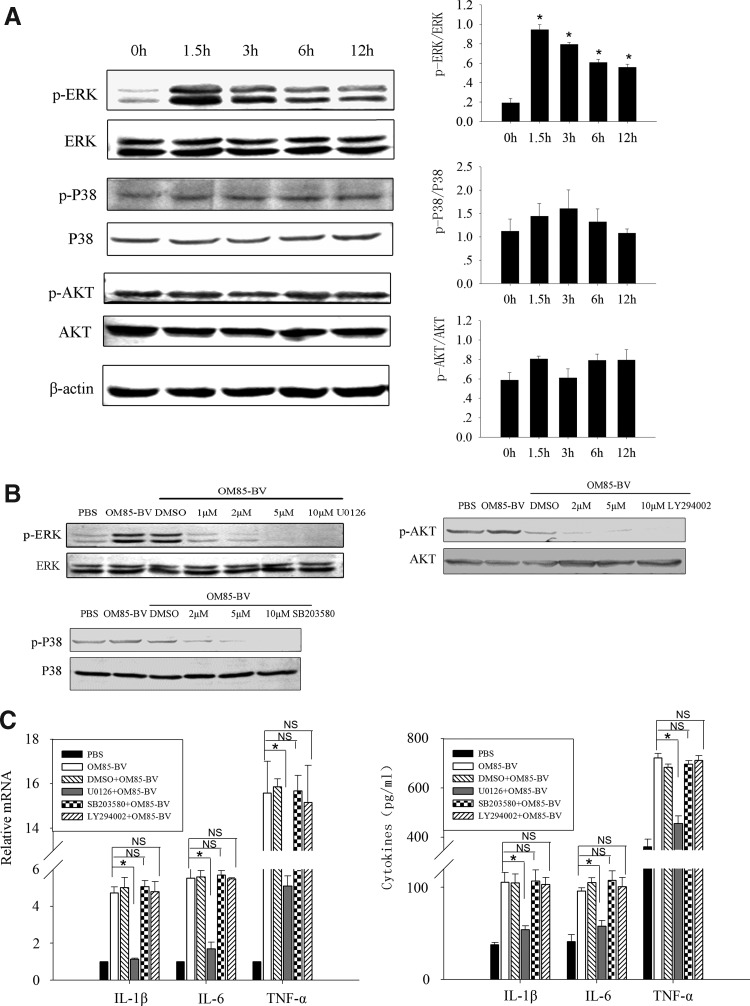
MAPK pathways are essential for the expression of a variety of cytokines. We hypothesized that they might play a role in the regulation of cytokine productions induced by OM85-BV. To test this hypothesis, RAW264.7 cells were stimulated with 100 μg/mL OM85-BV for 0, 1.5, 3, 6, and 12 h. It showed that OM85-BV induced ERK1/2 phosphorylation since 1.5 h, but not P38 and AKT phosphorylation (Fig. 4A). Further, we pretreated RAW264.7 cells with U0126 (Fig. 4B), a pharmacological inhibitor of MEK1/2, and the productions of IL-1β, IL-6, and TNF-α induced by OM85-BV significantly reduced at both mRNA and protein levels (Fig. 4C). However, SB203580 and LY294002, the specific inhibitor of P38 and AKT (Fig. 4B), had no effect on the expression of the cytokines (Fig. 4C). These results revealed that ERK1/2 pathway was involved in OM85-BV-induced cytokine expression.
FIG. 4.
ERK1/2 pathway was involved in OM85-BV-induced cytokine expression. (A) The activations of ERK1/2, P38, and AKT in response to OM85-BV were examined by western blot. n ≥3, *P<0.05 compared with 0 h. (B) The blocking effect of U0126, SB203580, and LY294002 on ERK1/2, P38, and AKT phosphorylation individually at different concentrations. (C) RAW264.7 cells were pretreated with 5 μM U0126, SB203580, and LY294002 for 2 h prior to OM85-BV treatment. RT-PCR was used to analyze IL-1β, IL-6, and TNF-α mRNA expression. ELISA was performed to measure the levels of cytokines in supernatant. n ≥3, *P<0.05. ERK1/2, extracellular signal-regulated kinase 1/2.
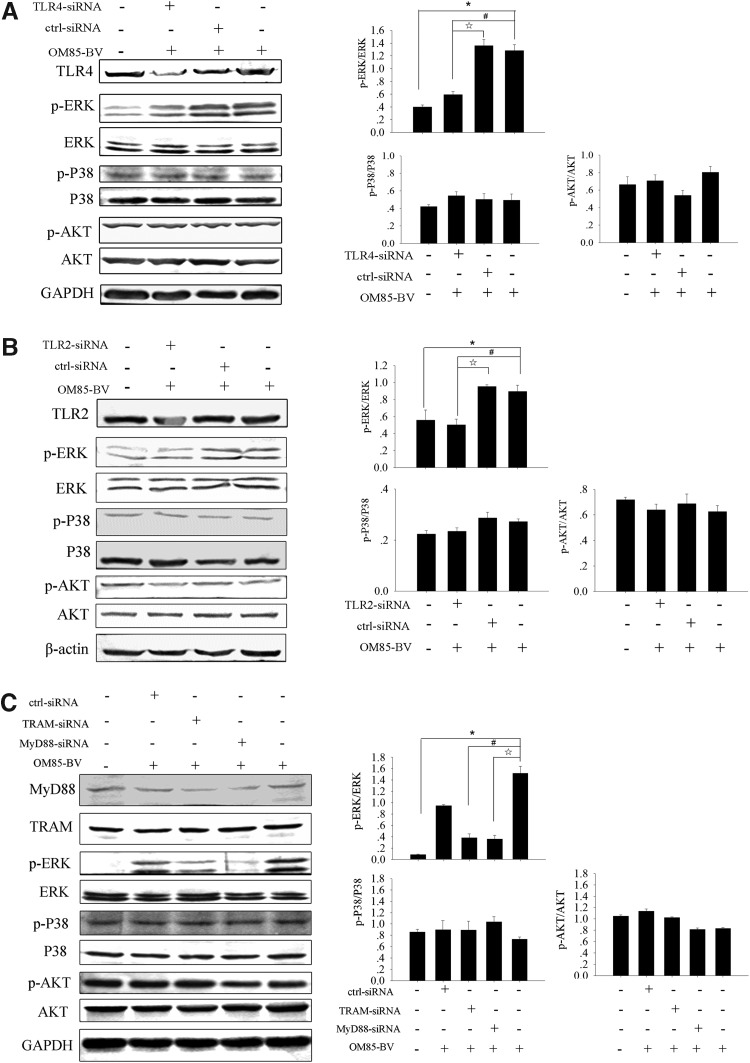
OM85-BV induced the activation of ERK1/2 via TLR4 and TLR2 signaling pathway
Our data revealed that OM85-BV-induced cytokine release was in a TLR4- and TLR2-dependent manner. Further, the activation of ERK1/2 contributed to OM85-BV-induced cytokine expression. Therefore, we hypothesized that OM85-BV induced the activation of ERK1/2 through TLR4 and TLR2 signaling pathway. RAW264.7 cells were pretreated with TLR4-siRNA, which induced the downregulation of ERK1/2 phosphorylation, but not P38 and AKT phosphorylation (Fig. 5A). The similar results were observed when the cells were transfected with TLR2-siRNA (Fig. 5B). Moreover, ERK1/2 phosphorylation induced by OM85-BV decreased when RAW264.7 cells were transfected with MyD88-siRNA or TRAM-siRNA individually (Fig. 5C). So, these data revealed that OM85-BV induced the activation of ERK1/2 via TLR4 and TLR2 signaling pathway.
FIG. 5.
OM85-BV induced the activation of ERK1/2 through TLR4 and TLR2 signaling pathway. (A, B) RAW264.7 cells transfected with TLR4-siRNA or TLR2-siRNA were cultured with 100 μg/mL OM85-BV for 6 h. The activation of ERK1/2, P38, and AKT in response to OM85-BV was examined by western blot. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. (C) RAW264.7 cells were transfected with MyD88-siRNA or TRAM-siRNA, respectively, for 48 h, and then cells were stimulated with 100 μg/mL OM85-BV for 6 h. The level of ERK1/2, P38, and AKT phosphorylation was tested by western blot. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05.
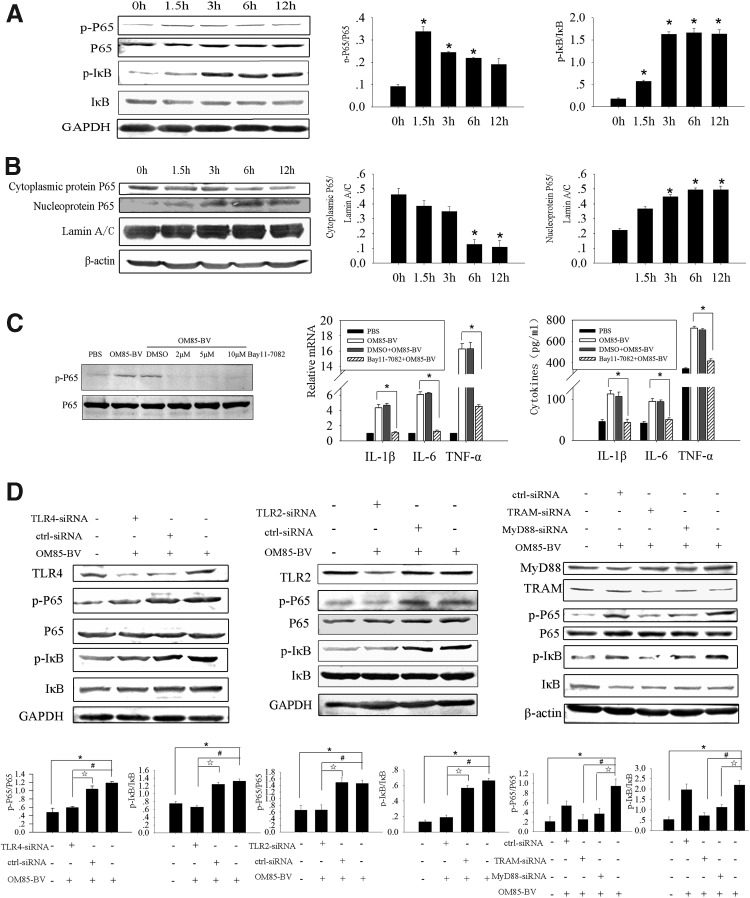
OM85-BV induced the activation and translocation of NF-κB via the TLR4 and TLR2 signaling pathway
Nuclear factor-kappa B (NF-κB), a key transcription factor of lymphocytes and macrophages, is a member of Rel family and plays an important role in regulating the immune system (Neurath and Pettersson 1997). NF-κB regulates the transcriptions of cytokines, chemokines, interferons, MHC proteins, growth factors, and cell adhesion molecules (Baeuerle and Henkel 1994). In addition, NF-κB is essential for the functions of proinflammatory factors, such as iNOS, COX-2, TNF-α, IL-1β, and IL-6 expression (Won and others 2006). OM85-BV could active murine bone marrow–derived macrophages by inducing nuclear translocation of NF-κB (Huber and others 2005). Accordingly, we hypothesized that NF-κB might play an essential role in OM85-BV-induced cytokine productions. First, we studied the role of P65 and IκB in the regulation of cytokine productions induced by OM85-BV. RAW264.7 cells were cocultured with 100 μg/mL OM85-BV for 0, 1.5, 3, 6, and 12 h separately. We discovered that OM85-BV-induced P65 and IκB phosphorylation reached the peak at 1.5 and 3 h individually (Fig. 6A). Moreover, cytoplasmic protein P65 decreased at 6 h and nucleoprotein P65 increased since 3 h and peaked at 6 h (Fig. 6B). Following that, we pretreated RAW264.7 cells with Bay11-7082, a specific inhibitor of P65, which could cause the remarkable decrease of IL-1β, IL-6, and TNF-α productions induced by OM85-BV (Fig. 6C). To demonstrate whether OM85-BV induced activation and translocation of NF-κB via TLR4 and TLR2 signaling pathway, we transfected RAW264.7 cells with siRNA and found that TLR4- or TLR2-siRNA partially downregulated P65 and IκB phosphorylation (Fig. 6D). The similar results were observed when cells were pretreated with MyD88-siRNA or TRAM-siRNA (Fig. 6D). Collectively, OM85-BV induced IL-1β, IL-6, and TNF-α productions via TLR4 and TLR2 signaling pathway–mediated activation and translocation of NF-κB.
FIG. 6.
OM85-BV induced activation and translocation of NF-κB via TLR4 and TLR2 signaling pathway. (A) The activation of P65 and IκB in response to OM85-BV was examined by western blot. n ≥3, *P<0.05 compared with 0 h. (B) Western blot was used to detect the cytoplasmic protein and nucleoprotein P65 in RAW264.7 cells. n ≥3, *P<0.05 compared with 0 h. (C) RAW264.7 cells were pretreated with 5 μM Bay11-7082 for 2 h before 100 μg/mL OM85-BV treatment. RT-PCR was used to analyze IL-1β, IL-6, and TNF-α mRNA expression. ELISA was performed to measure the levels of cytokines in supernatants. n ≥3, *P<0.05. (D) Cells were transfected with TLR4- or TLR2-siRNA and after 48 h they were stimulated with 100 μg/mL OM85-BV for 6 h. The level of P65 and IκB phosphorylation was discovered by western blot. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. Cells were transfected with MyD88-siRNA or TRAM-siRNA, respectively, for 48 h, and then they were stimulated with 100 μg/mL OM85-BV for 6 h. Western blot was used to detect the level of P65 and IκB phosphorylation. n ≥3, *P<0.05, #P<0.05, and ☆P<0.05. NF-κB, nuclear factor-kappa B.
Discussion
OM85-BV is an extract from 8 strains of Gram+ and Gram− bacteria. As a typical bacterial immunostimulator, OM85-BV has been proven to modulate the metabolism and function of macrophages, including the oxidative metabolism and nitric oxide release, and upregulation of adhesion molecules and proinflammatory cytokine productions, such as LFA-1, MAC-1, ICAM-1, TNF-α, and IL-8 (Mauël 1992, 1994; Broug-Holub and others 1995). In the present study, we demonstrated that OM85-BV stimulated IL-1β, IL-6, and TNF-α productions at mRNA and protein levels. IL-1β, IL-6, and TNF-α are critical inflammatory cytokines predominantly produced by macrophages, and have pleiotropic effects on regulating the immune response and acute-phase reaction (Bendtzen 1988). IL-1β and TNF-α recruit the inflammatory cells to the site of infection by the induction of the coordinated expression of leukocytes and vascular adhesion molecules in order to clear invading microorganisms. IL-6 has been shown to diminish tissue inflammation in hypersensitivity pneumonitis (Denis 1992) and relief lung injury induced by oxygen toxicity and endotoxin (Ulich and others 1991; Tsan and others 1992). This may further support the view that part of the therapeutic function of OM85-BV in respiratory tract infections could be ascribed to the enhanced clearance ability of macrophages against invading bacteria (Broug-Holub and others 1995).
However, the mechanism by which OM85-BV enhances the productions of the cytokines is not completely understood. Alyanakian and others (2006) reported that OM85-BV stimulated TGF-β production by dendritic cells in a MyD88-dependent fashion. But it remains controversial which TLR is essential for OM85-BV-induced cytokine productions (Huber and others 2005; Alyanakian and others 2006; Navarro and others 2011). TLR4 is a representative member of the TLR family and recognizes the LPS of Gram− bacteria. We hypothesized that OM85-BV might upregulate cytokines through TLR4. Although LPS is one of the components of OM85-BV, Alyanakian and others (2006) indicated the amount of LPS in OM85-BV was too low to activate mononuclear phagocytes (<0.1 pg of LPS per milligram of OM85-BV). So, LPS is not the major factor in TLR4-mediated stimulatory capacity of OM85-BV. To determine whether TLR4 contributed to the OM85-BV-induced cytokine productions, TLR4-siRNA was transfected into RAW264.7 cells. After the transfection of TLR4-siRNA, the expression of cytokines induced by OM85-BV decreased. Meanwhile, the levels of ERK1/2 phosphorylation declined following the knockdown of TLR4. Thus, this demonstrated that OM85-BV induced IL-1β, IL-6, and TNF-α productions and ERK1/2 activation in RAW264.7 cells in a TLR4-dependent manner. TLR4 is distinguished from other members of TLR family by the fact that it signals from 2 different subcellular locations; it activates the TRIAP/MyD88 pathway from the plasma membrane, while its interaction with TRAM and TRIF requires internalization into an endosomal compartment (Sheedy and O'Neill 2007; Kawai and Akira 2010). TRIF is involved in the TLR4- and TLR3-mediated signaling pathways, whereas TRAM is restricted to the TLR4 pathway (Fitzgerald and others 2003; Yamamoto and others 2003). Both the TRIAP/MyD88 and TRAM/TRIF pathways contribute to the inflammatory cytokine expression (Wang and others 2011). Interestingly, we found that both MyD88-dependent and TRAM-dependent signaling pathways were involved in the productions of cytokines and ERK1/2 activation induced by OM85-BV. To further disclose the link between TLR4 and MyD88/TRAM, the expression of MyD88 mRNA and TRAM mRNA at transcription level was tested following the transfection of TLR4-siRNA in macrophages. We found that MyD88 mRNA partly decreased but TRAM mRNA entirely reduced. It suggested that there may be other TLRs involved in OM85-BV-induced cytokine productions.
TLR2 is another representative member of the TLR family and recognizes a wide range of PAMPs derived from fungi, bacteria, parasites, and viruses (Kawai and Akira 2010). So we further hypothesized that TLR2 may be involved in the production of cytokines induced by OM85-BV. Consistent with TLR4-siRNA, the expression of cytokines and the level of ERK1/2 phosphorylation induced by OM85-BV decreased following the knockdown of TLR2. So, TLR2 as well as TLR4 signaling pathway was involved in OM85-BV-induced cytokine production. However, our study could not exclude the possibilities that other TLRs may participate in OM85-BV-induced cytokine productions.
NF-κB, as a key transcription factor of lymphocytes and macrophages, is a heterodimer composed of p50 and p65 subunits. In unstimulated cells, NF-κB is characterized by its sequestration in the cytoplasm by bounding to the IκB kinase (IKK) (Kholodenko 2007). Upon stimulation, IκB is phosphorylated by activated cellular kinase complexes IKK, triggering its degradation and leading to the rapid translocation of NF-κB into the nucleus (Baeuerle and Baltimore 1996). We here determined the macrophage immunostimulatory properties of OM85-BV by measuring the activation and translocation of NF-κB, and found that OM85-BV induced P65 and IκB phosphorylation and translocation of NF-κB. In addition, when transfection with TLR4- or TLR2-siRNA, the level of P65 and IκB phosphorylation and the expression of IL-1β, IL-6, and TNF-α induced by OM85-BV reduced. The similar results were obtained when applications of MyD88 or TRAM siRNA, respectively. Therefore, OM85-BV induced the activation and translocation of NF-κB via the TLR4 and TLR2 signaling pathway.
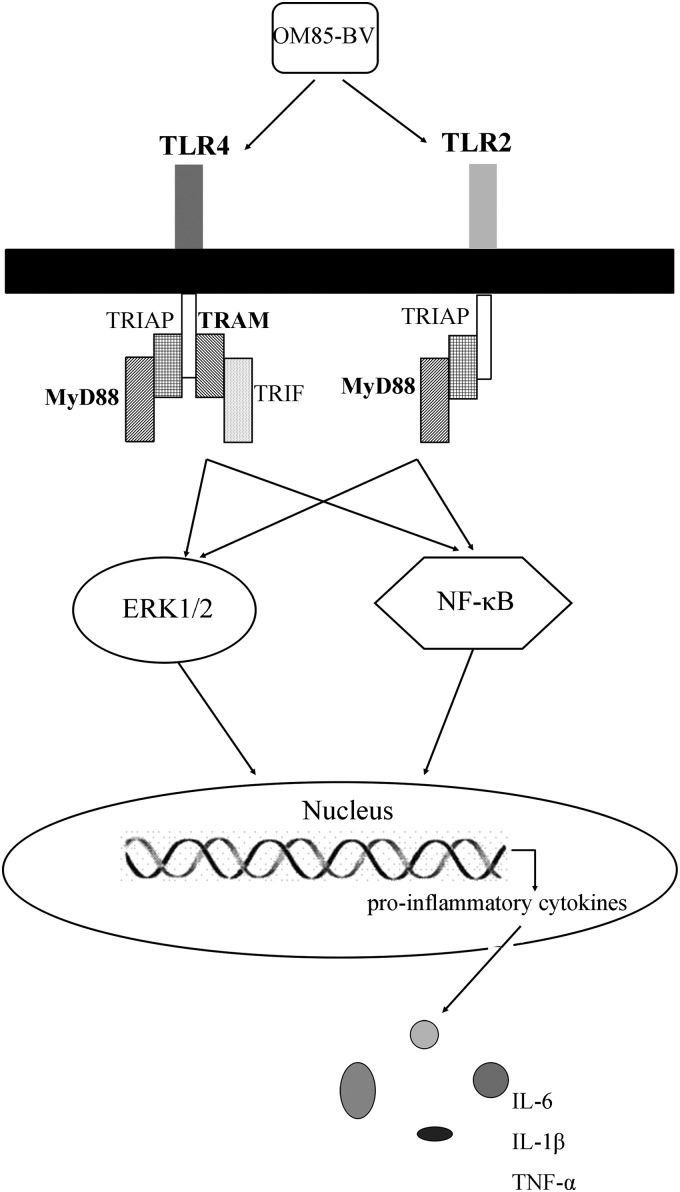
In conclusion, this study showed that OM85-BV could induce IL-1β, IL-6, and TNF-α productions as well as the activation of ERK1/2 and NF-κB in RAW264.7 cells. Meanwhile, the stimulatory capacity of OM85-BV was mediated by the TLR4 and TLR2 signaling pathway (Fig. 7). These observations may propose a new mechanism of OM85-BV in the prevention of infections. Simultaneously, it may provide the theoretical support for the applications of OM85-BV in the broader field.
FIG. 7.
OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2 and NF-κB pathway in RAW264.7 cells.
Acknowledgment
This work was supported by grant (81241025, 30800524, and 81000290) from the National Science Foundation of China.
Author Disclosure Statement
The authors confirm that there are no conflicts of interest.
References
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124(4):783–801 [DOI] [PubMed] [Google Scholar]
- Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, Normier G, Chatenoud L, Thieblemont N, Bach JF. 2006. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 55(1):179–185 [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. 1996. NF-kappa B: ten years after. Cell 87(1):13–20 [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. 1994. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179 [DOI] [PubMed] [Google Scholar]
- Bendtzen K. 1988. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett 19(3):183–191 [DOI] [PubMed] [Google Scholar]
- Bessler WG, Huber M, Baier W. 1997. Bacterial cell wall components as immunomodulators—II. The bacterial cell wall extract OM-85 BV as unspecific activator, immunogen and adjuvant in mice. Int J Immunopharmacol 19(9–10):551–558 [DOI] [PubMed] [Google Scholar]
- Bowman LM, Holt PG. 2001. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun 69(6):3719–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broug-Holub E, Kraal G. 1997. In vivo study on the immunomodulating effects of OM-85 BV on survival, inflammatory cell recruitment and bacterial clearance in Klebsiella pneumonia. Int J Immunopharmacol 19(9–10):559–564 [DOI] [PubMed] [Google Scholar]
- Broug-Holub E, Persoons JH, Schornagel K, Kraal G. 1995. Changes in cytokine and nitric oxide secretion by rat alveolar macrophages after oral administration of bacterial extracts. Clin Exp Immunol 101(2):302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. 1992. Interleukin-6 in mouse hypersensitivity pneumonitis: changes in lung free cells following depletion of endogenous IL-6 or direct administration of IL-6. J Leukoc Biol 52(2):197–201 [DOI] [PubMed] [Google Scholar]
- Emmerich B, Pachmann K, Milatovic D, Emslander HP. 1992. Influence of OM-85 BV on different humoral and cellular immune defense mechanisms of the respiratory tract. Respiration 59Suppl 3:19–23 [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, Monks B, Pitha PM, Golenbock DT. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med 198(7):1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Tarango MD, Berber A. 2001. Safety and efficacy of two courses of OM-85 BV in the prevention of respiratory tract infections in children during 12 months. Chest 119(6):1742–1748 [DOI] [PubMed] [Google Scholar]
- Huber M, Mossmann H, Bessler WG. 2005. Th1-orientated immunological properties of the bacterial extract OM-85-BV. Eur J Med Res 10(5):209–217 [PubMed] [Google Scholar]
- Jara-Pérez JV, Berber A. 2000. Primary prevention of acute respiratory tract infections in children using a bacterial immunostimulant: a double-masked, placebo-controlled clinical trial. Clin Ther 22(6):748–759 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11(5):373–384 [DOI] [PubMed] [Google Scholar]
- Keul R, Roth M, Papakonstantinou E, Nauck M, Perruchoud AP, Block LH. 1996. Induction of interleukin 6 and interleukin 8 expression by Broncho-Vaxom (OM-85 BV) via C-Fos/serum responsive element. Thorax 51(2):150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN. 2007. Untangling the signalling wires. Nat Cell Biol 9(3):247–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestroni GJ, Losa GA. 1984. Clinical and immunobiological effects of an orally administered bacterial extract. Int J Immunopharmacol 6(2):111–117 [DOI] [PubMed] [Google Scholar]
- Mauël J. 1992. Macrophage activation by OM-85 BV. Respiration 59Suppl 3:14–18 [DOI] [PubMed] [Google Scholar]
- Mauël J. 1994. Stimulation of immunoprotective mechanisms by OM-85 BV. A review of results from in vivo and in vitro studies. Respiration 61Suppl 1:8–15 [DOI] [PubMed] [Google Scholar]
- Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, Cazareth J, Sparwasser T, Dombrowicz D, Glaichenhaus N, Julia V. 2011. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol 4(1):53–65 [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S. 1997. Predominant role of NF-kappa B p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology 198(1–3):91–98 [DOI] [PubMed] [Google Scholar]
- Orcel B, Delclaux B, Baud M, Derenne JP. 1994. Oral immunization with bacterial extracts for protection against acute bronchitis in elderly institutionalized patients with chronic bronchitis. Eur Respir J 7(3):446–452 [DOI] [PubMed] [Google Scholar]
- Puigdollers JM, Serna GR, Hernandez del Rey I, Barruffet MT, Torroella JJ. 1980. Immunoglobulin production in man stimulated by an orally administered bacterial lysate. Respiration 40(3):142–149 [DOI] [PubMed] [Google Scholar]
- Razi CH, Harmancı K, Abacı A, Özdemir O, Hızlı S, Renda R, Keskin F. 2010. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol 126(4):763–769 [DOI] [PubMed] [Google Scholar]
- Rozy A, Chorostowska-Wynimko J. 2008. Bacterial immunostimulants—mechanism of action and clinical application in respiratory diseases. Pneumonol Alergol Pol 76(5):353–359 [PubMed] [Google Scholar]
- Schaad UB. 2010. OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr 6(1):5–12 [DOI] [PubMed] [Google Scholar]
- Schaad UB, Mütterlein R, Goffin H, BV-Child Study Group. 2002. Immunostimulation with OM-85 in children with recurrent infections of the upper respiratory tract: a double-blind, placebo-controlled multicenter study. Chest 122(6):2042–2049 [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, O'Neill LA. 2007. The Troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling. J Leukoc Biol 82(2):196–203 [DOI] [PubMed] [Google Scholar]
- Solèr M, Mütterlein R, Cozma G; Swiss-German OM-85 Study Group. 2007. Double-blind study of OM-85 in patients with chronic bronchitis or mild chronic obstructive pulmonary disease. Respiration 74(1):26–32 [DOI] [PubMed] [Google Scholar]
- Tsan MF, White JE, Del Vecchio PJ, Shaffer JB. 1992. IL-6 enhances TNF-alpha- and IL-1-induced increase of Mn superoxide dismutase mRNA and O2 tolerance. Am J Physiol 263(1 Pt 1):L22–L26 [DOI] [PubMed] [Google Scholar]
- Ulich TR, Yin S, Guo K, Yi ES, Remick D, del Castillo J. 1991. Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor beta inhibit acute inflammation. Am J Pathol 138(5):1097–1101 [PMC free article] [PubMed] [Google Scholar]
- Wang L, Trebicka E, Fu Y, Waggoner L, Akira S, Fitzgerald KA, Kagan JC, Cherayil BJ. 2011. Regulation of lipopolysaccharide-induced translation of tumor necrosis factor-alpha by the toll-like receptor 4 adaptor protein TRAM. J Innate Immun 3(5):437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JH, Im HT, Kim YH, Yun KJ, Park HJ, Choi JW, Lee KT. 2006. Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-kappaB inactivation. Br J Pharmacol 148(2):216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. 2003. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol 4(11):1144–1150 [DOI] [PubMed] [Google Scholar]
- Zhang M, Luan H, Zhang Q, Wang L, Lv YM, He F, Chen Y, Zeng HB, Yao Y, Liu Q. 2012. Prevention of infection in immunosuppressive patients with autoimmune nephrosis by using an immunostimulating bacterial lysate: Broncho-vaxom. Hum Vaccin Immunother 8(12):1802–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]