Abstract
Aims—To study correlations between the pattern of silver stained nucleolar organiser regions (AgNORs) in chronic lymphocytic leukaemia (CLL) and parameters of tumour kinetics. To investigate whether quantitation of the AgNOR pattern can be used to discriminate between patients with stable and progressive disease.
Methods—Peripheral blood smears from 48 patients with CLL, classified as having either stable or progressive disease (Rai stage III or IV; bulky lymph nodes or massive splenomegaly; or peripheral lymphocytes >100 × 109/1), were studied. For each patient, total tumour mass (TTM) and for patients undergoing a period of observation without treatment, the TTM duplication time (DT) and the lymphocyte doubling time (LDT) were calculated.
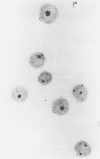
Results—Four cell types could be distinguished according to their AgNOR pattern: (1) cells with a single cluster; (2) cells with a single compact nucleolus; (3) cells with two compact nucleoli; and (4) cells with several scattered dots. The percentage of cells with clusters was the AgNOR parameter which correlated best with TTM and LDT. Correlations were also seen between the proportion of cells with clusters and age and haemoglobin concentration. A significant correlation with DT could be detected only when age was kept constant. Linear discriminant analysis revealed that the percentage of cells with clusters was the most important prognostic factor. This alone classified 94% of the patients correctly (jackknive procedure) as either stable or progressive CLL.
Conclusions—The percentage of circulating lymphocytes with clusters of AgNORs can be used as a parameter of tumour kinetics in CLL and helps to discriminate between patients with stable and progressive disease. For practical purposes, a value of more than 13% of cells with clusters is suggestive of progressive disease.
Keywords: nucleolar organiser regions
Keywords: discriminant analysis
Keywords: prognostic factors
Keywords: chronic lymphocytic leukaemia
Keywords: cell kinetics
Keywords: lymphocyte doubling time
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crocker J., Boldy D. A., Egan M. J. How should we count AgNORS? Proposals for a standardized approach. J Pathol. 1989 Jul;158(3):185–188. doi: 10.1002/path.1711580303. [DOI] [PubMed] [Google Scholar]
- Crocker J., Macartney J. C., Smith P. J. Correlation between DNA flow cytometric and nucleolar organizer region data in non-Hodgkin's lymphomas. J Pathol. 1988 Feb;154(2):151–156. doi: 10.1002/path.1711540207. [DOI] [PubMed] [Google Scholar]
- Erlanson M., Osterman B., Jonsson H., Lenner P. Chronic lymphocytic leukemia: a retrospective study of 122 cases. Eur J Haematol. 1994 Feb;52(2):108–114. doi: 10.1111/j.1600-0609.1994.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Gilberti M. F., Metze K., Lorand-Metze I. Changes of nucleolar organizer regions in granulopoietic precursors during the course of chronic myeloid leukemia. Ann Hematol. 1995 Dec;71(6):275–279. doi: 10.1007/BF01697979. [DOI] [PubMed] [Google Scholar]
- Grotto H. Z., Lorand-Metze I., Metze K. Nucleolar organizer regions in normal hematopoiesis: relationship to cellular proliferation and maturation. Nouv Rev Fr Hematol. 1991;33(1):1–4. [PubMed] [Google Scholar]
- Grotto H. Z., Metze K., Lorand-Metze I. Pattern of nucleolar organizer regions in human leukemic cells. Anal Cell Pathol. 1993 Jul;5(4):203–212. [PubMed] [Google Scholar]
- Hall P. A., Crocker J., Watts A., Stansfeld A. G. A comparison of nucleolar organizer region staining and Ki-67 immunostaining in non-Hodgkin's lymphoma. Histopathology. 1988 Apr;12(4):373–381. doi: 10.1111/j.1365-2559.1988.tb01952.x. [DOI] [PubMed] [Google Scholar]
- Han T., Rai K. R. Management of chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 1990 Apr;4(2):431–445. [PubMed] [Google Scholar]
- Iványi J. L., Kiss A., Telek B. Nucleolar organizer regions in acute and chronic leukaemias. Acta Histochem. 1992;93(2):453–461. doi: 10.1016/S0065-1281(11)80117-0. [DOI] [PubMed] [Google Scholar]
- Jaksić B., Vitale B. Total tumour mass score (TTM): a new parameter in chronic lymphocyte leukaemia. Br J Haematol. 1981 Nov;49(3):405–413. doi: 10.1111/j.1365-2141.1981.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Lachenbruch P. A. An almost unbiased method of obtaining confidence intervals for the probability of misclassification in discriminant analysis. Biometrics. 1967 Dec;23(4):639–645. [PubMed] [Google Scholar]
- Lanza F., Moretti S., Latorraca A., Scapoli G., Rigolin F., Castoldi G. Flow cytochemical analysis of peripheral lymphocytes in chronic B-lymphocytic leukemia. Prognostic role of the blast count determined by the H*1 system and its correlation with morphologic features. Leuk Res. 1992 Jun-Jul;16(6-7):639–646. doi: 10.1016/0145-2126(92)90014-x. [DOI] [PubMed] [Google Scholar]
- Leek R. D., Alison M. R., Sarraf C. E. Variations in the occurrence of silver-staining nucleolar organizer regions (AgNORs) in non-proliferating and proliferating tissues. J Pathol. 1991 Sep;165(1):43–51. doi: 10.1002/path.1711650108. [DOI] [PubMed] [Google Scholar]
- Metze K., Lorand-Metze I. G. Interpretation of the AgNOR pattern in hematologic cytology. Acta Haematol. 1993;89(2):110–112. doi: 10.1159/000204500. [DOI] [PubMed] [Google Scholar]
- Metze K., Lorand-Metze I. AgNOR staining in normal bone marrow cells. J Clin Pathol. 1991 Jun;44(6):526–526. [PMC free article] [PubMed] [Google Scholar]
- Molica S., Alberti A. Prognostic value of the lymphocyte doubling time in chronic lymphocytic leukemia. Cancer. 1987 Dec 1;60(11):2712–2716. doi: 10.1002/1097-0142(19871201)60:11<2712::aid-cncr2820601122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Molica S., Reverter J. C., Alberti A., Montserrat E. Timing of diagnosis and lymphocyte accumulation patterns in chronic lymphocytic leukemia: analysis of their clinical significance. Eur J Haematol. 1990 May;44(5):277–281. doi: 10.1111/j.1600-0609.1990.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Montserrat E., Sanchez-Bisono J., Viñolas N., Rozman C. Lymphocyte doubling time in chronic lymphocytic leukaemia: analysis of its prognostic significance. Br J Haematol. 1986 Mar;62(3):567–575. doi: 10.1111/j.1365-2141.1986.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Montserrat E., Viñolas N., Reverter J. C., Rozman C. Natural history of chronic lymphocytic leukemia: on the progression and progression and prognosis of early clinical stages. Nouv Rev Fr Hematol. 1988;30(5-6):359–361. [PubMed] [Google Scholar]
- Nikicicz E. P., Norback D. H. Spectrum of argyrophilic nucleolar organizer region (AgNOR) staining patterns in chronic and transformed B-cell leukemias. Arch Pathol Lab Med. 1992 Mar;116(3):265–268. [PubMed] [Google Scholar]
- Orfao A., Ciudad J., González M., San Miguel J. F., García A. R., López-Berges M. C., Ramos F., Del Cañizo M. C., Ríos A., Sanz M. Prognostic value of S-phase white blood cell count in B-cell chronic lymphocytic leukemia. Leukemia. 1992 Jan;6(1):47–51. [PubMed] [Google Scholar]
- Pich A., Chiusa L., Boccadoro M., Marmont F. AgNORs and myeloma prognosis. Leuk Lymphoma. 1994 Feb;12(5-6):383–394. doi: 10.3109/10428199409073779. [DOI] [PubMed] [Google Scholar]
- Rai K. R., Sawitsky A., Cronkite E. P., Chanana A. D., Levy R. N., Pasternack B. S. Clinical staging of chronic lymphocytic leukemia. Blood. 1975 Aug;46(2):219–234. [PubMed] [Google Scholar]
- Rüschoff J., Barth P. Theorie und Praxis der Silberfärbung Nukleolus organisierender Regionen (AgNOR). Pathologe. 1992 Feb;13(1):13–19. [PubMed] [Google Scholar]
- Smith R., Crocker J. Evaluation of nucleolar organizer region-associated proteins in breast malignancy. Histopathology. 1988 Feb;12(2):113–125. doi: 10.1111/j.1365-2559.1988.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Trerè D. Critical analysis of the methods commonly employed in the assessment of cell proliferation: advantages of the NOR silver-staining technique in routine cyto-histopathology. Anal Cell Pathol. 1993 Jul;5(4):191–201. [PubMed] [Google Scholar]
- Wachtler F., Schwarzacher H. G., Ellinger A. The influence of the cell cycle on structure and number of nucleoli in cultured human lymphocytes. Cell Tissue Res. 1982;225(1):155–163. doi: 10.1007/BF00216225. [DOI] [PubMed] [Google Scholar]