Abstract
Osteosarcoma (OS) is the most common malignant bone tumor affecting children and adolescents. Many patients are treated with a combination of chemotherapy, resection, and limb salvage protocols. Surgical reconstructions after tumor resection include structural allografts, non-cemented endoprostheses, and distraction osteogenesis (DO), which require direct bone formation. Although cisplatin (CDP) is extensively used for OS chemotherapy, the effects on bone regeneration are not well studied. The effects of CDP on direct bone formation in DO were compared using two dosing regimens and both C57BL/6 (B6) and tumor necrosis factor receptor 1 knockout (TNFR1KO) mice, as CDP toxicity is associated with elevated TNF levels. Detailed evaluation of the five dose CDP regimen (2mg/kg/day), demonstrated significant decreases in new bone formation in the DO gaps of CDP treated versus vehicle treated mice (P<0.001). Further, no significant inhibitory effects from the 5 dose CDP regimen were observed in TNFR1KO mice. The two dose regimen significantly inhibited new bone formation in B6 mice. These results demonstrate that CDP has profound short term negative effects on the process of bone repair in DO. These data provide the mechanistic basis for modeling peri-operative chemotherapy doses and schedules and may provide new opportunities to identify molecules that spare normal cells from the inhibitory effects of CDP.
Keywords: cisplatin, distraction osteogenesis, chemotherapy, mouse, limb salvage
INTRODUCTION
Osteosarcoma (OS) is the most common malignant bone tumor affecting children and adolescents1. Most patients are treated with a combination of chemotherapy and surgery with limb salvage being possible in many cases2. Surgical reconstruction utilizing limb salvage protocols are now the standard of care in most tertiary care centers, yet these patients continue to be at risk for postoperative complications related to poor bone regeneration1-4. Poor direct bone regeneration often leads to failure of the implanted endoprothesis, the engrafted bone, or of the lengthened bone1-4. Currently, anti RANKL therapy (Prolia®) and zoledronic acid (Zometa®) are the only FDA-approved therapies for cancer treatment induced bone loss (CTIBL) 5. However, few therapies are currently available for the treatment and/or repair of bone repair deficits 4.
Chemotherapeutic agents that are used widely and accepted as efficacious in patients with OS include doxorubicin, high-dose methotrexate and cisplatin (CDP, cis-diamminedichloroplatinum (II), cis-platinum) 6-9. Neoadjuvant chemotherapy followed by surgery with additional adjuvant chemotherapy has become a common treatment approach (Cure Search/COG). However, since CDP is extensively used for the treatment of OS, we believe the effects of CDP treatment on direct bone regeneration need further study10-12. Therefore, we tested the effects of two CDP dosing regimens on bone regeneration in a mouse model of distraction osteogenesis (DO) 13-16. During DO, bone formation process spatially well organized and temporally distinct from resorptive processes induced later. In addition, recent literature suggests that DO is emerging as an appropriate protocol for osteosarcoma patients and does provide significant benefit to to patients compared with other critical size defect repair procedures 17-20. As such, the studies described herein provide important new information regarding potential clinical applications 20. Based on previous studies, we observed that exposure to chemotherapy can occur preoperatively, postoperatively, and even simultaneously with bone repair/regeneration 21. Therefore, to develop and standardize a mouse model for CDP toxicity we chose to perform the DO surgery simultaneously with the first of five daily CDP injections. The dosage and delivery was also chosen based on previous experimental and clinical protocols (Cure Search: Children’s Oncology Group/COG) 21. The CDP dose was 2 mg/kg/day which is approximately equivalent to the pediatric dose of 60mg/m2/day. CDP administered during this protocol produced significant damage to the regenerating bone. Based on previous studies that demonstrated 1) excess levels of tumor necrosis factor alpha (TNFα) are inhibitory to bone regeneration in DO and act through TNF receptor 1 (TNFR1) 22, and 2) that CDP toxicity is associated with elevated TNFα levels23,24, we tested the hypothesis that the TNFR1 axis was mediating the negative effects of this regimen of CDP dosing using TNFR1 knockout mice22. Finally, to model more closely the current human therapeutic CDP dose common in clinical practice and to examine the persistence of the CDP effects, the same dose of CDP was administered for two consecutive days followed a week later by the initiation of the standard DO protocol. In sum, both the five dose and two dose regimens produced significant inhibitory effects on new bone formation, suggesting that the inhibition of bone repair in CDP-treated OS patients may be associated with CDP-mediated disruption of TNFα signaling.
MATERIALS & METHODS
Animals
Adult male C57BL/6 (B6, Jax #000664) and TNFR1 KO (Jax #002818) mice were purchased from Jackson Industries (Bar Harbor, ME). Animals were housed in individual cages in temperature (22°C) and humidity (50%) controlled rooms having a 12 h light/12 h dark cycle. All mice were acclimated by animal care personnel for 5-7 days prior to surgery. In all studies, the mice were assigned to respective experimental groups with mean body weights equal to that of the control group (± 4 g) for the study. All research protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Arkansas for Medical Sciences.
Study Designs
Study 1: Effects of CDP Treatment on DO in B6 Mice: Regimen 1
To study the effects of CDP administration on direct bone formation in the mouse DO model, nine week old C57BL/6 male mice (n=20) underwent the standard DO protocol (described below). All mice received an IP injection (under isoflurane anesthesia) of either vehicle (saline) or CDP (2mg/kg/day in saline) daily for five consecutive days beginning the day of surgery. The CDP was made fresh daily and at harvest distracted tibiae were collected.
Study 2: Effects of CDP Treatment on DO in TNFR1 Knockout Mice
Based on previous studies which suggest that TNFR1 mediates the negative effects of exogenous TNF, we performed a study identical to that above except we used nine week old male TNFR1 deficient mice (n=6: saline & n=6 CDP)22. Serum TNF levels were measured.
Study 3: Effects of CDP Treatment on DO in B6 Mice: Regimen 2
To better model clinical practice and to look at the persistence of the CDP effects, we administered the same dose of CDP (2mg/kg/day) for two consecutive days followed one week later by DO. The selected dose is equivalent of the clinical dose but may underestimate overall dose due to the higher metabolism of the mouse. Male 12 week old B6 mice were used (n=6: saline vs. n=6 CDP).
Distraction Protocol
Distraction was performed as we have previously described 14, 22, 25. Following acclimation and under Nembutal anesthesia, each mouse underwent placement of an external fixator and osteotomy to the left tibia22. Four 27-gauge, 1.25 in needles were manually drilled through the tibia (two proximally, two distally). The titanium external fixator was then secured to the pins. A small incision was made in the skin distal to the tibia crest and the soft tissue was carefully retracted to visualize the bone. A single hole was manually drilled through both cortices of the mid-diaphysis, and surgical scissors were used to fracture the cortex on either side of the hole. The fibula was fractured by direct lateral pressure. The periosteum and dermal tissues were closed with a single suture. Finally, buprenex (1.0mg/kg) was given by intramuscular injection post-surgery for analgesia. Distraction began three days after surgery (3 day latency) at a rate of 0.075 mm b.i.d. (0.15 mm/day) and continued for 11 days at which time the tibias were harvested.
MicroCT (μCT) Analysis
Following analytical radiography, bone formation was analyzed using a μCT40 (Scanco Medical AG, Bassersdorf, Switzerland) and the manufacturer’s software. The distracted tibia including the entire distraction gap was scanned in cross section with an isotropic voxel size of 12 μm at 55 kV, 114 μA, and 1000 projections per rotation with threshold and noise filter the same as that used for the trabecular analysis of intact mouse bones (sigma 0.8, support 1, threshold 245). The DO gaps and new bone formation were analyzed as we have described 25.
Radiographic and Histologic Analysis
After 48 hours of fixation in 10% neutral buffered formalin the left tibiae were removed from the fixators for high-resolution single beam radiography and subsequent histological processing. Radiographs were quantified as we have described 13, 15, 22.
Following radiography, the distracted tibiae were decalcified in 5% formic acid, dehydrated, and embedded in paraffin for histologic evaluation26. Five to seven micron longitudinal sections were cut on a microtome (Leitz 1512, Wetzlar, Germany) for hematoxylin and eosin staining (H&E). Sections were selected to represent a central or near central gap location as we have described 14, 22, 25. To be included in both radiographic and histologic analyses the DO samples had to 1) be well aligned, 2) have no broken pin sites, 3) have few bone chips within the DO gap, 4) have an intact ankle, and 5) have had no significant weight loss or health problems during the distraction period as we have described previously 14, 22, 25.
Serum mouse TNFα Bead Array
Murine serum samples were analyzed on a Luminex® 100™ Bioanalyzer in the Pediatric Endocrinology Core Facility, using the Linco mouse adipokine kits (MPXMCYTO-70K-1).
Statistics
Differences between individual group means were estimated by the Student’s t test. All data are reported as mean ± standard error of the mean (SEM). When assumptions of equal variances between groups failed, differences between group means were estimated using a generalized linear model assuming the underlying distribution of the outcome 27. Differences were considered significant when p < 0.05 and are reported as such.
RESULTS
Study 1: CDP Exposure During The Early Stages of DO Inhibits New Bone Formation: Regimen 1
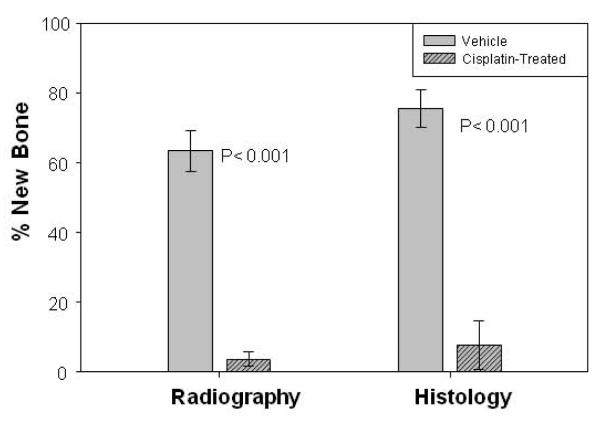
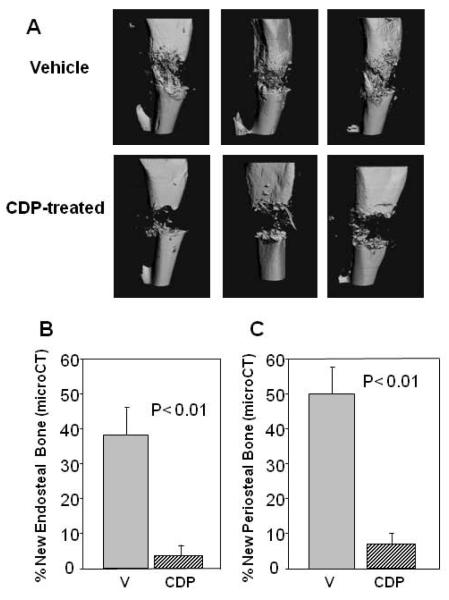
The study was designed to test the hypothesis that a five dose administration of CDP during the early stages of the distraction protocol would result in osteoinhibition in B6 mice. Comparison of the distracted tibial radiographs demonstrated a significant decrease in the mineralized area of distraction gaps of CDP treated mice (3.6% ± 2.1) versus vehicle treated mice (63.3% ± 5.8) (p<0.001) (Figure 1). Histological analysis of the DO gaps confirmed the significant decrease in cellular bone formation between the CDP treated mice (7.6% ± 7.0) versus vehicle treated mice (75.4% ± 5.4) (p<0.001) (Figure 1). Representative radiographs, and histograms are shown in Figures 2 A, B. Analysis of the same distracted tibiae by microCT (Figure 3A) demonstrated significant decreases in both new endosteal and periosteal bone formation in CDP-treated mice compared with vehicle–treated controls (Figure 3B, C). One mouse was excluded from both analyses.
Figure 1.
Five day CDP Regimen 1: Left: Comparison of the distracted tibial radiographs. A significant decrease in the mineralized area of distraction gaps of CDP treated mice (3.6% ± 2.1) versus vehicle treated mice (63.3% ± 5.8) (p<0.001). Right: Histology of the DO gaps. A significant decrease in cellular parameters of bone formation between the CDP treated mice (7.6% ± 7.0) versus vehicle treated mice (75.4% ± 5.4) (p<0.001).
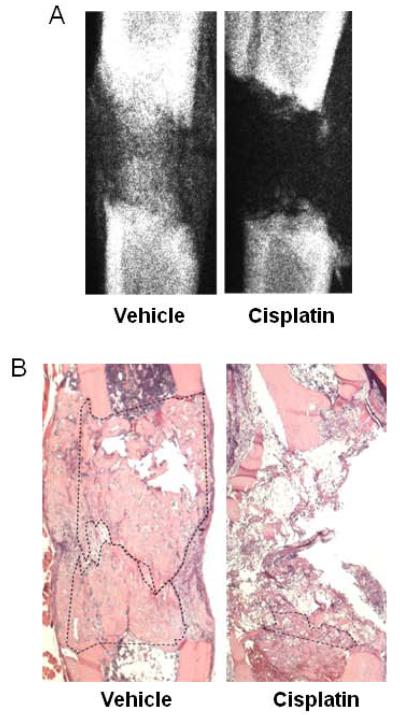
Figure 2.
Representative radiographs (A) and H&E histology (B) for B6 mice in Regimen 1 are shown. In 2B new bone is outlined by dashes.
Figure 3.
(A) Representative micro CT images of tibial DO gaps from vehicle and CDP-treated mice. (B) New mineralized endosteal bone formation is significantly inhibited by CDP (3% ± 3) vs. vehicle control (38% ± 10) (p<0.01) (C) New mineralized periosteal bone formation is significantly inhibited by CDP (8%±3) vs. vehicle control (50% ± 8) (p<0.01).
Study 2: Effects of CDP Treatment on DO in TNFR1 Knockout Mice
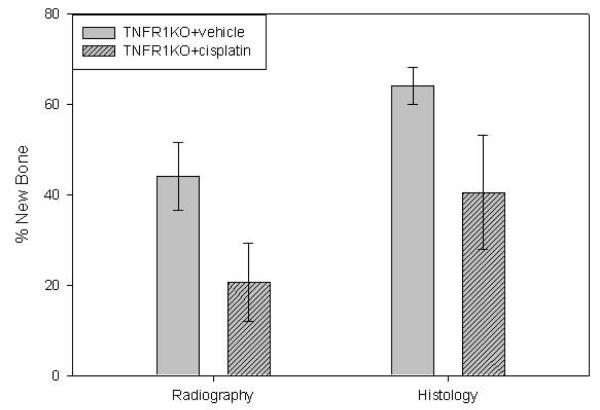
We next examined the role of TNF in this process using TNFR1-deficient mice. Comparison of the distracted tibial radiographs demonstrated no significant decrease in mineralized area in the distraction gaps of CDP treated TNFR1 knockout mice (20.6% ± 8.7) versus vehicle treated knockout mice (44.1% ± 7.5) (p=0.07). Similarly, histological analysis of the DO gaps showed no significant decrease in cellular bone formation between the CDP treated mice (40.5% ± 12.7) versus vehicle treated mice (64.1% ± 4.0) (p=0.09) (Figure 4). However, standard deviations between CDP treated mice and vehicle treated were statistically different (p=0.04). Therefore a generalized linear model assuming a beta distribution (i.e. beta regression) was used 27. Accounting for differences in variability between treatment groups indicates that the decrease in cellular bone formation between the CDP treated mice (37.6% ± 11.1) versus vehicle treated mice (64.0% ± 3.5) is statistically significant (p=0.024).
Figure 4.
Five day CDP Regimen 1. Left: Comparison of the distracted tibial radiographs. No significant decrease in mineralized area of CDP treated (20.6% ± 8.7) vs. vehicle-treated TNFR1 KO mice (44.1% ± 7.5) (p=0.09). Right: Comparison of the distracted tibial H&E histology. No significant decrease in cellular bone formation of CDP treated mice (40.5% ± 12.7) versus vehicle treated mice (64.1% ± 4) (p=0.07).
As previously reported 22, serum TNF analyses demonstrated relatively higher levels of TNF in both groups of the TNFR1 knockout mice (32.6 ± 7.7 pg.ml vs wild type below sensitivity at < 10 pg/ml) suggesting a TNF resistant state 22. These data suggest that the CDP inhibitory effect on bone formation requires functional TNFR1, which implicates the TNF signaling pathway.
Study 3: The Effects of CDP Treatments on DO in B6 Mice: Regimen 2
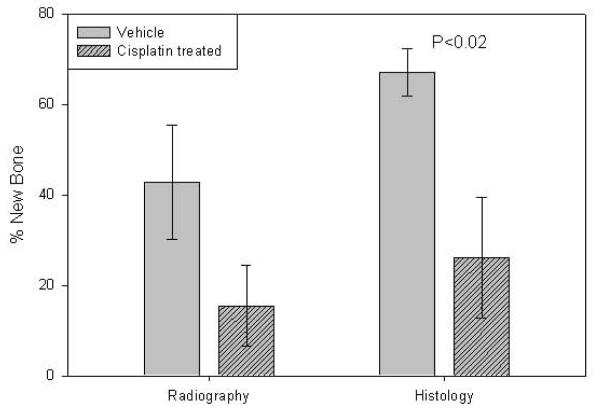
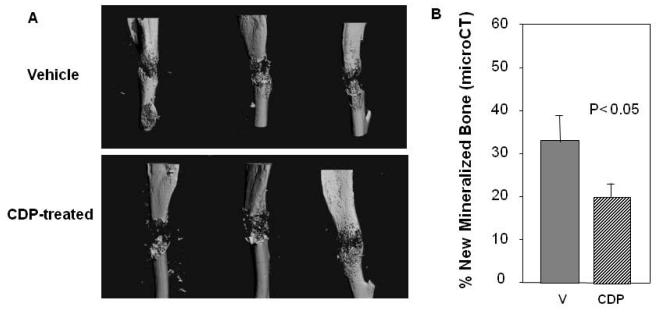
In order to approximate clinical CDP dose and to look at the persistence of the CDP effects, we administered the same dose of CDP for two consecutive days followed one week later by DO. Comparison of the distracted tibial radiographs demonstrated no decrease in mineralized area in the distraction gaps of CDP treated (15.5% ± 9.0) versus vehicle treated B6 mice (42.8% ± 12.67) (p=0.1). However, analysis of the distracted tibial histology demonstrated a significant decrease in the area of new bone formation in distraction gaps of CDP treated mice (26.1% ± 13.4) versus vehicle treated mice (67% ± 5.3) (p<0.02, Figure 5). Similarly, MicroCT analysis (Figure 6A) confirmed the significant decrease in total mineralized new bone in the DO gaps of CDP treated mice (19 % ± 3) vs. vehicle (33% ± 7 ) (p<0.05, Figure 6B).
Figure 5.
Two day CDP Regimen 2. Left: Comparison of the distracted tibial radiographs. No significant decrease in mineralized area in the distraction gaps of CDP treated (15.5% ± 9.0) versus vehicle treated B6 mice (42.8% ± 12.67) (p= 0.1). Right: Comparison of the distracted tibial H&E histology. A significant decrease in the area of new bone formation in distraction gaps of CDP treated mice (26.1% ± 13.4) versus vehicle treated mice (67% ± 5.3) (p<0.02)..
Figure 6.
(A) Representative microCT images of tibial DO gaps from vehicle and CDP-treated B6 mice in Regimen 2. (B) Total new mineralized endosteal bone formation is significantly inhibited by CDP (19% ± 3) vs. vehicle control (33% ± 7) (p<0.05).
DISCUSSION
OS is the most common type of malignant bone cancer, and the sixth most prevalent type of cancer in children and young adults1. Most OS patients are treated by a closely timed combination of chemotherapy (often CDP) and tumor resection, in many cases followed by a type of limb salvage procedure including DO (Cure Search: Children’s Oncology Group/COG). Surgical reconstruction, DO, and endoprostheses are also used commonly, yet these patients continue to be at risk for postoperative complications relating to inherent poor bone repair outcomes1-4, 10, 17.
Currently the three drugs most commonly used in the treatment of pediatric OS are CDP, methotrexate, and doxorubicin (COG). Although CDP is commonly used for the treatment of OS, the effects of CDP on direct bone regeneration models (endoprostheses, allografts, DO) in vivo are not well defined. Importantly, some experimental studies have demonstrated that CDP treatment adversely affects direct bone repair10,11. One study showed significantly decreased bone growth into a porous titanium implant after CDP administration10. Another study 11, demonstrated that bone defects in CDP-treated animals did heal, although the authors’ did not report if any delays were observed. Of note, in these authors’ 11 used a different species (dog), a different CDP dose, and more importantly perhaps, a different delivery (i.e. CDP was given only once every 21 days starting 3 days after surgery). In addition, the authors did note that the cisplatin-treated dogs had 1) decreased mineralized bone volume, 2) decreased percent of woven bone, 3) decreased percent of osteoblast covered bone, and 4) increased porosity when compared to values for control dogs, similar to our observed inhibition11. Indeed, these variables would be expected to impact DO in this model. Also, in a clinical study 12, the patients undergoing DO obtained good functional scores, although the time to resolution was not reported. We note that the patients were treated pre-operatively with cisplatin and caffeine +/− doxorubicin, and in some cases post operative chemotherapy was involved but was not detailed 12. Further, a recent publication examined the effects of CDP + Adriamycin on DO in rabbits, using a preoperative and a post-operative regimen similar to ours and identified significant deficits in bone formation and/or bone quality 28. Therefore, the focus of our study was to develop and standardize a mouse DO model for possible CDP toxicity and if appropriate begin to identify the mechanisms involved.
In the first study the hypothesis that overlapping administration of CDP and bone surgery would result in poor bone regeneration was confirmed. In fact, the inhibitory effects were profound and comparable when measured radiographically, histologically, or by microCT. This result is in contrast to a study of the effects of methotrexate, another potent chemotherapeutic agent that did not inhibit bone formation, given during distraction, in a rat model 21. Since many studies have shown that rat and mouse DO models behave similarly when used to study lead exposure, ethanol exposure, aging, and diabetes, we hypothesize that CDP has a more deleterious effect on bone repair than methotrexate 13-16.
The second study demonstrated that the TNF/TNFR1 axis is, at least, a partial mediator of CDP’s osteoinhibitory effects. Noting that although we failed to prove a difference (p=0.09) between the vehicle and the CDP treated KO mice, it would take ~n=1200 mice to expect to prove that (TNFR1KO +/− CDP) are statistically equivalent. Additionally, the TNFR1KO model was analyzed using beta regression, an extension of generalized linear models that assumes the outcome has an underlying distribution following the beta distribution. Most importantly, beta regression allows for differences in groups to be estimated in both mean as well as variance. As shown in Figure 4, the variances between the CDP treated and vehicle treated groups are different. Beta regression analysis indicated that the groups (TNFR1KO +/− CDP) are statistically different from each other with respect to new bone formation; however, this does not rule out a protective role for a TNFR1 deficit. We have noted that cross-study comparisons should be viewed with caution (especially with single beam radiography). However, we do show that the histological data for the vehicle controls (Figures 1 and 4) are not significantly different. We have reported previously that the histological data is more accurate than radiological data (22), since histology measures cell number and activity directly. Comparing the CDP effects between the two genetic strains, suggests that the KO strain is significantly different (i.e. protective) from the wild type mice (p< 0.04: t test or p< 0.007: beta regression). These findings are consistent with previous studies demonstrating that excess levels of TNF signaling via TNFR1 are inhibitory to bone regeneration and that CDP toxicity is associated with elevated TNF levels 22-24. However, blocking this pathway genetically does not fully restore new bone formation in CDP-treated mice. We conclude that there is a consistent reduction of bone formation in TNFR1KO mice due to CDP administration and that TNFR1KO mice have some degree of protection against the effects of CDP. Also, since the variation in the TNFR1KO + cisplatin group is much larger than the TNFR1KO + vehicle, we propose that this is indicative of a differential response in these mice compared to the other groups. These conclusions suggest possible efficacious combinations of the TNFR1 KO mice and other modulators to protect bone formation from the effects of CDP.
In the third study 1) we reduced the CDP treatment to two injections modeling current clinical practice. In addition, CDP treatment was given one week prior to surgery, thereby allowing evaluation of the persistence of the CDP effects. Interestingly, the negative effects of CDP were attenuated in comparison to the five day dosing regimen. Comparison of the distracted tibial histology and assessment by microCT demonstrated a significant decrease in the area of new bone formation in distraction gaps of CDP treated mice (p<0.02) suggesting that the negative CDP effects may persist for at least 1-2 weeks.
Although these studies were performed in vivo, some limitations persist. We note that single studies are reported here. The studies were not performed in humans and so need to be interpreted in this preclinical context. Routinely, multi-agent chemotherapy is performed and we only evaluated pre-operative/operative dosing of CDP and measured only direct bone formation. It is possible that increasing the time interval between the last CDP treatment and any subsequent surgical intervention may allow for normal/acceptable healing times.
In sum, the data presented here demonstrate the severe inhibition of direct bone formation following CDP treatment and suggest that the time interval between CDP dosing and the initiation of the surgical intervention may be critical to the magnitude of the negative bone healing effects. The results also indicate that TNFR1 deficient mice are protected from the early CDP effects and suggest the involvement of the TNF/TNFR1 axis in CDP-mediated inhibitory effects on bone formation. Ongoing studies are evaluating the clinical utility of these approaches for the protection of bone repair and are focused on multi-agent therapy and alternatively modeled clinical scenarios.
ACKNOWLEDGEMENTS
This research was supported, in part, by 1) the Children’s University Medical Group Fund Grant Program, the Arkansas Children’s Hospital Research Institute, 2) the Arkansas Biosciences Institute, funded by the Arkansas Tobacco Settlement Plan and administered by Arkansas Children’s Hospital Research Institute, 3) UAMS Pediatric Hematology/ Oncology Division, 4) Carl L. Nelson Endowed Chair in Orthopaedic Creativity, 5) the UAMS Translational Research Institute (TRI) supported by the National Institutes of Health National Center for Research Resources grant UL1 RR029884, and 6) NIH National Center for Research Resources Grant #1CORR16517-01
REFERENCES
- 1.Yang C, Ji D, Weinstein EJ, et al. The kinase Mirk is a potential therapeutic target in osteosarcoma. Carcinogenesis. 2010;31:552–8. doi: 10.1093/carcin/bgp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messerschmitt PJ, Garcia RM, Abdul-Karim FW, et al. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–27. doi: 10.5435/00124635-200908000-00005. Review. [DOI] [PubMed] [Google Scholar]
- 3.Bilariki K, Anagnostou E, Masse V, et al. Low bone mineral density and high incidences of fractures and vitamin D deficiency in 52 pediatric cancer survivors. Horm Res Paediatric. 2010;74:319–27. doi: 10.1159/000313378. [DOI] [PubMed] [Google Scholar]
- 4.Bertoldo F, Pancheri S, Zenari S, Boldini S. Emerging drugs for the management of cancer treatment induced bone loss. Expert Opin Emerg Drugs. 2010;15:323–42. doi: 10.1517/14728211003631385. Review. [DOI] [PubMed] [Google Scholar]
- 5.Bundred N. Antiresorptive therapies in oncology and their effects on cancer progression. Cancer Treat Rev. 2012;38:776–86. doi: 10.1016/j.ctrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Link MP, Goorin AM, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 7.Meyers PA, Heller G, Healey J, Huvos A, Lane J, Marcove R, Applewhite A, Vlamis V, Rosen G. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. 1992. J Clin Oncol. 10(1):5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 8.Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfürst C, Berger J, Ritter J, Jürgens H, Gerein V, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–37. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 9.Zalupski MM, Rankin C, Ryan JR, Lucas DR, Muler J, Lanier KS, Budd GT, Biermann JS, Meyers FJ, Antman K. Adjuvant therapy of osteosarcoma--A Phase II trial: Southwest Oncology Group study 9139. Cancer. 2004;100:818–25. doi: 10.1002/cncr.20021. [DOI] [PubMed] [Google Scholar]
- 10.Barth E, Roenningen H, Solheim LF, Saethren B. Effect of cis-platinum on bone ingrowth into porous fiber titanium. Mechanical and biochemical correlations. Int J Oral Maxillofac Implants. 1986;1:123–7. [PubMed] [Google Scholar]
- 11.Ehrhart N, Eurell JA, Tommasini M, et al. Effect of cisplatin on bone transport osteogenesis in dogs. Am J Vet Res. 2002;63:703–11. doi: 10.2460/ajvr.2002.63.703. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Tsuchiya H, Yamamoto N, Takeuchi A, Tomita K. Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. Int Orthop. 2008 Feb;32(1):63–8. doi: 10.1007/s00264-006-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronson J, Liu L, Liu Z, et al. Decreased endosteal intramembranous bone formation accompanies aging in a mouse model of distraction osteogenesis. J Regen Med. 2002;3:7–16. [Google Scholar]
- 14.Fowlkes JL, Bunn RC, Liu L, et al. Runt-related transcription factor 2 (RUNX2) and RUNX2-related osteogenic genes are down-regulated throughout osteogenesis in type 1 diabetes mellitus. Endocrinology. 2008;149:1697–1704. doi: 10.1210/en.2007-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrien D, Brown E, Zhendong L, et al. Ethanol induces tnf-α and inhibits osteoblastic proliferation in the rat distraction gap. Cytokine. 2003;23:179–189. doi: 10.1016/s1043-4666(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 16.Ronis M, Aronson J, Gao G, et al. Skeletal effects of developmental lead exposure in rats. Toxicol Sci. 2001;62:321–329. doi: 10.1093/toxsci/62.2.321. [DOI] [PubMed] [Google Scholar]
- 17.McCoy TH, Jr, Kim HJ, Cross MB, Fragomen AT, Healey JH, Athanasian EA, Rozbruch SR. Bone tumor reconstruction with the Ilizarov method. J Surg Oncol. 2013;107:343–352. doi: 10.1002/jso.23217. [DOI] [PubMed] [Google Scholar]
- 18.Sabharwal S, Fragomen A, Iobst CJ. What’s New in Limb Lengthening and Deformity Correction. Bone Joint Surg Am. 2013;95:1527–34. doi: 10.2106/JBJS.M.00599. [DOI] [PubMed] [Google Scholar]
- 19.Betz M, Dumont CE, Fuchs B, Exner GU. Physeal distraction for joint preservation in malignant metaphyseal bone tumors in children. Clin Orthop Relat Res. 2012;470:1749–54. doi: 10.1007/s11999-011-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe K, Tsuchiya H, Yamamoto N, Shirai T, Nishida H, Hayashi K, Takeuchi A, Matsubara H, Nomura I. Over 10-year follow-up of functional outcome in patients with bone tumors reconstructed using distraction osteogenesis. J Orthop Sci. 2013;18:101–9. doi: 10.1007/s00776-012-0327-4. [DOI] [PubMed] [Google Scholar]
- 21.Jarka DE, Nicholas RW, Aronson J. Effect of methotrexate on distraction osteogenesis. Clin Orthop Relat Res. 1998:209–15. doi: 10.1097/00003086-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Wahl EC, Aronson J, Liu L, et al. Direct bone formation during distraction osteogenesis does not require TNFα receptors and elevated serum TNFα fails to inhibit bone formation in TNFR1 deficient mice. Bone. 2010;46:410–7. doi: 10.1016/j.bone.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benedetti G, Fredriksson L, Herpers B, Meerman J, van de Water B, de Graauw M. TNF-α-mediated NF-κB survival signaling impairment by cisplatin enhances JNK activation allowing synergistic apoptosis of renal proximal tubular cells. Biochem Pharmacol. 2013;85:274–86. doi: 10.1016/j.bcp.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Zhong YJ, Zheng XL, Wang Q, Yang L, Shi F, Yan JQ, He F, Liao LC, Lin Y, Zhang L, Wang X. A critical role of CD40-mediated autocrine TNF-α in potentiation of cisplatin-induced cytotoxicity in cancer cells. Cancer Sci. 2012;103:197–202. doi: 10.1111/j.1349-7006.2011.02122.x. [DOI] [PubMed] [Google Scholar]
- 25.Perrien DS, Nicks KM, Liu L, Akel NS, Bacon AW, Skinner RA, Swain FL, Aronson J, Suva LJ, Gaddy D. Inhibin A enhances bone formation during distraction osteogenesis. J Orthop Res. 2012;30:288–95. doi: 10.1002/jor.21501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner RA, Hickmon SG, Lumpkin CK, Jr, et al. Decalcified bone: twenty years of successful specimen management. J Histotech. 1997;20:267–277. [Google Scholar]
- 27.Swearingen CJ, Tilley BC, Adams RJ, Rumboldt Z, Nicholas JS, Bandyopadhyay D, Woolson RF. Application of beta regression to analyze ischemic stroke volume in NINDS rt-PA clinical trials. Neuroepidemiology. 2011;37:73–82. doi: 10.1159/000330375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monsell, et al. Strat Traum Limb Recon. PMID; p. 24105429. [Google Scholar]