Abstract
Mutations in retinoid isomerase, RPE65, or lecithin-retinol acyltransferase (LRAT) disrupt 11-cis-retinal recycling and cause Leber congenital amaurosis (LCA), the most severe retinal dystrophy in early childhood. We used Lrat−/−, a murine model for LCA, to investigate the mechanism of rapid cone degeneration. We found that mislocalized M-opsin was degraded whereas mislocalized S-opsin accumulated in Lrat−/− cones before the onset of massive ventral/central cone degeneration. Since the ventral and central retina expresses higher levels of S-opsin than the dorsal retina in mice, our results may explain why ventral and central cones degenerate more rapidly than dorsal cones in Rpe65−/− and Lrat−/− LCA models. In addition, human blue opsin and mouse S-opsin, but not mouse M-opsin or human red/green opsins, aggregated to form cytoplasmic inclusions in transfected cells, which may explain why blue cone function is lost earlier than red/green-cone function in LCA patients. The aggregation of short-wavelength opsins likely caused rapid cone degenerations through an ER stress pathway as demonstrated in both the Lrat−/− retina and transfected cells. Based on this mechanism, we designed a new therapy of LCA by reducing ER stress. We found that systemic injection of an ER chemical chaperone, tauroursodeoxycholic acid (TUDCA), is effective in reducing ER stress, preventing apoptosis, and preserving cones in Lrat−/− mice.
Keywords: aggregation, RPE65, LRAT, Leber congenital amaurosis (LCA), Short-wavelength sensitive opsins (SWS), Medium/long-wavelength sensitive opsins (M/LWS), Cone degeneration
99.1 Introduction
Both Retinoid isomerase (RPE65) and lecithin-retinol acyltransferase (LRAT) are required for 11-cis-retinal recycling in the RPE. Mutations in RPE65 or LRAT cause Leber congenital amaurosis (LCA) [1]. Two mouse models, Rpe65−/− and Lrat−/−, capture many salient pathologic features of human LCA [2–4]. Both rod and cone function are severely compromised due to the lack of 11-cis-retinal [2, 3]. The cone opsins (S-opsin and M-opsin) fail to traffic from the cone inner segment to the cone outer segment and the cone photoreceptors in the central/ventral regions degenerate rapidly (< 4 weeks). Early loss of foveal cones also occur in RPE65-deficient patients [5]. The mechanisms responsible for early cone death in both mouse models and human patients are not well understood. Specifically it is unclear why ventral and central cones in mouse models die much more rapidly than dorsal cones. Similarly, it is unclear why blue cone function is lost early in patients [5, 6]. Here, we used the Lrat−/− mouse, a model for LCA, to investigate the mechanism and treatment for cone photoreceptor degeneration.
99.2 Materials and Methods
99.2.1 Animals and TUDCA Injection
Lrat−/− mice were generated and described previously [3]. WT (C57BL/6J) mice were purchased from Jackson Laboratory. All animal experiments were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Utah and were performed in accordance with the ARVO Statement for the Use of Animal in Ophthalmic and Vision Research. Mice were reared under cyclic light (12 h light/12 h dark). Lrat−/− and WT mice were treated with TUDCA (TCI America) or vehicle (0.15 M NaHCO3, pH 7.0) following a published procedure [7].
99.2.2 Statistics
Data were presented as mean ± SEM, and the differences were analyzed with unpaired two-sample Student's t-test. P values < 0.05 were considered statistically significant.
99.3 Results
99.3.1 Different Protein Stability of M and S Opsins in the Lrat−/− Retina
By immunohistochemistry, we found that Lrat−/− cones accumulated more mislocalized S-opsin than M-opsin [8]. We proceeded to verify this finding by western blot at three stages of cone degeneration: (1) P14, pre-degeneration [9]; (2) P18, early-stage degeneration; (3) P30, late-stage degeneration. In P14 Lrat−/−, the protein levels of both M-opsin and S-opsin were similar to those in WT (Fig. 99.1a) although their mRNA levels were slightly reduced [8]. However, in P18 Lrat−/−retina, the M-opsin protein was markedly reduced (∼ 1.9 times) whereas the S-opsin level remained the same, as compared with WT (Fig. 99.1a). After we normalized the protein levels of cone opsins against their mRNA levels (i.e. protein/mRNA), this ratio almost doubled (∼ 1.9 times) for S-opsin in P18 Lrat−/− compared to WT (Fig. 99.1b). In contrast, the protein/mRNA ratio of M-opsin in P18 Lrat−/− was similar to that in WT. Assuming the protein synthesis for cone opsins is minimally affected in the early stage of Lrat−/− cone degeneration, our results suggest that mislocalized S-opsin was more resistant to proteasome degradation than mislocalized M-opsin. Since the ventral and central retina express higher levels of S-opsin than the dorsal retina in mice [10], our results suggest that accumulation of mislocalized S-opsin may underlie the fast ventral/central cone degeneration in Lrat−/− mice. Cones at the far-dorsal region, which express more M-opsin than S-opsin, degenerated much more slowly, corresponding to less accumulation of mislocalized M-opsin. In P30 Lrat−/−, both the protein (Fig. 99.1a) and mRNA [8] levels of S-opsin were drastically reduced, due to significant loss of ventral and central cones [4, 9] (Fig. 99.1c).
Fig. 99.1.
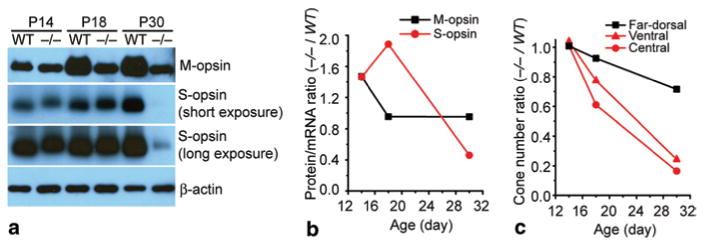
Expression of M and S opsins in Lrat −/− and WT retinas. a Western blot analysis of M and S opsins in the retinas of Lrat −/− and WT mice at P14, P18, and P30. Equal loading was indicated by β-actin. b The average protein/mRNA ratios of M and S opsins of Lrat−/− were normalized against those of WT. c The average cone numbers from ventral, central, and far-dorsal retina of Lrat−/− were normalized against those of WT. (Data were from Fig. 2 of [8]. Copyright National Academy of Sciences, USA)
99.3.2 Mouse and Human SW Opsins Aggregate and Cause ER Stress in Transfected Cells
By using a cell culture system (COS-7), we found that mouse S-opsin and human blue opsin showed prominent aggregations manifesting as green dots of varying sizes in the perinuclear region (Fig. 99.2a). This was not observed for mouse M-opsin and human red/green opsins. To examine the possibility that the aggregation of SW opsins may induce UPR and ER stress, COS-7 cells were co-transfected with various opsins and a CHOP-GFP reporter plasmid. CHOP (C/EBP homology protein) is a well characterized UPR (unfolded protein response) target gene and an ER stress marker associated with apoptosis. It has been shown that CHOP-GFP can track the expression of endogenous CHOP [11]. The expression of mouse S and human blue opsins induced significant CHOP activation compared with the expression of rhodopsin, mouse M-opsin or human red/green opsins (Fig. 99.2b, p < 0.001). Consistent with the cell culture result, the CHOP signal was markedly increased in the ONL of the ventral retina of P18 Lrat −/−, coinciding with the maximal S-opsin accumulation and the early stage of cone degeneration [8]. The CHOP signal was lower in the ventral retina of P14 Lrat −/− when S-opsin accumulation was much less. In the far-dorsal retina of Lrat −/− (P14 and P18) that mainly expresses M-opsin, no CHOP upregulation was observed [8].
Fig. 99.2.
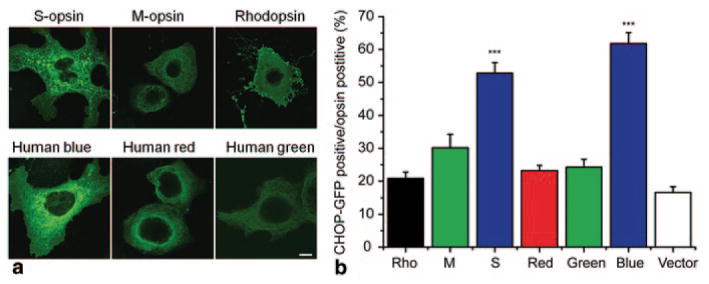
Mouse S-opsin and human blue opsin aggregated and induced CHOP activation in COS-7 cells. a COS-7 cells were transfected with plasmids encoding various opsins, and were labeled with opsin antibodies (green). Scale bar, 10 μm. b Quantification of CHOP-GFP positive cells as percentages of opsin expressing cells. COS-7 cells were co-transfected with plasmids encoding CHOP-GFP and various opsins (or control vector pRK5 and pmCherry-N1), and were labeled with opsin antibodies. The numbers of vector transfected cells were estimated from mCherry positive cells. N = 10 for Rho; N = 11 for M, red, blue; N = 12 for S and green opsins; N = 6 for vector control. Both mouse S-opsin (S) and human blue opsin induced significant more CHOP activation than mouse M-opsin (M), human red/green opsins, bovine rhodopsin, and vector control. ***, p < 0.001. (Data were from Figs. 5 and 6 of [8]. Copyright National Academy of Sciences, USA)
99.3.3 TUDCA Significantly Slows Down Cone Degeneration in Lrat−/− Mice
To determine whether TUDCA can prevent or slow down this rapid cone degeneration, Lrat−/− mice were injected subcutaneously once every 3 days starting at P9. Treated mice were euthanized at P28 and cone cell densities were determined at different retinal regions from fluorescence images of cone-arrestin stained retinal flatmounts. There was significant cone degeneration in the ventral and central regions of retina in vehicle injected Lrat−/− mice (Fig. 99.3). TUDCA treatment resulted in a significant increase (∼ 3-fold, p < 0.001) in cone density in the ventral and central retina compared with the vehicle (Fig. 99.1). Cone cell morphology was greatly improved as evidenced by the presence of intact cone structures [12]. TUDCA treatment also increased cone numbers (25 %) in the dorsal retina although the difference was not statistically significant (p = 0.057). Although TUDCA provided significant protection of central and ventral cones of Lrat−/− mice, the cone densities are still lower than those of WT (54.5 and 62.7 % lower for central cones and ventral cones, respectively). We observed a substantial reduction of CHOP in the ventral retina of TUDCA-treated Lrat−/− mice compared to that in vehicle-treated littermates at P18 [12]. Furthermore, TUDCA injection virtually eliminated apoptotic signals in the ONL of Lrat−/− mice judged by caspase-3 activation [12]. Our data suggest that TUDCA slows down cone degeneration by reducing ER stress and preventing apoptosis.
Fig. 99.3.
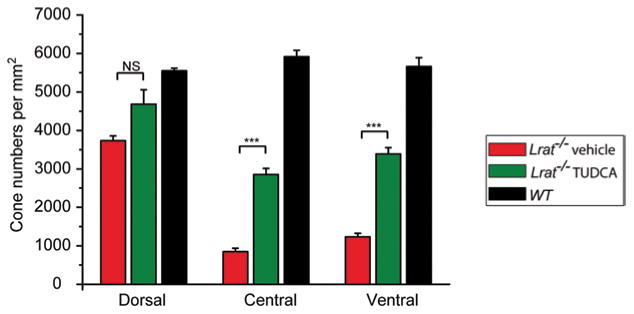
Quantification on the protective effect of TUDCA on Lrat−/− cones. Cone photoreceptors were counted in the ventral, central, and dorsal sections in TUDCA injected Lrat−/− (n = 8), vehicle injected Lrat−/− (n = 6), and untreated WT (n = 5). Data were expressed as the average numbers of cones per mm2 (mean ± SEM). Untreated WT mice were included as controls to evaluate the efficacy of TUDCA. ***p < 0.001, NS, not significant. (From Fig. 1 of [12]. Copyright Association for Research in Vision and Ophthalmology)
99.4 Discussion
Our studies showed that M and S opsins face very different fates in Lrat−/− mice: A significant amount of mislocalized M-opsin is degraded whereas S-opsin is resistant to proteasome degradation, resulting in far more toxic aggregation of S-opsin in the ventral and central cones than that of M-opsin in the dorsal cones. In addition, we found that aggregation of S-opsin activated the unfolded protein response (UPR) and caused ER stress. It is likely that the UPR in cones copes with mislocalized M-opsin more effectively than with mislocalized S-opsin. Thus, M-opsin is significantly degraded by the ER-associated degradation (ERAD) pathway, which relieves ER stress. On the other hand, S-opsin is resistant to ERAD resulting in aggregation/ accumulation, which induces apoptosis. As the ventral and central retina express higher levels of S-opsin than the dorsal retina in mice [10], our results explain the region-specific cone degeneration pattern in Lrat−/− and Rpe65−/− retinas. In addition, we demonstrated that systemic injection of TUDCA is effective in reducing ER stress, preventing apoptosis, and preserving cones in Lrat−/− mice. TUDCA has the potential to lead to the development of a new class of therapeutic drugs for treating LCA.
Acknowledgments
We thank W. Baehr for providing the Lrat−/− mice. This study was supported by NIH grant EY022614, E. Matilda Ziegler Award, Career Initiation Research Grant Award from Knights Templar, Career Development Award from Research to Prevent Blindness, RPB), and an unrestricted grant to the Department of Ophthalmology at the University of Utah from RPB.
Contributor Information
Yingbin Fu, Email: Yingbin.fu@hsc.utah.edu.
Tao Zhang, Email: tao0905@gmail.com.
References
- 1.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27(4):391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 3.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279(11):10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan J, Rohrer B, Frederick JM, Baehr W, Crouch RK. Rpe65-/- and Lrat-/- mice: comparable models of leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2008;49(6):2384–2389. doi: 10.1167/iovs.08-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, et al. Human cone photoreceptor dependence on RPE65 isomerase. Proc Natl Acad Sci U S A. 2007;104(38):15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz B, Poliakov E, Schambeck M, Friedburg C, Preising MN, Redmond TM. A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest Ophthalmol Vis Sci. 2008;49(12):5235–5242. doi: 10.1167/iovs.07-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips MJ, Walker TA, Choi HY, Faulkner AE, Kim MK, Sidney SS, et al. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Invest Ophthalmol Vis Sci. 2008;49(5):2148–2155. doi: 10.1167/iovs.07-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Zhang N, Baehr W, Fu Y. Cone opsin determines the time course of cone photoreceptor degeneration in Leber congenital amaurosis. Proc Natl Acad Sci U S A. 2011;108(21):8879–8884. doi: 10.1073/pnas.1017127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Fan J, Li S, Karan S, Rohrer B, Palczewski K, et al. Trafficking of membraneassociated proteins to cone photoreceptor outer segments requires the chromophore 11-cis-retinal. J Neurosci. 2008;28(15):4008–4014. doi: 10.1523/JNEUROSCI.0317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, et al. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27(3):513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 11.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang T, Baehr W, Fu Y. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2012;53(7):3349–3356. doi: 10.1167/iovs.12-9851. [DOI] [PMC free article] [PubMed] [Google Scholar]


