INTRODUCTION
Clinicians in tropical settings face great challenges when addressing neurological cases. Many parasites have evolved and adapted to infect the central nervous system, but those less common tend to be a major difficulty, due to potentially obscure clinical features or lack of diagnostic methods. Here we present three rare types of cestodiasis that are present across the tropics.
SPARGANOSIS
Sparganosis is a zoonotic parasitosis discovered by Patrick Manson in China in 1882 and later described in humans by Stiles in the USA in 1908. It is caused by infection with the plerocercoid (second) larval stage of several cestodes of the class Cestoidea, order Pseudophyllida, family Diphyllobothriidae, within the genus Spirometra. Cats and dogs are the final hosts of the adult stage and there are two intermediate stages; the first hosted by small crustaceans and the second by vertebrates, usually fish, frogs, snakes, or other reptiles. Humans can become infected by ingestion of raw or undercooked intermediate hosts or contaminated water. Larva cannot develop into adult worms within the human intestine, but become lodged in different tissues, such as the brain. In the CNS, larvae typically lodge in the brain parenchyma, spinal cord or eye, producing seizures, headache, hemiparesis, blindness, paralysis, or death.
The genus Spirometra is present throughout the world, but is most common in China, Korea, and Japan. The US CDC describes sparganosis as endemic in North America, but infections in the USA are uncommon, with the majority caused by S. mansonoides in the Gulf States. Infections occur most often in Asia due to regional preferences for the ingestion of exotic dishes containing snails, frogs, or snakes and other traditional practices, including applying poultices containing raw frog and raw snake or consumption during masculinity rituals. Local residents and adventurous travelers, particularly those visiting family and relatives (VFRs), may be a high-risk group.
Life cycle
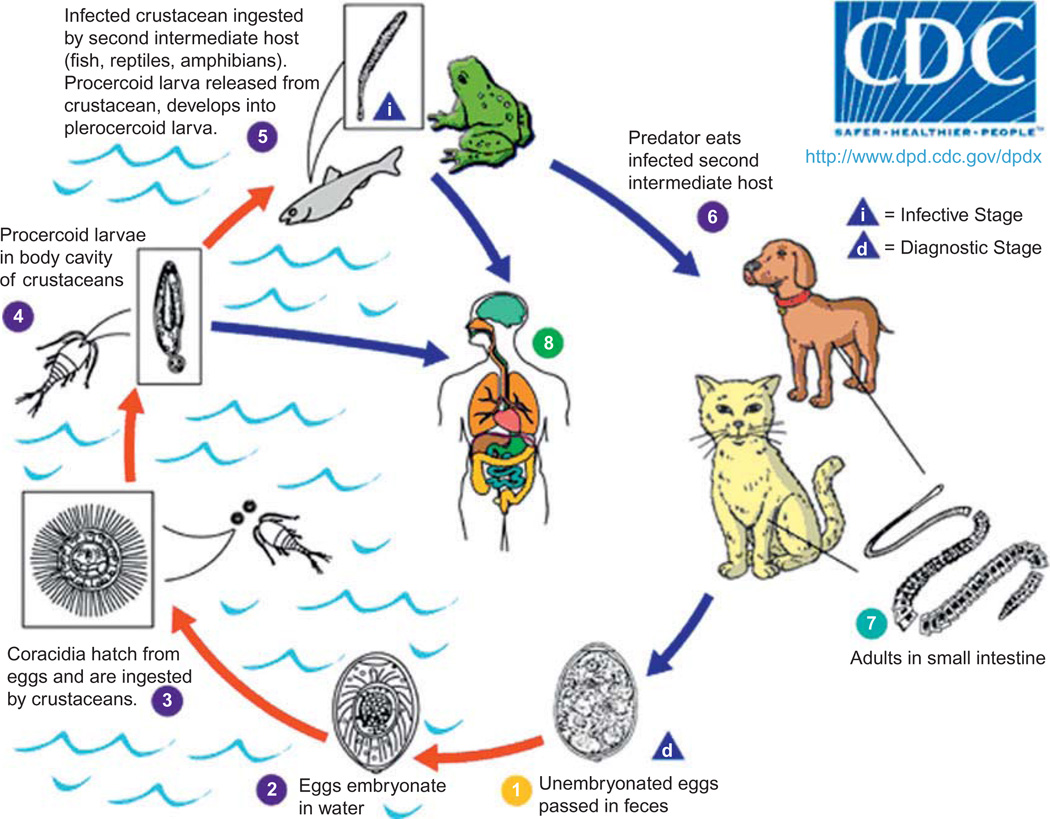
Sparganosis is a parasitosis that affects humans and animals and is caused by infection with the plerocercoid larval stage (spargana) of multiple tapeworms of the genus Spirometra, including S. mansoni, S. ranarum, S. mansonoides, S. erinaceieuropaei, and S. proliferum. The two primary causative agents are S. erinaceieuropaei in Asia and S. mansonoides in the Americas. These parasites have a complex life cycle that includes three different hosts (Fig. 27.1) (Centers for Disease Control and Prevention (CDC), 2011). Canines and felines serve as the final host, with the larvae developing into adult tapeworms within the hosts’ small intestine (Li et al., 2011). The adult tapeworm sheds unembryonated eggs that are excreted with the feces, embryonate in the environment, and hatch in water, releasing the coracidia. Oncospheres are ingested by freshwater cyclopoid copepod crustaceans including water fleas and cyclops (the first intermediate host), and later develop into procercoid larvae inside crustacean hosts. Infected copecods are then eaten by secondary intermediate hosts: fish, snakes, and frogs. Inside the vertebrate hosts, procercoid larvae cross the intestinal wall and migrate to tissue and organs, where they develop into the second intermediate stage, the plerocercoid larvae. The life cycle is complete when a carnivore eats a secondary intermediate host infected by spargana. Two to three weeks later, adult tapeworms are formed (Anders et al., 1984) and reportedly can survive up to 30 years (Mueller, 1968). Additionally, infected secondary intermediate hosts can be eaten by paratenic hosts such as nonhuman primates, pigs, mice, and birds.
Fig. 27.1.
Sparganosis transmission cycle. From Centers for Disease Control and Prevention (CDC), 2011 (http://www.dpd.cdc.gov/DPDx/HTML/Sparganosis.htm). Use approved by CDC DPDx.
Spargana are white flat worms 2–115 mm in length and 1–2 mm wide (Li et al., 2011). The adult S. mansoni worm has a scolex with two longitudinal channels and a chain of immature and gravid proglottids with a spiral-shaped uterus in each proglottid (Cui et al., 2011a). In the Henan province of China, Spirometra mansoni eggs were found in 19% of dogs (n = 31) and one of three cats (Cui et al., 2011b). Successful experimental infection of S. mansoni in a cat resulted in the identification of eggs in stool on days 12–25 and of adult worms on day 25. In the Henan province of China, studies reported 8% to 16% of tadpoles were infected with Spirometra spp. and 4% of Mesocyclops leuckarti cyclops contained 3–5 procercoids in the hemocele of each cyclop (Cui et al., 2011b). Infection rates in frogs ranged from 3% to 91%, with an average infection rate of 22% across seven species of frogs in eight provinces of China (Li et al., 2011). Up to 131 worms were found in R. nigromaculata frogs, with an average of 1.3 to 7.8 worms per frog across different species.
Human infection
Humans can develop sparganosis as an accidental host, but Spirometra spp. cannot complete the life cycle in the human host. Infection can occur through (a) eating raw or undercooked meat containing secondary intermediate hosts or paratenic hosts, (b) applying frog or snake poultice on the eyes, wounds, vagina, or other tissue, (c) eating raw tadpoles (Cui et al., 2011b), or (d) drinking water contaminated with procercoid-infected crustaceans. The first three mechanisms are particularly common in Asia, while drinking contaminated water is the most common mechanism of infection in other regions. Cerebral sparganosis most often occurs in adults, with fewer cases in adolescents and children (Nobayashi et al., 2006; Song et al., 2007; Anantaphruti et al., 2011). A disproportionate 10:1 male:female seroprevalence ratio for sparganosis has been reported (Lee et al., 2002) but the male: female ratio in published cases of cerebral sparganosis is 3:2 (Holodniy et al., 1991; Kim et al., 1996; Song et al., 2007; Choi et al., 2010; Anantaphruti et al., 2011).
The parasite migrates, often asymptomatically, from the digestive system through the capillaries to diverse locations in the human body. The parasite then lodges in subcutaneous tissue (primarily the face, abdomen, or limbs), eyes and less frequently in organs, such as the liver, lungs or kidney or CNS. In the CNS, spargana can migrate into the brain and eventually develop into a granulomatous lesion. Clinical symptoms depend upon the location of the lesions and often include headache, seizures, and weakness. Infection by the proliferative form of sparganosis associated with S. proliferum has the highest mortality. The larvae of this species grow by branching and budding, causing massive proliferation that can affect multiple tissues including the brain.
Until the 1980s, experts considered CNS sparganosis a rare event (Mineura and Mori, 1980; Chan et al., 1987) but with the increasing availability of brain imaging, CNS sparganosis is now reported more frequently and more than 160 cases have been reported in scientific literature. Human cases of CNS sparganosis have been reported in China, South Korea, Japan, Thailand, India, the Philippines, and the USA, among many other countries (Anders et al., 1984; Chan et al., 1987; Yamashita et al., 1990; Rengarajan et al., 2008; Shirakawa et al., 2010; Anantaphruti et al., 2011; Cui et al., 2011b). While it is difficult to assess the role of reporting bias, it is likely that CNS sparganosis is a more frequent condition than previously described. Over 1000 cases of sparganosis have been reported over an 80-year span in China, covering 25 provinces but mainly concentrated in the Guangdong province (Qiu and Qiu, 2009). A review of 110 recent articles published in Chinese describes 42 human cases of CNS sparganosis over 11 years, with patients ranging from 2 to 80 years of age (Li et al., 2011).A treatment case series in Korea additionally reported 17 cases over 8 years in a single hospital (Kim et al., 1996).
Diagnosis
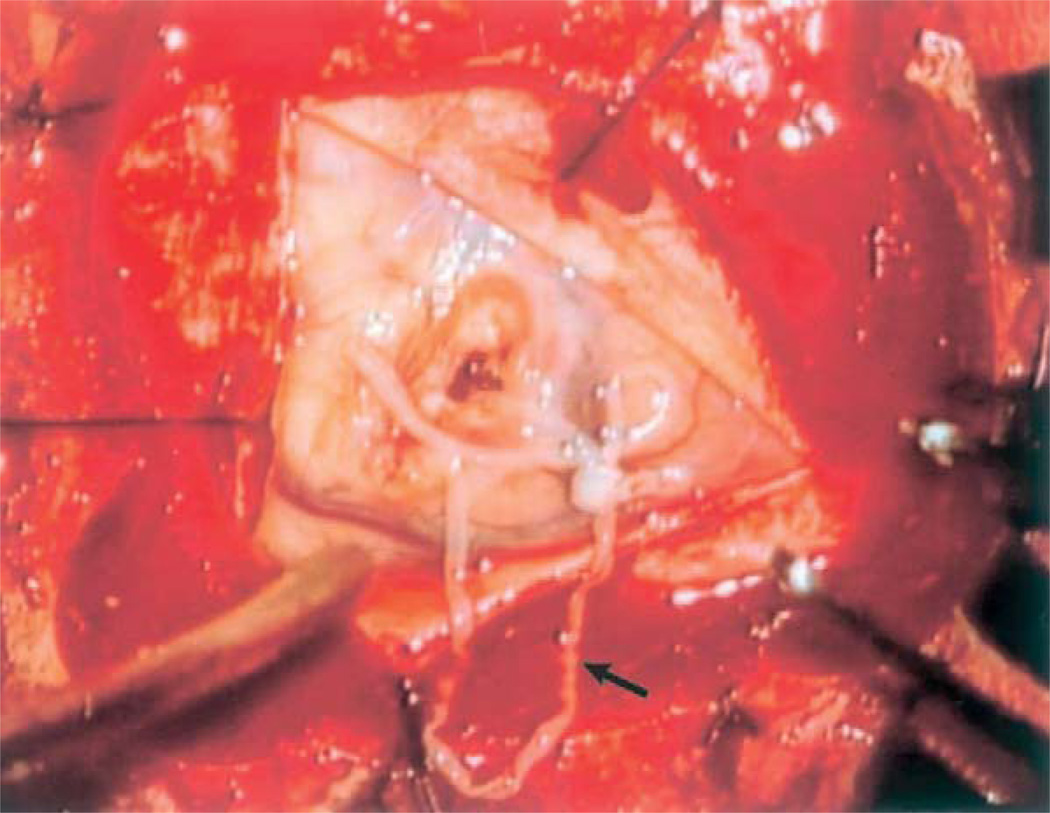
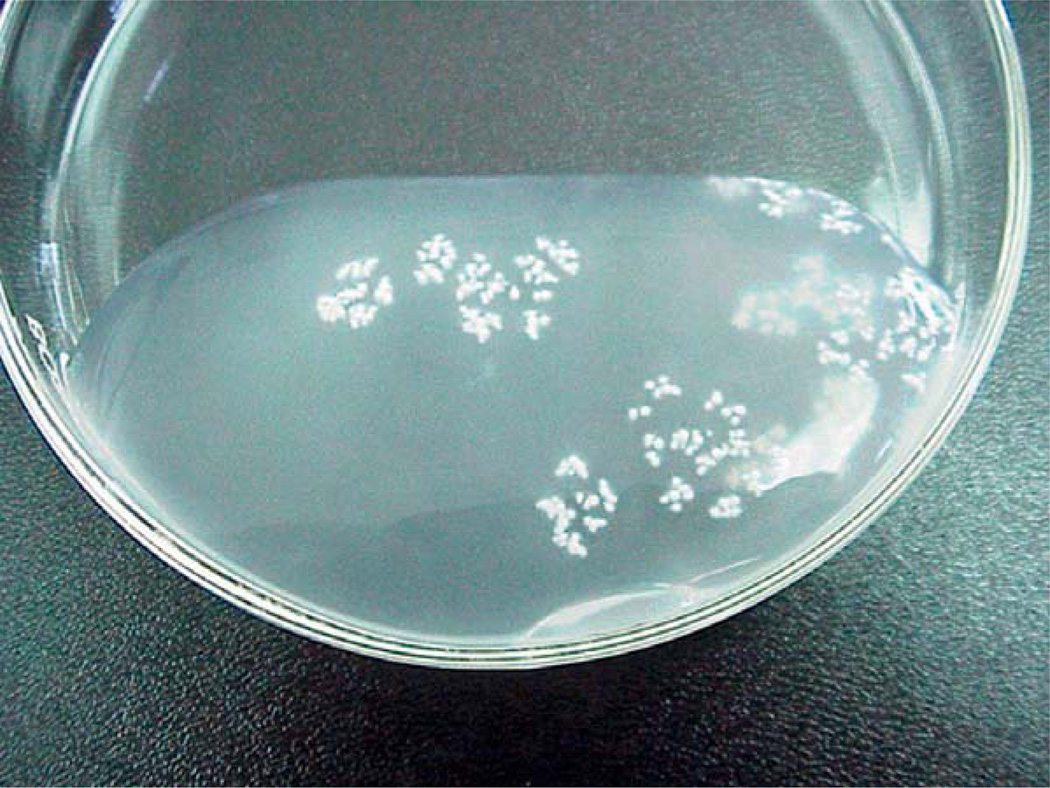
Definitive diagnosis is made by identification of the spargana within infected tissue obtained through biopsy. The spargana is a thin, white solid flatworm ranging from a few millimeters to several centimeters in length (Fig. 27.2)(Nobayashi et al., 2006), enclosed within a gliotic wall and surrounded by inflammatory exudates (Anders et al., 1984; Rengarajan et al., 2008). Histologically, spargana differ from other cysticerci by the absence of bladder walls, a hooked scolex or suckers, and the presence of a solid noncavitated body.
Fig. 27.2.
Live Spirometra mansoni worm found during parietal craniotomy. (From Nobayashi et al., 2006, with permission.)
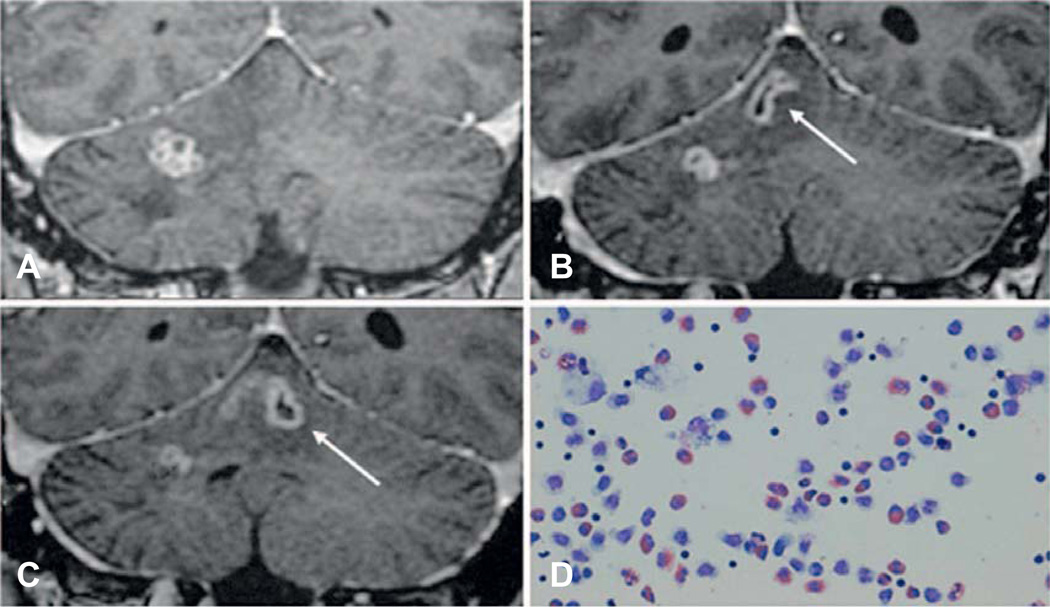
Imaging is an important diagnostic tool, but is not specific and cannot provide definitive diagnosis. Neuroimaging, in combination with biopsy or serological testing described below, can provide presumptive diagnosis. Neuroimaging of 23 confirmed cases of cerebral sparganosis in China demonstrated edema, white matter degeneration, and cortical atrophy in all patients (Song et al., 2007). Magnetic resonance imaging (MRI) is more specific than computed tomography (CT) for sparganosis, but not pathognomonic: lesions are typically hypointense and hyperintense on T1- and T2-weighted images, respectively, with variable enhancement. Ipsilateral ventricular dilation was observed in 20 of 23 patients, while the other three patients had ipsilateral ventricular compression. Interestingly, a tunnel sign was observed in 10 subjects on postcontrast images, highlighting the migrating path of the moving worm (2–6 cm length by 0.8 cm width). CT images, on the other hand, typically reveal hypo- or isodense images, without the tunnel sign (Song et al., 2007). Figure 27.3 provides a clear view of the tunnel sign (Shirakawa et al., 2010).
Fig. 27.3.
Migrating Spirometra erinaceieuropaei larva in the brain and tunnel sign on magnetic resonance imaging after application of contrast. Original legend as published: “MRIs demonstrate the migration of the lesion from the cerebellar hemisphere to the vermis over a period of 7 weeks. (A) Initial image, (B) 4 weeks later, and (C) 7 weeks later. The tunnel sign appearing as a hollow tube (arrow) represents the moving track of a migrating worm. Giemsa-stained CSF (D) shows a high number of eosinophils.” (From Shirakawa et al., 2010; use approved by corresponding author.)
The differential diagnosis should include neurocysticercosis, tuberculoma, fungal or bacterial abscess, and neoplastic lesions. Stereotactic imaging increases the likelihood of localizing the sparganum during biopsy (Chan et al., 1987).
Immunological tests are a valuable complement to diagnosis but can cross-react with other cestodes common in sparganosis-endemic settings (Rengarajan et al., 2008). A colorimetric ELISA assay for S. mansoni IgG reportedly has a sensitivity of 100% and 97% specificity in subjects with potentially cross-reacting parasitic coinfections (Nishiyama et al., 1994).
Clinical features
Case series and individual reports describe a wide range of incubation periods between presumptive infection and development of neurological symptoms. Some patients develop symptoms decades after the initial ingestion of raw frog or snake or application of poultices containing raw frog (Song et al., 2007). On the other hand, some infections in residents from nonendemic regions have become symptomatic within months of traveling through endemic areas (Chan et al., 1987). Symptoms can persist for years, and such prolonged presentations have been observed both in subjects with live and dead parasites detected at biopsy.
Cerebral sparganosis often presents with generalized tonic–clonic seizures and headaches, but paresthesias, weakness, and brisk tendon reflexes are also common (Mineura and Mori, 1980; Rengarajan et al., 2008). A slowly progressive hemiparesis has been reported (Pampiglione et al., 2003). Involvement of the eye often produces considerable pain, irritation of the conjunctival tissue and invasion of periorbital tissues, resulting in proptosis and blindness (Botterel and Bouree, 2003). Literature describing cerebral sparganosis is probably biased by reports of patients with highly symptomatic infection and confirmed diagnoses. The low occurrence of this infection and the challenges of definitive diagnosis have limited the ability of defining the spectrum of infection. No reports have documented a higher risk of infection among immunocompromised individuals (Walker and Zunt, 2005).
Prognosis and treatment
There is no effective medical treatment for CNS sparganosis and treatment of peripheral sparganosis is often ineffective, with reports of resistance to praziquantel and mebendazole when immature forms of the parasite are present (Moulinier et al., 1982; Sohn et al., 1993; Kim et al., 1996). Complete or partial excision of the parasite is considered the treatment of choice (Mineura and Mori, 1980; Chan et al., 1987) and is often curative. Surgical excision can also prevent further white matter degeneration, cognitive loss, permanent neurological deficits, and seizure remission. A review of 11 Chinese patients who underwent cyst excision reported significant improvement, with 10 patients reporting complete remission of seizures and resolution of hemiparesis in all four patients (Deng et al., 2011). In another case series, six of seven Thai patients with cerebral sparganosis had complete recovery after surgery, except one individual with deep brain infection who died after an attack of apnea and cardiac arrest (Anantaphruti et al., 2011). Autopsy findings from this individual identified a 2 × 0.1 cm sparganum that produced a migratory tunnel through the brainstem from the upper-left to the lower-right pontine tegmentum. A subarachnoidal hemorrhage in the right pontomedullary junction, likely caused by the parasite migration, was apparently the cause of death. Additionally, this individual had a Taenia solium cyst identified in the lateral pontomedullary area, which caused a year of chronic vertigo reported earlier by the patient (Anantaphruti et al., 2011).
COENUROSIS
Human coenurosis is caused by infection with the coenuri (metacestode or larval stage) of Taenia multiceps, T. serialis, T. brauni, and T. glomerata; although only T. multiceps coenuri are believed to be able to infect the CNS. These four Taenia species are present in the Americas, Europe, and Africa. Canids are the definitive hosts and a variety of mammals including rodents, horses, cattle, and sheep serve as intermediate hosts. Each coenurus contains many protoscolices, either floating or attached inside a vesicle-shaped cyst. Human infection results from accidental ingestion of eggs in contaminated food or water. Eggs hatch, lodge into subcutaneous tissue, eyes or brain, and coenuri develop over approximately 90 days. Neurological symptoms of cerebral coenurosis range from headache to seizures, hemiparesis and hydrocephalus, frequently with fatal consequences. Surgical excision is the most common treatment.
Life cycle
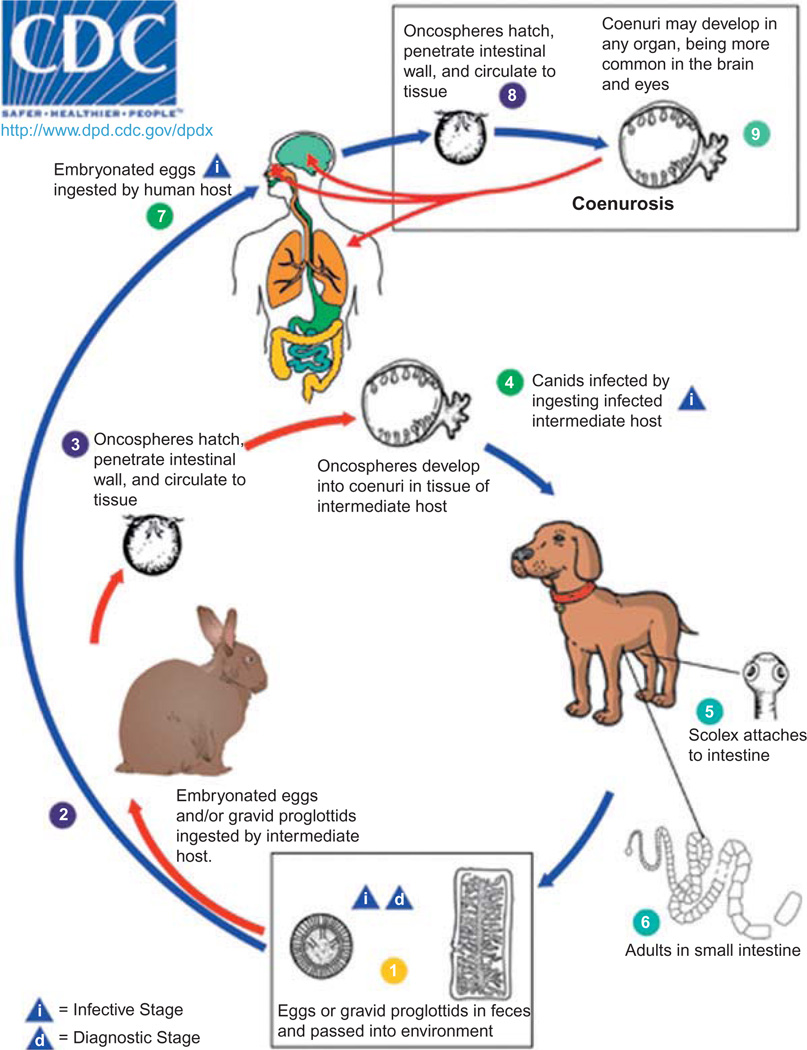
Figure 27.4 displays the life cycle of the four taeniid tapeworms that cause coenurosis (T. multiceps, T. serialis, T. brauni, and T. glomerata), although only T. multiceps is known to cause CNS infection. Carnivorous hosts, such as dogs, foxes, and wolves, harbor the adult tapeworm while several herbivorous mammals, mainly sheep, serve as intermediate hosts, with each tapeworm species often infecting specific hosts. Tapeworms develop in 15–42 days after ingestion of coenuri, with 3–4 proglottids maturing daily and producing more than 35 000 eggs per proglottid. Eggs are released from proglottids before leaving the final host in the feces, and are resistant to a wide range of temperatures and humidities (Scala and Varcasia, 2006). Eggs are ingested by humans accidentally and distributed via the bloodstream, eventually lodging in subcutaneous tissue, in the eyes or brain. Coenuri are typically 2–5 cm in diameter, although on occasion exceed 10 cm, and contain anywhere from a few to a hundred or more scolices. Figure 27.5 shows coenuri extracted from the brain of a sheep in Peru.
Fig. 27.4.
Life cycle of coenurosis-causing taeniid species. From Centers for Disease Control and Prevention (CDC) 2011 (http://www.dpd.cdc.gov/dpdx/html/Coenurosis.htm). Use approved by CDC DPDx.
Fig. 27.5.
Coenurus larvae excised from the brain of a sheep in Huancayo, Peru, showing multiple scolices on its wall. (Courtesy of Dr. Saul Santivañez.)
Epidemiology
Coenurosis is present worldwide, mainly in sheep-farming regions of Europe, the Americas, Africa and Asia, but also has numerous other definitive and intermediate hosts. There are approximately 30 cases of human CNS coenurosis reported in the scientific literature in South Africa, Europe, India, the USA, Brazil, and Israel (Table 27.1) (Brumpt, 1913; Clapham, 1941; Roger, 1942; Cluver quoted by Craig, 1943; Landells, 1949; Johnstone and Jones, 1950; Becker and Jacobson, 1951a,b; Watson and Laurie, 1955; Ranque and Nicoli, 1955; Bertrand et al., 1956; Correa et al., 1962; D’Andrea and Morello, 1964; Hermos et al., 1970; Michal et al., 1977; Schellhas and Norris, 1985; Pau et al., 1987, 1990; Malomo et al., 1990; Sabattani et al., 2004; Benifla et al., 2007). Becker describes at least 14 additional similar cases (Becker and Jacobson, 1951a). Taenia multiceps is the only species reported to cause human CNS infection. Cerebral infection due to T. serialis has been reported in sheep but not in humans in Europe, the Americas, and less commonly in Africa. Taenia brauni has only been reported in Central and South Africa (Fain, 1956; Vanderick et al., 1964; Collomb et al., 2007) and CNS cases have only been observed in rats. One human case of T. glomeratus has been reported, presenting as a subcutaneous cyst in a Nigerian patient (Turner and Leiper, 1919); coenuri of this species have been detected in the brain of nonhuman primates, (Fain, 1956).
Table 27.1.
Documented cases of human coenurosis of the central nervous system
| Author, year | Infection site | Age, sex, and country | Species |
|---|---|---|---|
| 1. Brumpt, 1913 | Brain, lateral ventricles, epilepsy, died | 40 M, France | T. multiceps |
| 2. Spencer, 1936 (described by Correa, no reference) | Brain, outcome unknown | Age/sex unknown, Africa | Unknown |
| 3. Cluver, 1940 | Brain, left lateral ventricle, died | Age/sex unknown, South Africa | T. multiceps |
| 4. Clapham, 1941 | Brain, lateral ventricle, coma, died | 39 M, England | T. multiceps |
| 5. Parkinson, 1942 (described by Correa, no reference) | Brain, outcome unknown | Age/sex unknown, England | Unknown |
| 6. Roger, 1942 | Brain, posterior cerebral fossa, died | 42 F, France | T. multiceps |
| 7. Buckley, 1947/Landells, 1949 | Spinal cord, paraplegia, outcome unknown | 14 F, England | T. multiceps |
| 8. Johnstone, 1950 | Brain, pons, medulla. Hemiparesis, hydrocephalus, died | 2 M, USA | T. multiceps |
| 9. Becker, 1951a | Brain, lateral and third ventricle, outcome unknown | 55 M, South Africa | T. multiceps |
| 10. Becker, 1951a | Brain, left ventricle, outcome unknown | 34 M, South Africa | T. multiceps |
| 11. Becker, 1951a | Brain, cerebellum and spinal cord, outcome unknown | 33 M, South Africa | T. multiceps |
| 12. Becker, 1951b | Brain, fourth ventricle, outcome unknown | 28 M, South Africa | T. multiceps |
| 13. Watson, 1955 | Brain, cerebellum, hydrocephalus, died | 32 M, South Africa | T. multiceps |
| 14. Watson, 1955 | Brain, left and right occipital lobe, right temporal lobe, died | 33 M, South Africa | T. multiceps |
| 15. Ranque, 1955 | Brain, outcome unknown | Age/sex unknown, France | T. multiceps |
| 16. Bertrand, 1956 | Brain, outcome unknown | Age/sex unknown, France | T. multiceps |
| 17. Correa, 1962 | Brain, generalized seizures, arachnoidal, died | 42 F, Brazil | T. multiceps |
| 18. D’Andrea, 1964 | Brain, outcome unknown | Age/sex unknown, Italy | T. multiceps |
| 19. Hermos, 1970 | Brain, spinal cord. Hydrocephalus and died | 2 M, USA | T. multiceps |
| 20. Michal, 1977 | Brain, paresthesia, headache | 37 F, Switzerland | T. multiceps |
| 21. Jung, 1981 | Brain, focal seizure, survived | 48 M, USA | T. multiceps, T. serialis or T. solium |
| 22. Schellhas, 1985 | Brainstem, fourth ventricle, spinal cord. Hydrocephalus, weakness, died | 3 F, USA | T. serialis??? |
| 23. Pau, 1987 | Brain, parenchyma and interpeduncular cistern, generalized seizures, hemiparesis, survived | 54 M, Italy | T. multiceps |
| 24. Pau, 1990 | Brain, paresthesia, 6-year survival postsurgery | 28 M, Italy | T. multiceps |
| 25. Pau, 1990 | Brain, seizures, blindness, 10-year survival postsurgery | 51 F, Italy | T. multiceps |
| 26. Pau, 1990 | Brain, headache, drowsiness, 20-year survival postsurgery | 32 M, Italy | T. multiceps |
| 27. Malomo, 1990 | Brain, outcome unknown | Age/sex unknown, Nigeria | T. multiceps |
| 28. Sabattani, 2004 | Brain, in the pons. Facial palsy, paresthesia, ataxia, outcome unknown | 46 F, Italy | T. multiceps |
| 29. Benifla, 2007/El-On, 2008 | Brain, morning headache, nausea, hemiparesis, survived surgery | 4 F, Israel | T. multiceps |
| 30. Mahadevan, 2011/Ambekar, 2011 | Brain, hemiparesis, sensory impairment, survived surgery | 55 M, India | T. multiceps |
| 31. Mahadevan, 2011 | Brain, headache and vomiting, survived surgery | 36 M, India | T. multiceps |
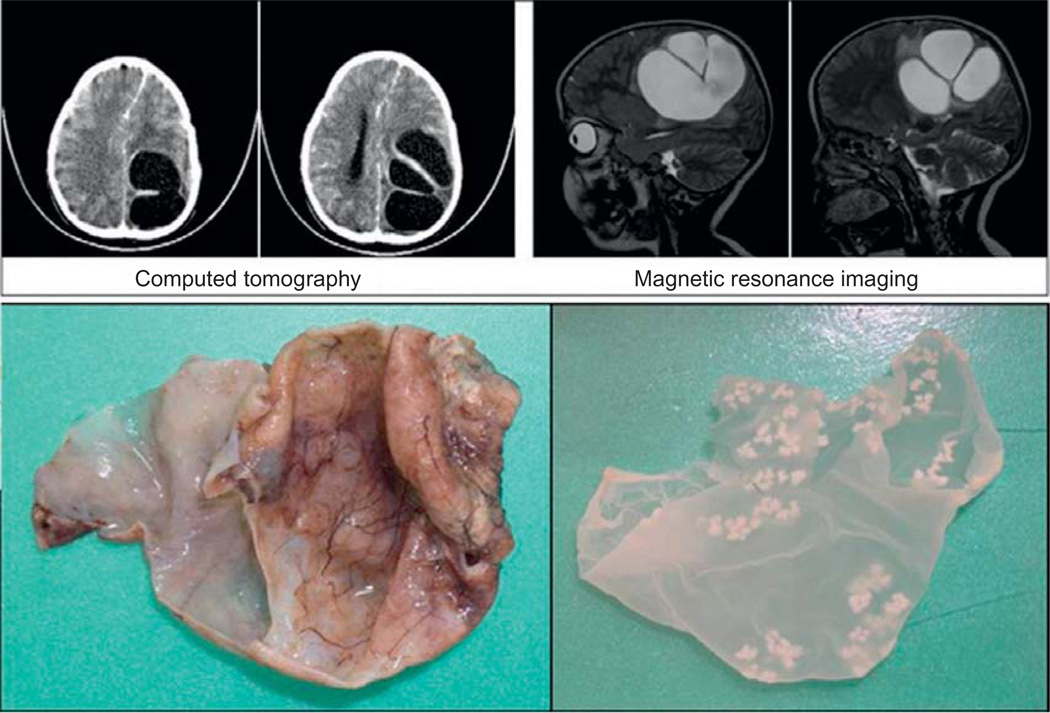
Diagnosis of the specific species causing coenurosis is challenging due to the lack of unique features of each species. The number and size of hooklets, the location and arrangement of the cysts, and the location of potential exposure can aid in the diagnosis. Taenia multiceps is enzootic in Europe and Asia but has not been reported in North America for 60 years (Ing et al., 1998). Taenia serialis is more common in North America. A report from 1998 noted adult tapeworms were present in 29% of wild mammals while coenurosis was detected in 19% of a rodent population (Ing et al., 1998). For this reason, some believe that cerebral coenurosis in the Americas could be caused by T. serialis (Ing et al., 1998) although no confirmatory evidence yet supports this view. Figure 27.6 shows neuroimaging and the actual coenurus excised from a 4-year-old girl in Israel (El-On et al., 2008).
Fig. 27.6.
Computed tomography and magnetic resonance imaging (upper panel) and coenurus cysts excised from a 4-year-old girl in Israel. The outer and inner cysts are 13 and 6 cm in diameter, respectively. (Reprinted with kind permission from Veterinaria Italiana, 44(4), 621–31, 2008, El-On et al.)
Cases of coenurosis often occur in adults who have had many years of contact with dogs and sheep. Four cases have been reported in children less than 5 years of age, the first three in the USA between 1950 and 1983, all with fatal consequences.
Clinical features
Human CNS infection usually presents with severe headache, disturbances of personality, weight loss, nuchal rigidity, and intracranial hypertension (Becker and Jacobson, 1951a). Hemiparesis, weakness, and papilledema are also common. The location of cysts is typically diverse and intraventricular cysts are often present. Coenuri are also commonly detected in the cerebellum and subarachnoid spaces. Two cases of spinal cord infection have been reported. Clinical manifestations with neurological symptoms have been observed up to 15 years prior to seeking medical attention (Hermos et al., 1970).
Diagnosis, prognosis, and treatment
Racemose T. solium cysticercosis and echinococcosis are the main differential diagnoses for coenurosis, and morphology remains a valuable aid for distinction between these parasites. Coenuri are often larger than T. solium cysticerci. Serological tests can cross-react with other taeniid species including T. solium. Diagnosis of coenurosis, particularly differentiation from T. solium racemose cysticercosis depends upon the morphological analysis of the excised coenuri. The presence of inverted cysts, budding or linear arrangement of daughter scolices provides definitive diagnosis of coenurosis. As explained above, discrimination between species is not possible due to the large overlap in number and size of hooklets (Johnstone and Jones, 1950). Clinically, T. multiceps coenurosis presents more often as a unilocular infection while T. serialis is more commonly multilocular. Surgery is the most effective treatment, and is often used to alleviate intracranial hypertension and remove the source of inflammation. Antiparasitic therapy with albendazole and praziquantel has been successful in some patients. Praziquantel can produce inflammation of undetected ocular coenurosis, so a dilated ophthalmological examination is recommended prior to initiating treatment. Prognosis before the advent of advanced imaging diagnosis was poor, but in the last three decades patients have reportedly survived more than 20 years after surgery (Pau et al., 1990).
TAENIA CRASSICEPS CYSTICERCOSIS
Taenia crassiceps is a cosmopolitan cestode parasite endemic to the northern hemisphere, including Europe, North America, and Asia. Similar to the life cycle of T. multiceps, T. crassiceps has a fully zoonotic transmission life cycle that includes foxes, dogs, and felids as the hosts of the adult tapeworm. Small mammals including mice, rabbits, and other rodents are intermediate hosts of the larval stage (Wunschmann et al., 2003). In rodents, cysts usually lodge subcutaneously in muscle, but not in the eyes or nervous system (Freeman et al., 1973). Animal neurocysticercosis has been documented in the brain of mice and cats (Fankhauser et al., 1967; Rietschel, 1981; Wunschmann et al., 2003) and in the spinal cord of nonhuman primates (Baer and Scheidegger, 1946). It can produce incoordination of movement (Rietschel 1981) and “circling disease.” The only case of brain infection in a cat is suspected to be related to immunosuppression, potentially resulting from treatment-resistant periodontitis (Fig. 27.7) (Wunschmann et al., 2003).
Fig. 27.7.
Domestic cat brain with approximately 20 – 25 intraventricular T. crassiceps cysticerci. (From Wunschmann et al., 2003, with permission from Journal of Veterinary Diagnostic Investigation.)
Taenia crassiceps differs from other tapeworms because of its ability to bud exogenously inside the host and generate new buds outside the cysts’ bladder that in turn become additional infective cysticerci. Bud multiplication can reach the thousands, diminishing the health status of the host and eventually killing it.
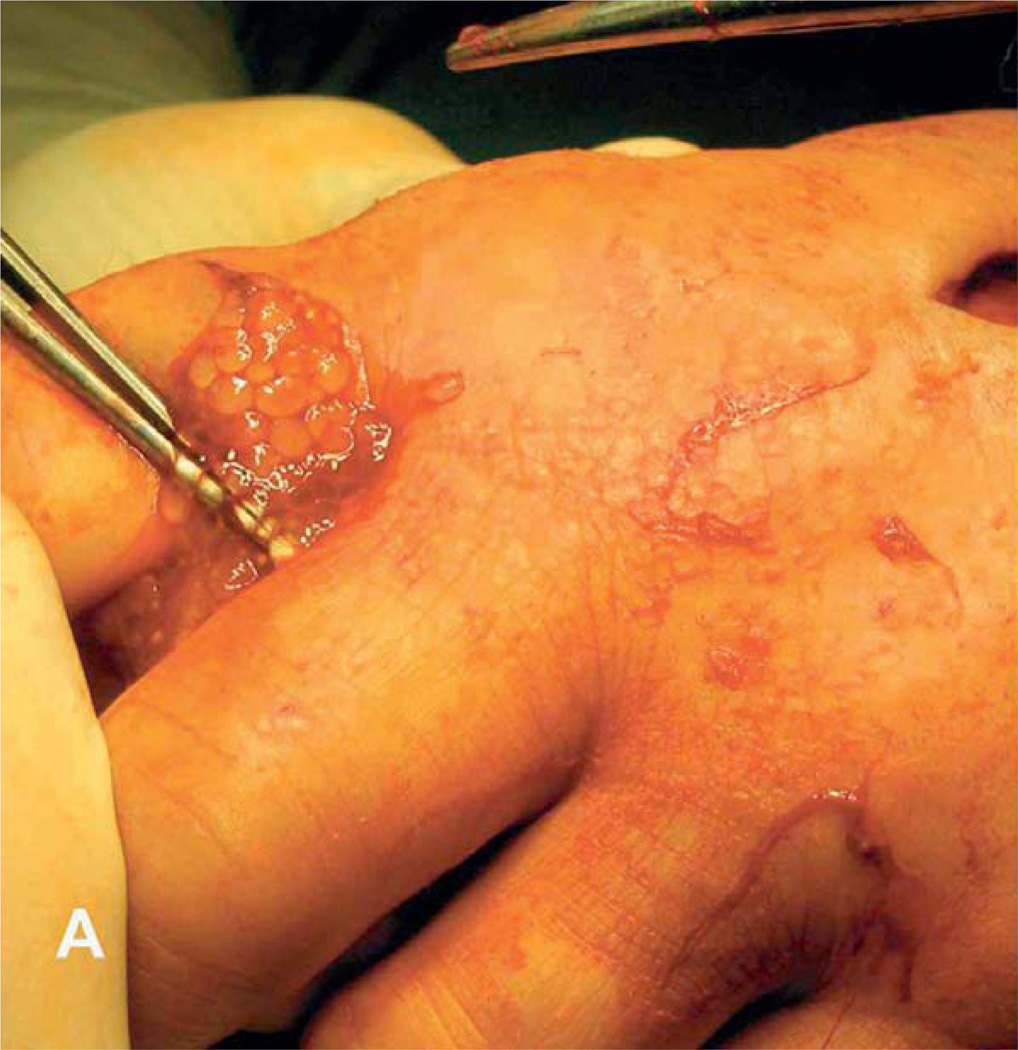
Humans are a dead-end, intermediate host, and human infection is extremely rare, with only 10 confirmed human cases described (Shea et al., 1973; Klinker et al., 1992; Arocker-Mettinger et al., 1992; Chermette et al., 1995; Mougeot et al., 1996; Chuck et al., 1997; Francois et al., 1998; Maillard et al., 1998; Heldwein et al., 2006; Goesseringer et al., 2011) mainly from France and Germany (Table 27.2).A depressed host immune system is believed to play a role in facilitating the encysting of the parasite, and six of the ten confirmed cases had some form of immunosuppression. In immunosuppressed individuals, T. crassiceps cysticercosis has been observed mainly in the arms (Fig. 27.8), while in immuncompetent people the parasite infects the eyes, although one patient presented with subcutaneous cysts in the paravertebral area. Most patients are young adults or teenagers, with the exception of an 82-year-old female receiving treatment for non-Hodgkin lymphoma. A case of spinal cord infection with a strobilated tapeworm causing paraplegia was reported in Thailand, and T. crassiceps and T. multiceps were proposed as potential etiologies, although no definitive evidence was obtained (Bamrungphol et al., 1972).
Table 27.2.
Documented cases of T. crassiceps cysticercosis in humans
| Author, year | Infection site | Age, sex, and country | Immunocompromised |
|---|---|---|---|
| 1. Shea, 1973 | Intraocular | 17F, Canada | No |
| 2. Klinker, 1992 | Subcutaneous paravertebral area | 33M, Germany | AIDS |
| 3. Arocker-Metinger, 1992 | Intraocular | 15F, Germany | No |
| 4. Chermette, 1995 | Subcutaneous | 33M, France | AIDS |
| 5. Mougeot, 1996 | Intraocular | 14M, France | Unknown |
| 6. Chuck, 1997 | Intraocular | 38F, USA | No |
| 7. Francois, 1998 | Intramuscular, arm | 38M, France | AIDS |
| 8. Maillard, 1998 | Subcutaneous, arm | 34M, France | AIDS |
| 9. Heldwein, 2006 | Subcutaneous, arm and hand | 82F, German | Non-Hodgkin lymphoma |
| 10. Goesseringer, 2011 | Subcutaneous, arm | 47F, Switzerland | AIDS |
Fig. 27.8.
Subcutaneous T. crassiceps infection in the hand presenting with characteristic proliferation. (From Heldwein et al., 2006, with permission from the American Journal of Tropical Medicine & Hygiene.)
To our knowledge, there has been no description of T. crassiceps infection within the human CNS, but this possibility is not completely unlikely (Tanowitz et al., 2001), particularly based on evidence of animal T. crassiceps neurocysticercosis observed in multiple species. Taenia crassiceps infection should be considered particularly when neurocysticercosis presents in an immunosuppressed individual and T. solium cysts have been ruled out. Serological tests and parasitological and pathological evaluation for morphology of the cyst can guide the diagnosis, particularly if budding is present. Differentiation of the rostellar hooks based on their size is also useful for Taenia spp. discrimination. Evidence of contact with tapeworm-carrying dogs or foxes provides additional epidemiological support for suspecting T. crassiceps infection. The treatment of choice is surgical excision, as relapses have been observed after antiparasitic therapy with mebendazole, albendazol, and praziquantel.
References
- Ambekar S, Prasad C, Dwarakanath S, et al. MRS findings in cerebral coenurosis due to Taenia multiceps. J Neuroimaging. 2013;23:149–151. doi: 10.1111/j.1552-6569.2011.00616.x. [DOI] [PubMed] [Google Scholar]
- Anantaphruti MT, Nawa Y, Vanvanitchai Y. Human sparganosis in Thailand: an overview. Acta Trop. 2011;118:171–176. doi: 10.1016/j.actatropica.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Anders K, Foley K, Stern E, et al. Intracranial sparganosis: an uncommon infection Case report. J Neurosurg. 1984;60:1282–1286. doi: 10.3171/jns.1984.60.6.1282. [DOI] [PubMed] [Google Scholar]
- Arocker-Mettinger E, Huber-Spitzy V, Auer H, et al. Taenia crassiceps in the anterior chamber of the human eye. A case report. Klin Monbl Augenheilkd. 1992;201:34–37. doi: 10.1055/s-2008-1045865. [DOI] [PubMed] [Google Scholar]
- Baer JG, Scheidegger S. Not available. Schweiz Z Pathol Bakteriol. 1946;9:61–66. [PubMed] [Google Scholar]
- Bamrungphol V, Little MD, Virayavejakul A, et al. Juvenile strobilate tapeworm from the human spinal cord: report of a case. Am J Trop Med Hyg. 1972;21:435–439. doi: 10.4269/ajtmh.1972.21.435. [DOI] [PubMed] [Google Scholar]
- Becker BJ, Jacobson S. Infestation of the human brain with Coenurus cerebralis; a report of three cases. Lancet. 1951a;2:198–202. doi: 10.1016/s0140-6736(51)91440-7. [DOI] [PubMed] [Google Scholar]
- Becker BJ, Jacobson S. Infestation of the human brain with Coenurus cerebralis in human brain. Lancet. 1951b;2:1202–1204. doi: 10.1016/s0140-6736(51)93205-9. [DOI] [PubMed] [Google Scholar]
- Benifla M, Barrelly R, Shelef I, et al. Huge hemispheric intraparenchymal cyst caused by Taenia multiceps in a child Case report. J Neurosurg. 2007;107:511–514. doi: 10.3171/PED-07/12/511. [DOI] [PubMed] [Google Scholar]
- Bertrand I, Callot J, Terrasse J, et al. A new case of cerebral coenurosis. Presse Med. 1956;64:333–335. [PubMed] [Google Scholar]
- Botterel F, Bouree P. Ocular sparganosis : a case report. J Travel Med. 2003;10:245–246. doi: 10.2310/7060.2003.40468. [DOI] [PubMed] [Google Scholar]
- Brumpt E. Précis de parasitologie. 2nd edn. Paris: Masson and Co; 1913. [Google Scholar]
- Buckley JJC. Coenurus from human spinal cord. Trans Roy Soc Trop Med & Hyg. 1947;41:7. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) DPDx, Laboratory Identification of Parasites of Public Health Concern. Center for Global Health; 2011. [cited November 24 2011]. 2011. Available from, http://www.dpd.cdc.gov/dpdx. [Google Scholar]
- Chan ST, Tse CH, Chan YS, et al. Sparganosis of the brain. Report of two cases. J Neurosurg. 1987;67:931–934. doi: 10.3171/jns.1987.67.6.0931. [DOI] [PubMed] [Google Scholar]
- Chermette R, Bussieras J, Marionneau J, et al. Invasive cysticercosis due to Taenia crassiceps in an AIDS patient. Bull Acad Natl Med. 1995;179:777–780. discussion 780–3. [PubMed] [Google Scholar]
- Choi WH, Chu JP, Jiang M, et al. Analysis of parasitic diseases diagnosed by tissue biopsy specimens at KyungHee Medical Center (1984–2005) in Seoul, Korea. Korean J Parasitol. 2010;48:85–88. doi: 10.3347/kjp.2010.48.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuck RS, Olk RJ, Weil GJ, et al. Surgical removal of a subretinal proliferating cysticercus of taeniaeformis crassiceps. Arch Ophthalmol. 1997;115:562–563. doi: 10.1001/archopht.1997.01100150564028. [DOI] [PubMed] [Google Scholar]
- Clapham PA. An English case of Coenurus cerebralis in the human brain. J Helmintk. 1941;19:84–86. [Google Scholar]
- Craig CF, Faust EC, editors. Cluver EH quoted by Craig CF. Clinical Parasitology. 3rd edn. Philadelphia, PA: Lea & Febiger; 1943. [Google Scholar]
- Cluver EH, Rep S. Afr Inst med Res. 1940;49 [Google Scholar]
- Collomb J, Machouart M, Biava MF, et al. Contribution of NADH dehydrogenase subunit I and cytochrome C oxidase subunit I sequences toward identifying a case of human coenuriasis in France. J Parasitol. 2007;93:934–937. doi: 10.1645/GE-1160R.1. [DOI] [PubMed] [Google Scholar]
- Correa FM, Ferriolli Filho F, Forjaz S, et al. Cerebral coenurosis. Apropos of a human case. Rev Inst Med Trop Sao Paulo. 1962;4:38–45. [PubMed] [Google Scholar]
- Cui J, Li N, Wang ZQ, et al. Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigens of Spirometra mansoni spargana. Parasitol Res. 2011a;108:1551–1556. doi: 10.1007/s00436-010-2206-2. [DOI] [PubMed] [Google Scholar]
- Cui J, Lin XM, Zhang HW, et al. Sparganosis, Henan Province, central China. Emerg Infect Dis. 2011b;17:146–147. doi: 10.3201/eid1701.101095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea F, Morello G. La cenurosi cerebrale. Acta Neurol. 1964;19:245–257. [Google Scholar]
- Deng L, Xiong P, Qian S. Diagnosis and stereotactic aspiration treatment of cerebral sparganosis: summary of 11 cases. J Neurosurg. 2011;114:1421–1425. doi: 10.3171/2010.4.JNS1079. [DOI] [PubMed] [Google Scholar]
- El-On J, Shelef I, Cagnano E, et al. Taenia multiceps: a rare human cestode infection in Israel. Vet Ital. 2008;44:621–631. [PubMed] [Google Scholar]
- Fain A. Coenurus of Taenia brauni Setti parasitic in man and animals from the Belgian Congo and Ruanda-Urundi. Nature. 1956;178:1353. doi: 10.1038/1781353a0. [DOI] [PubMed] [Google Scholar]
- Fankhauser R, Horning B, Waelchli P. Cysticercus longicollis in the brain of a field mouse (Microtus arvalis) Schweiz Arch Tierheilkd. 1967;109:525–532. [PubMed] [Google Scholar]
- Francois A, Favennec L, Cambon-Michot C, et al. Taenia crassiceps invasive cysticercosis: a new human pathogen in acquired immunodeficiency syndrome? Am J Surg Pathol. 1998;22:488–492. doi: 10.1097/00000478-199804000-00015. [DOI] [PubMed] [Google Scholar]
- Freeman RS, Fallis AM, Shea M, et al. Intraocular Taenia crassiceps (Cestoda). II. The parasite. Am J Trop Med Hyg. 1973;22:493–495. doi: 10.4269/ajtmh.1973.22.493. [DOI] [PubMed] [Google Scholar]
- Goesseringer N, Lindenblatt N, Mihic-Probst D, et al. Taenia crassiceps upper limb fasciitis in a patient with untreated acquired immunodeficiency syndrome and chronic hepatitis C infection: the role of surgical debridement. J Plast Reconstr Aesthet Surg. 2011;64:e174–e176. doi: 10.1016/j.bjps.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Heldwein K, Biedermann HG, Hamperl WD, et al. Subcutaneous Taenia crassiceps infection in a patient with non-Hodgkin’s lymphoma. Am J Trop Med Hyg. 2006;75:108–111. [PubMed] [Google Scholar]
- Hermos JA, Healy GR, Schultz MG, et al. Fatal human cerebral coenurosis. JAMA. 1970;213:1461–1464. [PubMed] [Google Scholar]
- Holodniy M, Almenoff J, Loutit J, et al. Cerebral sparganosis: case report and review. Rev Infect Dis. 1991;13:155–159. doi: 10.1093/clinids/12.5.155. [DOI] [PubMed] [Google Scholar]
- Ing MB, Schantz PM, Turner JA. Human coenurosis in North America: case reports and review. Clin Infect Dis. 1998;27:519–523. doi: 10.1086/514716. [DOI] [PubMed] [Google Scholar]
- Johnstone HG, Jones OW., Jr Cerebral coenurosis in an infant. Am J Trop Med Hyg. 1950;30:431–441. doi: 10.4269/ajtmh.1950.s1-30.431. [DOI] [PubMed] [Google Scholar]
- Jung RC, Rodriguez MA, Beaver PC, et al. Racemose cysticercus in human brain. A case report. Am J Trop Med Hyg. 1981;30:620–624. doi: 10.4269/ajtmh.1981.30.620. [DOI] [PubMed] [Google Scholar]
- Kim DG, Paek SH, Chang KH, et al. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg. 1996;85:1066–1071. doi: 10.3171/jns.1996.85.6.1066. [DOI] [PubMed] [Google Scholar]
- Klinker H, Tintelnot K, Joeres R, et al. Taenia crassiceps infection in AIDS. Dtsch Med Wochenschr. 1992;117:133–138. doi: 10.1055/s-2008-1062291. [DOI] [PubMed] [Google Scholar]
- Landells JW. Intra-medullary cyst of the spinal cord due to the cestode Multiceps multiceps in the coenurus stage; report of a case. J Clin Pathol. 1949;2:60–63. [PubMed] [Google Scholar]
- Lee KJ, Bae YT, Kim DH, et al. A seroepidemiologic survey for human sparganosis in Gangweon-do. Korean J Parasitol. 2002;40:177–180. doi: 10.3347/kjp.2002.40.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Song HQ, Li C, et al. Sparganosis in mainland China. Int J Infect Dis. 2011;15:e154–e156. doi: 10.1016/j.ijid.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Mahadevan A, Dwarakanath S, Pai S, et al. Cerebral coenurosis mimicking hydatid disease - report of two cases from South India. Clin Neuropathol. 2011;30:28–32. doi: 10.5414/npp30028. [DOI] [PubMed] [Google Scholar]
- Maillard H, Marionneau J, Prophette B, et al. Taenia crassiceps cysticercosis and AIDS. AIDS. 1998;12:1551–1552. doi: 10.1097/00002030-199812000-00019. [DOI] [PubMed] [Google Scholar]
- Malomo A, Ogunniyi J, Ogunniyi A, et al. Coenurosis of the central nervous system in a Nigerian. Trop Geogr Med. 1990;42:280–282. [PubMed] [Google Scholar]
- Michal A, Regli F, Campiche R, et al. Cerebral coenurosis. Report of a case with arteritis. J Neurol. 1977;216:265–272. doi: 10.1007/BF00314050. [DOI] [PubMed] [Google Scholar]
- Mineura K, Mori T. Sparganosis of the brain. Case report. J Neurosurg. 1980;52:588–590. doi: 10.3171/jns.1980.52.4.0588. [DOI] [PubMed] [Google Scholar]
- Mougeot G, Cambon M, Dimeglio V, et al. Intraocular Cestode larva in a 14-year-old boy in Auvergne. Presse Med. 1996;25:1168. [PubMed] [Google Scholar]
- Moulinier R, Martinez E, Torres J, et al. Human proliferative sparganosis in Venezuela: report of a case. Am J Trop Med Hyg. 1982;31:358–363. doi: 10.4269/ajtmh.1982.31.358. [DOI] [PubMed] [Google Scholar]
- Mueller JF. Growth stimulating effect of experimental sparganosis in thyroidectomized and hypophysectomized rats, and comparative activity of different species of Spirometra. J Parasitol. 1968;54:795–801. [PubMed] [Google Scholar]
- Nishiyama T, Ide T, Himes SR, Jr, et al. Immunodiagnosis of human sparganosis mansoni by micro-chemiluminescence enzyme-linked immunosorbent assay. Trans R Soc Trop Med Hyg. 1994;88:663–665. doi: 10.1016/0035-9203(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Nobayashi M, Hirabayashi H, Sakaki T, et al. Surgical removal of a live worm by stereotactic targeting in cerebral sparganosis Case report. Neurol Med Chir (Tokyo) 2006;46:164–167. doi: 10.2176/nmc.46.164. [DOI] [PubMed] [Google Scholar]
- Pampiglione S, Fioravanti ML, Rivasi F. Human sparganosis in Italy. Case report and review of the European cases. APMIS. 2003;111:349–354. doi: 10.1034/j.1600-0463.2003.1110208.x. [DOI] [PubMed] [Google Scholar]
- Pau A, Turtas S, Brambilla M, et al. Computed tomography and magnetic resonance imaging of cerebral coenurosis. Surg Neurol. 1987;27:548–552. doi: 10.1016/0090-3019(87)90153-4. [DOI] [PubMed] [Google Scholar]
- Pau A, Perria C, Turtas S, et al. Long-term follow-up of the surgical treatment of intracranial coenurosis. Br J Neurosurg. 1990;4:39–43. doi: 10.3109/02688699009000680. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Qiu MD. Human plerocercoidosis and sparganosis: II. A historical review on pathology, clinics, epidemiology and control. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27:251–260. [PubMed] [Google Scholar]
- Ranque J, Nicoli RN. Parasitological aspects of cerebral coenurosis; a new case. Ann Parasitol Hum Comp. 1955;30:22–42. [PubMed] [Google Scholar]
- Rengarajan S, Nanjegowda N, Bhat D, et al. Cerebral sparganosis: a diagnostic challenge. Br J Neurosurg. 2008;22:784–786. doi: 10.1080/02688690802088073. [DOI] [PubMed] [Google Scholar]
- Rietschel G. Contribution to the knowledge of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda, Taeniidae) (author’s transl) Z Parasitenkd. 1981;65:309–315. doi: 10.1007/BF00926726. [DOI] [PubMed] [Google Scholar]
- Roger H, Sautet J, Paillas JE. Un cas cénurose de la fosse cérébrale postérieure. Rev Neurologie. 1942;74:319–321. [Google Scholar]
- Sabattani S, Marliani AF, Roncaroli F, et al. Cerebral coenurosis. Case illustration. J Neurosurg. 2004;100:964. doi: 10.3171/jns.2004.100.5.0964. [DOI] [PubMed] [Google Scholar]
- Scala A, Varcasia A. Updates on morphobiology, epidemiology and molecular characterization of coenurosis in sheep. Parassitologia. 2006;48:61–63. [PubMed] [Google Scholar]
- Schellhas KP, Norris GA. Disseminated human subarachnoid coenurosis: computed tomographic appearance. AJNR Am J Neuroradiol. 1985;6:638–640. [PMC free article] [PubMed] [Google Scholar]
- Shea M, Maberley AL, Walters J, et al. Intraocular Taenia crassiceps (Cestoda) Trans Am Acad Ophthalmol Otolaryngol. 1973;77:OP778–OP783. [PubMed] [Google Scholar]
- Shirakawa K, Yamasaki H, Ito A, et al. Cerebral sparganosis: the wandering lesion. Neurology. 2010;74:180. doi: 10.1212/WNL.0b013e3181c91a15. [DOI] [PubMed] [Google Scholar]
- Sohn WM, Hong ST, Chai JY, et al. Infectivity of the sparganum treated by praziquantel, gamma-irradiation and mechanical cutting. Korean J Parasitol. 1993;31:135–139. doi: 10.3347/kjp.1993.31.2.135. [DOI] [PubMed] [Google Scholar]
- Song T, Wang WS, Zhou BR, et al. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol. 2007;28:1700–1705. doi: 10.3174/ajnr.A0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz HB, Weiss LM, Wittner M. Tapeworms. Curr Infect Dis Rep. 2001;3:77–84. doi: 10.1007/s11908-001-0062-z. [DOI] [PubMed] [Google Scholar]
- Turner M, Leiper RT. On the occurrence of coenurus glomeratus in Man in West Africa. Trans R Soc Trop Med Hyg. 1919;13:23–24. [Google Scholar]
- Vanderick F, Fain A, Langi S, et al. 2 New cases of human coenurosis caused by Taenia Brauni in Rwanda, with orbital localization of the coenurus. Ann Soc Belges Med Trop Parasitol Mycol. 1964;44:1077–1079. [PubMed] [Google Scholar]
- Walker MD, Zunt JR. Neuroparasitic infections: cestodes, trematodes, and protozoans. Semin Neurol. 2005;25:262–277. doi: 10.1055/s-2005-917663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KC, Laurie W. Cerebral coenuriasis in man. Lancet. 1955;269:1321–1322. doi: 10.1016/s0140-6736(55)93116-0. [DOI] [PubMed] [Google Scholar]
- Wunschmann A, Garlie V, Averbeck G, et al. Cerebral cysticercosis by Taenia crassiceps in a domestic cat. J Vet Diagn Invest. 2003;15:484–488. doi: 10.1177/104063870301500517. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Akimura T, Kawano K, et al. Cerebral sparganosis mansoni. Report of two cases. Surg Neurol. 1990;33:28–34. doi: 10.1016/0090-3019(90)90221-a. [DOI] [PubMed] [Google Scholar]