Abstract
Excessive alcohol use increases the risk of acute lung injury and pneumonia. Chronic alcohol ingestion causes oxidative stress within the alveolar space, including near depletion of glutathione (GSH), which impairs alveolar epithelial and macrophage function, in experimental animals and human subjects. However, the fundamental mechanism(s) by which alcohol induces such profound lung oxidative stress is unknown. Nuclear factor (erythroid-derived 2)–like 2 (Nrf2) is a redox-sensitive master transcription factor that regulates activation of the antioxidant response element (ARE). As the alveolar epithelium controls GSH levels within the alveolar space, we hypothesized that alcohol also decreases Nrf2 expression and/or activation within the alveolar epithelium. In this study, we determined that alcohol ingestion in vivo or direct alcohol exposure in vitro down-regulated the Nrf2–ARE pathway in lung epithelial cells, decreased the expression of antioxidant genes, and lowered intracellular GSH levels. RNA silencing of Nrf2 gene expression in alveolar epithelial cells in vitro decreased expression of these same antioxidant genes, and likewise lowered intracellular GSH levels, findings that mirrored the effects of alcohol. In contrast, treating alcohol-exposed alveolar epithelial cells in vitro with the Nrf2 activator, sulforaphane, preserved Nrf2 expression, ARE activation, intracellular GSH levels, and epithelial barrier function. These new experimental findings implicate down-regulation of the Nrf2–ARE signaling pathway as a fundamental mechanism by which alcohol causes profound oxidative stress and alveolar epithelial dysfunction, and suggest that treatments, such as sulforaphane, that activate this pathway could mitigate the pathophysiological consequences of alcohol on the lung and other organs.
Keywords: acute respiratory distress syndrome, glutathione, lung, redox signaling, oxidative stress
Clinical Relevance
Alcohol abuse causes oxidative stress and alveolar epithelial and macrophage dysfunction, and renders individuals at high risk for pneumonia and acute respiratory distress syndrome. This article provides the first comprehensive evidence that alcohol causes these effects within the alveolar epithelium by inhibiting the expression and actions of nuclear factor (erythroid-derived 2)–like 2 (Nrf2), the master transcription factor that activates the antioxidant response element (ARE). In parallel, this study provides evidence that treatment with phytochemicals, such as sulforaphane, which activate the Nrf2–ARE signaling pathway, can reverse the pathophysiological effects of alcohol on the alveolar epithelium, and therefore could enhance lung health in people suffering from chronic alcohol use disorders.
Alcohol is the most commonly used and abused drug throughout the United States and the rest of the world. Although the overuse of alcohol is commonly associated with disorders of the liver, brain, and gastrointestinal tract, the role of alcohol in diseases of the respiratory system is becoming more widely recognized. Although a connection between alcohol abuse and pneumonia has been known for centuries, it was only in 1996 that an epidemiological association with the acute respiratory distress syndrome (ARDS) was recognized (1), an observation later validated in a prospective study (2). ARDS is a severe form of acute lung injury, characterized by alveolar epithelial barrier disruption and flooding of the alveolar space with proteinaceous fluid that interferes with gas exchange and causes profound respiratory failure. ARDS can result from a wide range of critical illnesses, including pneumonia, sepsis, massive gastric aspiration, severe burns, and trauma; recent estimates place its incidence at approximately 200,000 cases per year in the United States and, despite aggressive supportive care, is associated with a mortality rate of approximately 40% (3). Since its description in the 1960s (4), there have been ongoing laboratory and clinical investigations into the mechanisms underlying this common and deadly disorder. However, despite these efforts, the treatment of ARDS remains largely supportive and, to date, no therapeutic agents have been shown to have any significant effect on its outcome.
Since the discovery of the association between alcohol ingestion and ARDS, our research group has focused its efforts on elucidating the pathophysiological effects of alcohol in the lung. For example, although an otherwise healthy alcohol-fed experimental animal or human subject with alcoholism has no grossly apparent pulmonary dysfunction attributable to the direct effects of alcohol, alveolar epithelial function is, in fact, impaired when examined more closely. Chronic alcohol ingestion increases protein leak across the alveolar epithelial barrier and renders the lung susceptible to injury (5, 6). In addition, chronic alcohol ingestion produces severe oxidative stress in the lung, as best reflected by dramatic decreases in the levels of glutathione (GSH) within the alveolar space of experimental animals and humans (6, 7), a central feature of the alcohol-mediated susceptibility to acute lung injury that we have termed the “alcoholic lung” (8).
Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a redox-sensitive master transcription factor that regulates the activation of the antioxidant response element (ARE), a group of antioxidant gene targets that have a significant role in cellular responses to oxidative stress (9). Nrf2 is a member of the cap’n’collar–basic region/leucine zipper transcription factor family, and is expressed abundantly in many tissues, including the lung (9, 10). Nrf2 induces antioxidant and detoxifying enzymes and proteins by its binding to the ARE, and is regulated via sequestration by Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm (9). When activated, Nrf2 dissociates from Keap1 and moves into the nucleus, where it activates the expression of antioxidant genes (9, 10). Many respiratory diseases, including ARDS, idiopathic pulmonary fibrosis, asthma, emphysema, and cystic fibrosis, are associated with oxidative stress and GSH depletion, and, therefore, Nrf2 has been posited to have a role in their pathogenesis. As evidence in support of this, Nrf2-knockout mice exposed to hyperoxia had enhanced lung permeability, inflammation, and epithelial cell injury and, in parallel, decreased expression of numerous antioxidant enzymes, including glutathione-S-transferase (GST) and reduced nicotinamide adenine dinucleotide phosphate-dehydrogenase-quinone-1 (NQO1) (11).
Therefore, as a fundamental mechanism by which alcohol causes oxidative stress within the airway remained unknown, we investigated the role of the Nrf2–ARE pathway in mediating the pathophysiological effects of alcohol on alveolar epithelial cells (AECs). Using both an established animal model of chronic alcohol ingestion in vivo as well as an AEC line (L2 cells) exposed to alcohol in vitro, we tested the hypothesis that alcohol inhibits the expression and/or activity of Nrf2, thereby dampening the alveolar epithelium’s ability to activate the ARE. In parallel, we determined whether or not increasing the expression and/or activation of Nrf2 pharmacologically could mitigate the detrimental effects of alcohol on the alveolar epithelium, and thereby offer a potential therapy to enhance lung health in these highly vulnerable individuals.
Materials and Methods
Rat Model of Chronic Alcohol Ingestion
Adult male Sprague-Dawley rats (initial weight, ∼250–350 g; Charles River Laboratory, Wilmington, MA) were fed the Lieber-DeCarli liquid diet (Research Diets, New Brunswick, NJ) with and without alcohol (36% of total calories) for 10 weeks. All work was approved by the Emory University Institutional Care and Use of Animals Committee.
AECs
Primary AECs were isolated from rats fed an isocaloric liquid diet with and without alcohol, as previously described (12). The rat alveolar epithelial cell line (L2 cells) was obtained from ATCC (Manassas, VA).
Alcohol Exposure with and without Sulforaphane Treatment In Vitro
L2 cells were exposed to alcohol at concentrations of 0.1% (wt/vol) or 0.2% (wt/vol) for 72 hours. In some experiments, sulforaphane (SPN; 5 μM) was added during the final 24 hours of alcohol exposure. Primary AECs derived from rats fed with alcohol were treated with and without SPN (1 μM) for 5 days before measuring epithelial monolayer barrier function.
Nrf-2 Silenced RNA Transfection
L2 cells or primary AECs from control-fed rats were seeded in 12-well transwell plates and transfected the next day with rat Nrf-2 (Nfe2l2) silencing RNA (siRNA; Invitrogen, Carlsbad, CA) at 5 nM final siRNA concentration, or the same concentration of negative control siRNA (Invitrogen) using HiPerFect (Qiagen, Valencia, CA), as described in the Qiagen protocol. Cells were analyzed 48 hours after transfection for gene expression and 5 days after transfection for epithelial monolayer permeability.
RNA Isolation and Real-Time PCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen) and treated with DNase I (Qiagen) to remove contaminating genomic DNA. Reverse transcription was performed with 0.5 μg of total RNA in a total volume of 10 μl per reaction with Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time PCR was performed using the Bio-Rad iCycler, with amplification reactions performed in 20 μl containing primers at 0.3 μM along with iQ SYBR Green Supermix (Bio-Rad). PCR products from Nrf2, glutathione synthetase (GS), glutamate-cysteine ligase, catalytic subunit (GCLC), GST, and NQO1 were normalized to 9S ribosomal RNA, and expressed relative to expression in untreated control cells.
ELISA-Based Electrophoretic Mobility Shift Assay for Nrf2–DNA Binding
Nuclear protein was isolated and then analyzed by the TransAm Nrf2 assay (Active Motif, Carlsbad, CA), as instructed in the Active Motif protocol, with absorbance measured at 430 nm.
Nrf2–ARE Activity Assay
L2 cells were seeded (10,000/well) in 96-well plates and, the next day, transfected with the Cignal ARE reporter using HiPerFect transfection reagent (Qiagen). Transfected cells were then exposed to alcohol with and without SPN, as described previously here. A dual-luciferase reporter assay was performed as described by the manufacturer (Promega, Madison, WI), and Nrf2–ARE promoter activity values were expressed as ratios of arbitrary units of firefly luciferase/Renilla luciferase activity.
GSH Assay
GSH was measured with the DetectX Glutathione Detection kit (Arbor Assays, Ann Arbor, MI). In each sample, the levels of GSH and “total GSH” (the sum of GSH and GSH disulfide or GSSG, the predominant form of oxidized GSH) were determined.
Measurement of Epithelial Monolayer Barrier Function
Epithelial monolayer barrier function was assessed by quantifying two complementary functions, namely, transepithelial electrical resistance (TER) and FITC–dextran (4 kD) paracellular flux. After epithelial cells were cultured for 6 days on transwell plates, TER was determined using an EVOM V/Ω meter with STX2 electrodes (World Precision Instruments, Sarasota, FL), and FITC–dextran paracellular flux over 2 hours was quantified, as we have previously described (13).
Statistical Analysis
One-way analyses of variance were performed, followed by Student-Newman-Keuls post hoc tests using SigmaStat v2.0 software (Northampton, MA) or student t test by Prism (GraphPad, San Diego, CA). In all cases, data shown represent the mean (±SEM) of three or more determinations. Significance was accepted at P less than 0.05.
Results
Chronic Alcohol Ingestion In Vivo, or Chronic Alcohol Exposure In Vitro, Decreased Nrf2 Gene Expression in AECs
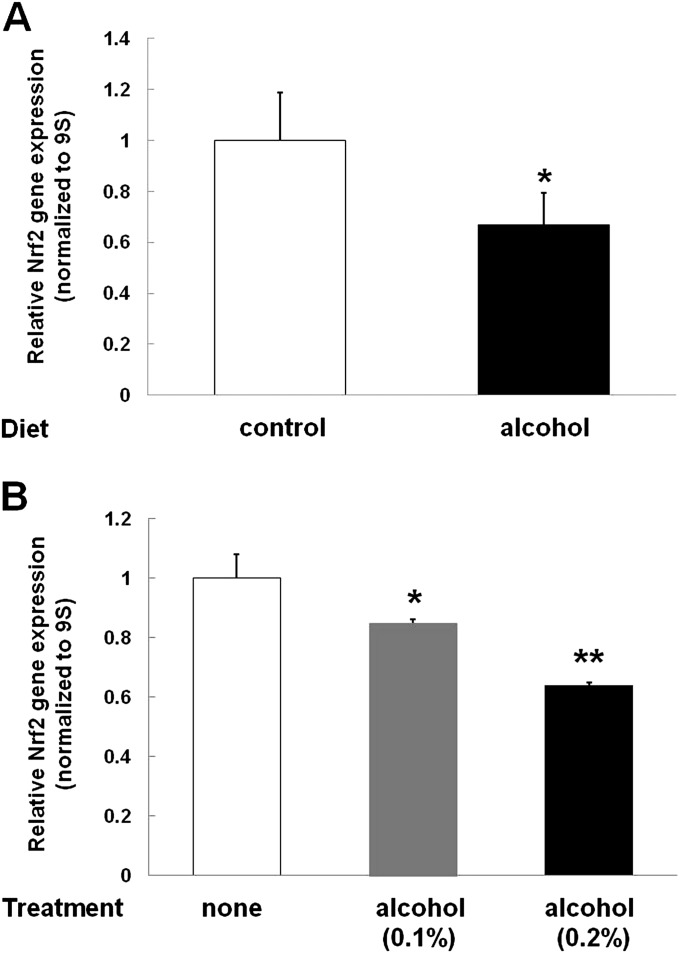
A cardinal feature of the alcoholic lung phenotype is oxidative stress within the alveolar epithelium that causes barrier dysfunction. As shown in Figure 1A, chronic alcohol ingestion significantly (P < 0.05) decreased Nrf2 gene expression, as determined by RT-PCR for Nrf2 mRNA in primary AECs freshly isolated from these animals. In parallel, exposing cultured L2 cells (rat AEC line) to alcohol, either at a concentration of 0.1% (wt/vol) or 0.2% (wt/vol), for 72 hours also significantly (P < 0.05) decreased Nrf2 mRNA expression to 81 and 65%, respectively, of the expression in untreated cells (Figure 1B). As the relative decrease in Nrf2 gene expression in L2 cells induced by an alcohol concentration of 0.2% (wt/vol) was comparable to the degree of inhibition observed in the primary AECs from alcohol-fed rats, this concentration was used for all subsequent experiments in which L2 cells were exposed to alcohol. Of note, a blood alcohol concentration of 0.08% (∼20 mM) is the legal threshold for alcohol intoxication in the United States, and blood alcohol concentrations in the range of 0.2–0.4% are commonly seen in intoxicated individuals.
Figure 1.
Alcohol decreases nuclear factor (erythroid-derived 2)–like 2 (Nrf2) gene expression. (A) Sprague-Dawley rats were fed isocaloric diets ± alcohol for 10 weeks. Type II alveolar epithelial cells (AECs) were isolated and grown in culture for 7 days. RNA was isolated and RT-PCR was then performed for Nrf2 gene expression and normalized to 9S. Control Nrf2 mRNA gene expression was arbitrarily set at 1.0. Nrf2 gene expression was significantly decreased (*P < 0.05) to 67% of control levels. (B) AECs in culture (L2 cells) were treated with alcohol (either 0.1 or 0.2%) for 3 days to simulate chronic alcohol exposure. Alcohol treatment significantly decreased Nrf2 expression in a dose-dependent fashion (*P < 0.05 decreased compared with no treatment and **P < 0.05 decreased compared with treatment with 0.1% alcohol).
In Parallel, Chronic Alcohol Ingestion In Vivo, or Chronic Alcohol Exposure In Vitro, Decreased Gene Expression of the Nrf2–ARE–Regulated Antioxidant Enzymes
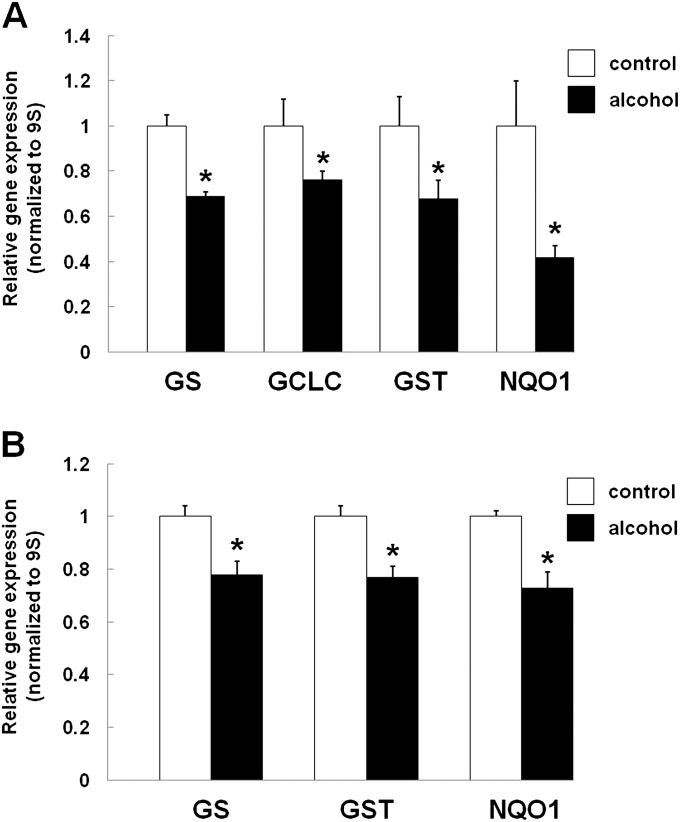
To determine whether or not the alcohol-mediated inhibition of Nrf2 expression, as shown in Figure 1, had any functional consequences on ARE activation, we next quantified the relative expression of GS, GCLC, GST, and NQO1. As shown in Figure 2, GS, GCLC, GST, and NQO1 expression were all significantly (P < 0.05) decreased in primary AECs from alcohol-fed rats (Figure 2A). In parallel, the expression of GS, GST, and NQO1 were likewise significantly (P < 0.05) decreased in alcohol-exposed L2 cells (Figure 2B; GCLC expression was not assessed in the L2 cells).
Figure 2.
Alcohol inhibits the expression of antioxidant response element (ARE)–regulated antioxidants. (A) Sprague-Dawley rats were fed an isocaloric liquid diet ± alcohol for a minimum of 10 weeks. Type II AECs were isolated and grown in culture for 7 days and the relative gene expression of glutathione (GSH) synthetase (GS), glutamate-cysteine ligase, catalytic subunit (GCLC), glutathione-S-transferase (GST), and reduced nicotinamide adenine dinucleotide phosphate-dehydrogenase-quinone-1 (NQO1) quantified by PCR, as described in the Materials and Methods. GS, GCLC, GST, and NQO1 expression were significantly decreased (*P < 0.05) in the AECs from alcohol-fed rats. (B) L2 cells (rat AEC line) were exposed to alcohol (0.2%) for 72 hours and the relative gene expression of GS, GST, and NQO1 quantified by PCR, as described in the Materials and Methods. The expression of GS, GST, and NQO1 were significantly decreased (*P < 0.05) to 77, 77, and 73% of control levels, respectively.
Direct Inhibition of Nrf2 Gene Expression by RNA Interference Reproduced the Effects of Alcohol Exposure in Lung Epithelial Cells
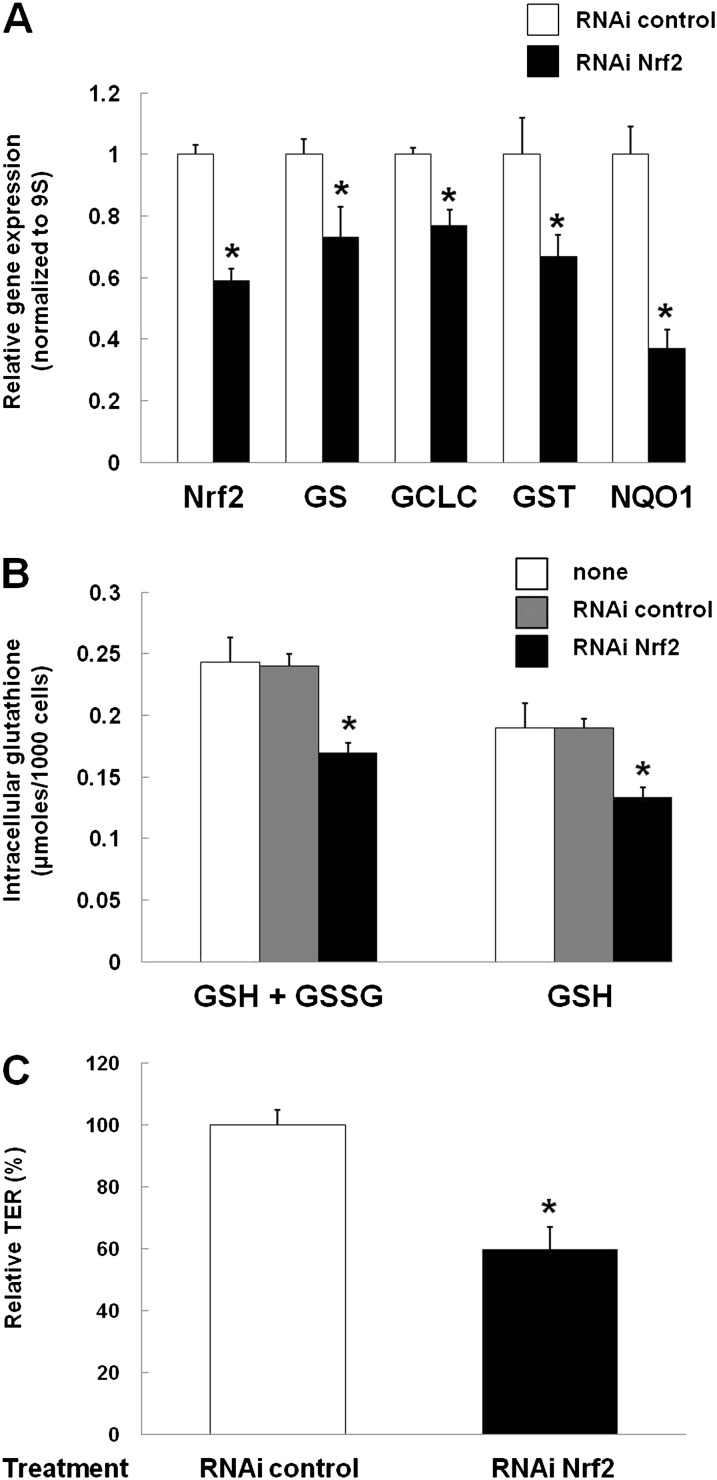
As shown in Figure 3A, we were able to titrate the Nrf2 siRNA such that Nrf2 gene expression was decreased by approximately 50–60% in L2 cells. This degree of inhibition of Nrf2 gene expression was comparable to that caused by alcohol exposure of these cells (as already shown in Figure 1B), and also produced comparable inhibition in the expression of the ARE-regulated gene targets, GS, GCLC, GST, and NQO-1 (Figure 3A). As a reflection of an important functional consequence of these inhibitory effects on Nrf2–ARE–mediated activation of antioxidant defenses, the intracellular levels of GSH were also significantly (P < 0.05) decreased in these cells, as shown in Figure 3B. Furthermore, the barrier function of primary epithelial monolayers in which Nrf2 had been silenced was significantly (P < 0.05) impaired, as reflected by a 40% decrease in the TER (Figure 3D).
Figure 3.
RNA interference (RNAi) of Nrf2 recapitulates the effects of alcohol exposure. (A) L2 cells plated to 50% confluence were treated with short interfering RNA (siRNA; 5 μM) against Nrf2 or a negative control siRNA (CTL) at the same concentration. Nrf2 gene expression, as measured by RT-PCR, was reduced (*P < 0.05) to 42% of CTL RNAi levels. In parallel, expression of the ARE-regulated genes, GS, GCLC, GST, and NQO1, were reduced (*P < 0.05) to 59, 73, 77, 67, and 37% of control levels, respectively; the effects of Nrf2 RNAi on the expression of Nrf2, GST, and NQO1 were comparable to the effects of alcohol presented in Figures 1 and 2. (B) Nrf2 RNAi treatment also decreased (*P < 0.05) the intracellular GSH pools in the L2 cells. (C) Nrf2 RNAi treatment also decreased epithelial monolayer barrier function, as reflected by a significantly (P < 0.05) decreased transepithelial electrical resistance (TER).
Treatment with SPN Restored Nrf2 Expression, DNA Binding, and Activation of the ARE, and, in Parallel, Restored the Intracellular GSH Pools in L2 Epithelial Cells Exposed to Alcohol
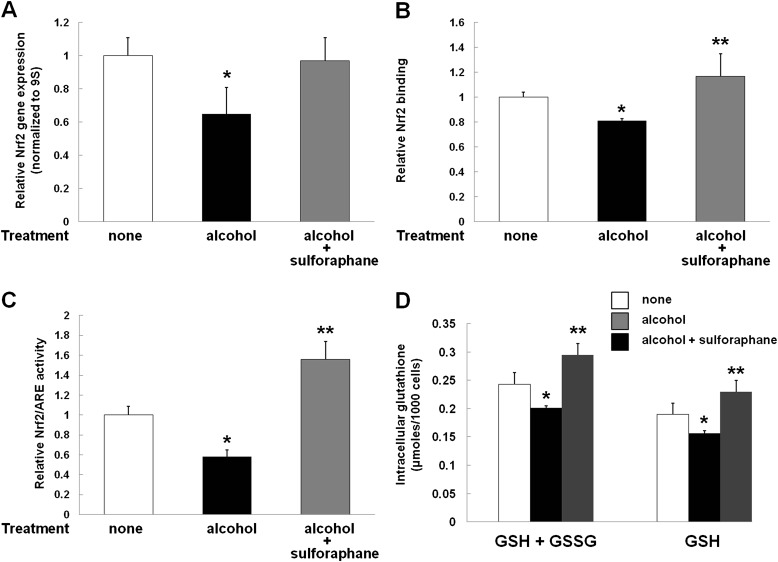
SPN, a naturally occurring compound found in cruciferous vegetables, has been shown to increase the expression and activation of Nrf2. Therefore, we treated alcohol-exposed L2 cells with SPN, as described in the Materials and Methods. As shown in Figure 4, SPN treatment restored Nrf2 gene expression (Figure 4A) and, in parallel, increased relative Nrf2–DNA binding (Figure 4B) and activation of the ARE (Figure 4C). Consistent with multiple previous studies by our research group, alcohol exposure significantly (P < 0.05) decreased the intracellular GSH pools in L2 epithelial cells, as shown in Figure 4D. In contrast, treatment with SPN actually increased these intracellular GSH pools to levels that were significantly (P < 0.05), albeit modestly, higher than those in the untreated control cells (Figure 4D). Taken together, the results shown in Figure 4 show that SPN can reverse the inhibitory effects of alcohol on the Nrf2–ARE signaling pathway and the subsequent oxidative stress and GSH depletion within the alveolar epithelium that produce the “alcoholic lung” phenotype.
Figure 4.
The Nrf2 activator, sulforaphane (SPN), restored Nrf2 expression, activation of the ARE, and the intracellular GSH pool in alcohol-exposed cells. (A) As in the experiments shown in Figure 1B, alcohol exposure of L2 cells significantly decreased (*P < 0.05) Nrf2 gene expression. In contrast, concomitant treatment with SPN restored Nrf2 gene expression in alcohol-exposed cells to the same levels (P > 0.05) as those in untreated control cells. (B) Alcohol exposure of L2 cells significantly decreased (*P < 0.05) Nrf2 DNA binding to and activation of the ARE to 80% of control levels; in contrast, SPN treatment increased Nrf2 binding in alcohol-treated cells to levels that were slightly greater (116%; **P < 0.05) than in untreated control cells. (C) Alcohol exposure of L2 cells significantly decreased (*P < 0.05) Nrf2–ARE activity to 56% of the activity in untreated cells; in contrast, concomitant treatment with SPN actually increased Nrf2–ARE activity to levels that were 158% of the levels in untreated control cells (**P < 0.05). (D) Intracellular “total GSH” (GSH + GSSG) and GSH, as measured using a colorimetric assay (see Materials and Methods), in L2 AECs treated with alcohol ± SPN. Alcohol treatment significantly decreased (*P < 0.05) both total GSH and GSH intracellular levels, whereas treatment with SPN increased (**P < 0.05) GSH levels in alcohol-treated cells to levels that were approximately 25% higher than those in untreated control cells.
In Parallel to Restoring Nrf2–ARE Signaling in L2 Cells Exposed to Alcohol, SPN Treatment Reversed Alcohol-Induced Epithelial Barrier Dysfunction
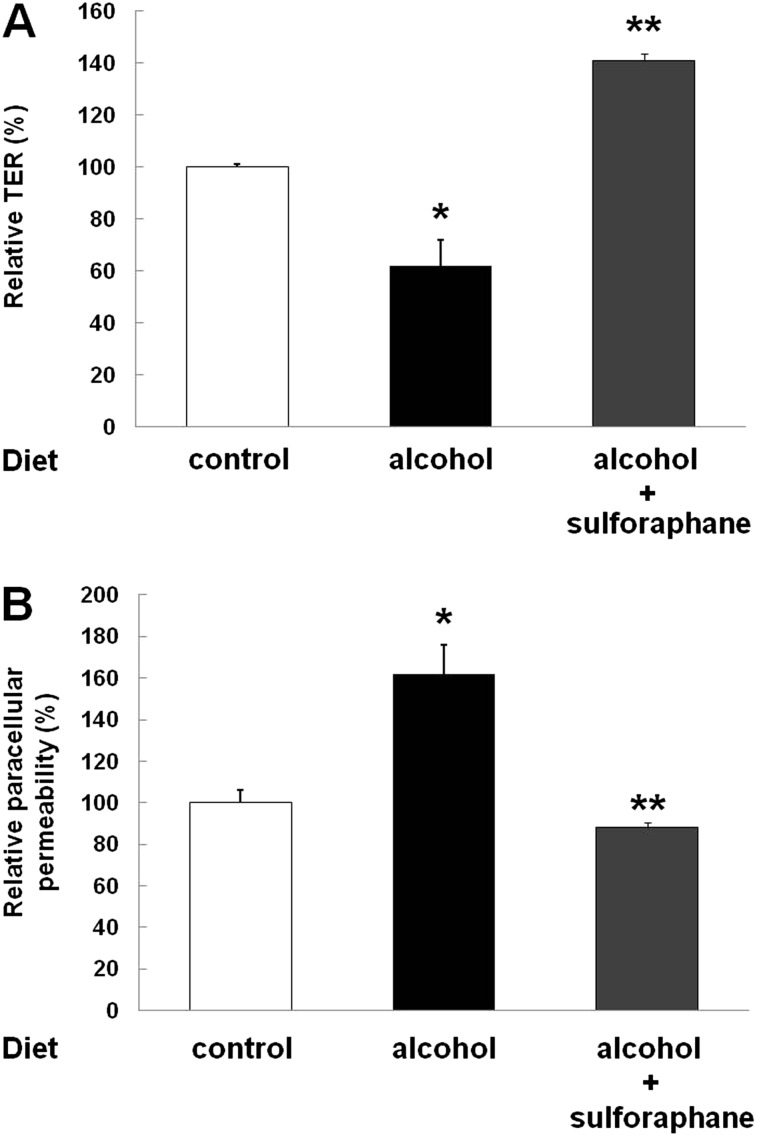
Alveolar epithelial monolayers derived from primary AECs isolated from alcohol-fed rats had impaired barrier integrity, as reflected by decreased TER (Figure 5A) and increased paracellular flux of FITC-labeled dextran (Figure 5B); these findings are consistent with those in our previous report (5). In contrast, SPN treatment reversed these effects of chronic alcohol ingestion, and even increased alveolar epithelial barrier function, as reflected both by increasing the TER to 141% (Figure 5A) and decreasing FITC–dextran flux to 88% (Figure 5B) of that in monolayers derived from control-fed rats, respectively.
Figure 5.
SPN treatment reversed alcohol-induced epithelial barrier dysfunction. Primary AECs were isolated from alcohol-fed rats and cultured for 5 days ± SPN (1 μl). Consistent with our previously published studies, alveolar epithelial monolayers derived from alcohol-fed rats had decreased barrier function, as reflected by decreased TER (A) and increased paracellular flux of FITC–dextran (B) compared with monolayers derived from control-fed rats (*P < 0.05 in each case). In contrast, SPN treatment reversed these effects of chronic alcohol ingestion, and even increased alveolar epithelial barrier function, as reflected both by increasing the TER to 141% (A) and decreasing FITC–dextran flux to 88% (B) of that in monolayers derived from control-fed rats, respectively (**P < 0.05 in each case).
Discussion
In light of the rapidly growing evidence that alterations in Nrf2 activity may contribute to a wide range of lung pathologies, including acute lung injury (9–11, 14–16), we sought to determine a role for the Nrf2–ARE pathway in the oxidative stress and alveolar epithelial barrier dysfunction caused by chronic alcohol ingestion that we first identified more than 15 years ago (6). In this study, we determined that chronic alcohol ingestion in vivo, as well as alcohol exposure in vitro, significantly decreased expression of Nrf2, the master transcription factor that is required to activate ARE, in the lung epithelium. In parallel, and consistent with the functional role of Nrf2, alcohol inhibited the expression of the ARE-regulated antioxidants, GS, GCLC, GST, and NQO1. Furthermore, this alcohol-mediated inhibition of GS, GCLC, GST, and NQO1 expression could be reproduced by directly inhibiting Nrf2 expression by RNA silencing, which consequently compromised epithelial barrier function. In contrast, treating alcohol-exposed lung epithelial cells with SPN, which is known to increase Nrf2 expression and/or activation, restored Nrf2 expression and DNA binding and, in parallel, activated the ARE and normalized intracellular GSH levels. More importantly, SPN treatment reversed alcohol-induced impairment of barrier function in alveolar epithelial monolayers derived from alcohol-fed rats. Taken together, these results provide compelling evidence that alcohol inhibits the Nrf2–ARE pathway and thereby induces oxidative stress and consequent AEC dysfunction. In addition, the salutary effects of SPN in restoring signaling through this critical antioxidant defense pathway raise the intriguing possibility that Nrf2 activators could enhance lung health in individuals with alcohol-use disorders and reduce their susceptibility to acute lung injury.
The first and perhaps most fundamental observation is that alcohol somehow inhibits the expression of Nrf2 in the lung epithelium. This appears to be a relatively direct effect that does not require hepatic metabolism of alcohol and/or other systemic effects, as Nrf2 was equally inhibited in the AEC line by direct exposure to alcohol as in the alveolar epithelium of alcohol-fed rats. We then determined that the gene expression of antioxidant enzymes that are activated by the Nrf2–ARE pathway—specifically, GS, GCLC, GST, and NQO1—were also diminished, indicating that the inhibition of Nrf2 had downstream functional consequences on cellular antioxidant defenses. In parallel, selective silencing of Nrf2 gene expression to levels that were comparable to those in alcohol-treated cells recapitulated the inhibition of GS, GCLC, GST, and NQO1, and significantly decreased intracellular GSH levels. Overall, these are remarkable findings in that they alter our understanding of how alcohol causes such profound oxidative stress within the lower airway. Specifically, it had seemed paradoxical that alcohol abuse causes such profound GSH depletion within the alveolar space, whereas cigarette smoking, which one would expect to be a much greater oxidative stress in the airways, actually increases alveolar GSH levels (7). Although we have previously identified that chronic alcohol ingestion induces the expression of NADPH oxidase in the lung and could thereby increase the local production of reactive oxygen species (17), it remained perplexing that this chronic low-level oxidative stress did not induce a robust antioxidant response, as is seen in the airways of smokers. The current study provides compelling and provocative evidence that alcohol somehow dampens the antioxidant response at the level of its “master switch,” and thereby may induce oxidative stress, not primarily by increasing reactive oxygen species production, but rather by inhibiting the production of antioxidant defenses, including GSH. At present, we can only speculate as to the mechanism(s) by which alcohol inhibits Nrf2 expression and activation of the ARE. We do know that it has protean effects, including inducing transforming growth factor-β1 expression in the lung through the actions of angiotensin II (18, 19), and inhibits the transport of zinc, an important factor in the regulation of gene expression, into the alveolar space (20). However, further investigation will be necessary to determine if alcohol acts through one or more such intermediates, which we believe is likely, or if the alcohol molecule itself has direct effects on Nrf2 expression.
Conversely, this study provides evidence that restoring Nrf2 expression, even in the presence of ongoing alcohol exposure, can restore activation of the ARE and intracellular GSH pools in the alveolar epithelium. We initially attempted to overexpress Nrf2 in alcohol-exposed cells by transfecting them with an Nrf2–cytomegalovirus expression vector, but we could not achieve consistent results with this technique. Therefore, we used SPN, as it is one of several phytochemicals that have been shown to activate Nrf2 (21). Interestingly, SPN treatment not only increased Nrf2 binding to DNA and activation of the ARE, as would be predicted by its putative mechanisms of action (21), but it also increased Nrf2 gene expression in alcohol-exposed cells. It is still not clear how SPN stimulates and/or activates the Nrf2–ARE pathway. It has been reported that SPN interacts with cysteines in Keap1, and consequently disrupts Keap1 ubiquitination and degradation of Nrf2 (22). Therefore, it appears that, at least within this context, SPN either has additional effects on Nrf2 expression or that its ability to stabilize Nrf2 promotes a feedback mechanism to induce the expression of more Nrf2. Either way, the net result of SPN treatment was complete restoration of the intracellular GSH pools, which we have previously determined translates to normalization of critical cellular alveolar epithelial functions, including surfactant secretion (23), resistance to apoptosis and necrosis (24), and barrier formation (5).
This study raises the provocative possibility that pharmacological activation of Nrf2, using phytochemicals, such as Nrf2, or the more recently developed composition of synergistic activators, known as Protandim (9), could enhance the antioxidant defenses in the alcoholic lung in a more broad-based and effective manner than could be achieved with selected supplementation with GSH precursors alone. Specifically, although such a strategy is effective in preventing alcohol-mediated lung dysfunction in animal models (6, 25), in the more complex and often acute clinical settings the ability to rapidly augment the proximal Nrf2–ARE signaling pathway and its subsequent induction of hundreds of genes that have antioxidant as well as immune functions is likely to be more effective. Alternatively, such a strategy could complement the effects of GSH precursors and/or the benefits of dietary zinc supplementation, which we recently determined increases Nrf2 nuclear binding in the alveolar macrophages of alcohol-fed rats and restores their ability to clear a lung bacterial challenge in vivo (26). Although, clearly, these and other experimental findings must be translated and tested in the clinical situation, our study provides further evidence that the “alcoholic lung” may be amenable to relatively simple dietary supplementation that can augment antioxidant and immune functions and thereby limit the risk of pneumonia, acute lung injury, and perhaps other serious diseases.
In summary, we provide novel evidence that alveolar epithelial oxidative stress and GSH depletion, which are cardinal features of the alcoholic lung phenotype that confers increased susceptibility to acute lung injury, is mediated by inhibition of the Nrf2–ARE signaling pathway. Therefore, although we had previously identified that chronic alcohol ingestion perturbs multiple homeostatic mechanisms within the lung that appear to exert oxidative stress by increasing the production of reactive oxygen species, the profound redox imbalance in the alcoholic lung airway also involves a targeted lowering of its antioxidant defenses. These observations help explain why alcohol abuse causes almost complete depletion of the GSH pool within the alveolar space, whereas a seemingly greater airway oxidative stress (i.e., cigarette smoking) induces a compensatory and substantial increase in the same GSH pool. Furthermore, these observations suggest a new therapeutic opportunity in that dietary supplementation with phytochemicals, such as SPN, may be able to augment or even restore the Nrf2–ARE pathway, and thereby replenish the antioxidant defenses in the lungs of individuals with alcohol use disorders. Specifically, although abstinence is clearly the primary goal for such individuals, treatments that enhance their overall health could decrease their tragically high morbidity and mortality from acute lung injury, pneumonia, and possibly a wide range of other complications.
Footnotes
This work was supported by Emory Alcohol and Lung Biology Center and Training Program grants P50 AA 013757 and T32 AA 013528, and by a Veterans Affairs Merit Review Award.
Originally Published in Press as DOI: 10.1165/rcmb.2012-0334OC on January 10, 2013
References
- 1.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996;275:50–54 [PubMed] [Google Scholar]
- 2.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877 [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693 [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319–3234143721 [Google Scholar]
- 5.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 2000;279:L127–L135 [DOI] [PubMed] [Google Scholar]
- 6.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moss M, Guidot DM, Wong-Lambertina M, Ten HT, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000;161:414–419 [DOI] [PubMed] [Google Scholar]
- 8.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol 2007;292:L813–L823 [DOI] [PubMed] [Google Scholar]
- 9.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 2011;32:234–246 [DOI] [PubMed] [Google Scholar]
- 10.Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal 2006;8:76–87 [DOI] [PubMed] [Google Scholar]
- 11.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, Kleeberger SR. Role of Nrf2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol 2002;26:175–182 [DOI] [PubMed] [Google Scholar]
- 12.Fernandez AL, Koval M, Fan X, Guidot DM. Chronic alcohol ingestion alters claudin expression in the alveolar epithelium of rats. Alcohol 2007;41:371–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Joshi PC, Koval M, Guidot DM. Chronic alcohol ingestion exacerbates lung epithelial barrier dysfunction in HIV-1 transgenic rats. Alcohol Clin Exp Res 2011;35:1866–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HY, Kleeberger SR. Nrf2 protects against airway disorders. Toxicol Appl Pharmacol 2010;244:43–56 [DOI] [PubMed] [Google Scholar]
- 15.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor Nrf2 protects against pulmonary fibrosis. FASEB J 2004;18:1258–1260 [DOI] [PubMed] [Google Scholar]
- 16.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN, Aplenc R, Yamamoto T, Yamamoto M, Cho HY, et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 2007;21:2237–2246 [DOI] [PubMed] [Google Scholar]
- 17.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol 2006;34:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, Raynor R, Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2005;289:L363–L370 [DOI] [PubMed] [Google Scholar]
- 19.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor β1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 2004;170:188–194 [DOI] [PubMed] [Google Scholar]
- 20.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol 2009;41:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Paonessa JD, Zhang Y. Mechanism of chemical activation of Nrf2. PLoS ONE 2012;7:e35122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, Kensler TW. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat 2012;132:175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res 2000;24:1070–1076 [PubMed] [Google Scholar]
- 24.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator–induced apoptosis. Alcohol Clin Exp Res 2001;25:1078–1085 [PubMed] [Google Scholar]
- 25.Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res 2002;26:1245–1251 [DOI] [PubMed] [Google Scholar]
- 26.Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, Guidot DM. Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res 2011;35:1519–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author disclosures