Abstract
Immunodeficient mice have been used as recipients of human peripheral blood mononuclear cells (PBMC) for in vivo analyses of human xeno-graft-versus-host disease (GVHD). This xeno-GVHD model system in many ways mimics the human disease. The model system is established by intravenous or intraperitoneal injection of human PBMC or spleen cells into unconditioned or irradiated immunodeficient recipient mice. Recently, the development of several stocks of immunodeficient Prkdcscid (scid), or recombination activating 1 or 2 gene (Rag1 or Rag2) knockout mice bearing a targeted mutation in the gene encoding the IL2 receptor gamma chain (IL2rγ) have been reported. The addition of the mutated IL2rγ gene onto an immunodeficient mouse stock facilitates heightened engraftment with human PBMC. Stocks of mice with mutations in the IL2rγ gene have been studied in several laboratories on NOD-scid, NOD-Rag1null, BALB/c-Rag1null, BALB/c-Rag2null, and Stock-H2d-Rag2null strain backgrounds. Parameters to induce human xeno-GVHD in H2d-Rag2null IL2rγnull mice have been published, but variability in the frequency of disease and kinetics of GVHD were observed. The availability of the NOD-scid IL2rγnull stock that engrafts more readily with human PBMC than does the Stock-H2d-Rag2null IL2rγnull stock should lead to a more reproducible humanized mouse model of GVHD and for the use in drug evaluation and validation. Furthermore, GVHD in human PBMC-engrafted scid mice has been postulated to result predominately from a human anti-mouse major histocompatibility complex (MHC) class II reactivity. Our recent development of NOD-scid IL2rγnull β2mnull and NOD-scid IL2rγnull Abnull stocks of mice now make it possible to investigate directly the role of host MHC class I and class II in the pathogenesis of GVHD in humanized mice using NOD-scid IL2rγnull stocks that engraft at high levels with human PBMC and are deficient in murine MHC class I, class II, or both classes of MHC molecules.
Keywords: scid, Rag, humanized mice, GVHD, immunodeficient mice, MHC class I, MHC class II
INTRODUCTION
1.0 Hematopoietic Stem Cell Transplantation and Graft-versus-Host Disease
Hematopoietic stem cell (HSC) transplantation has the potential to be curative for a variety of human diseases. These include diseases of the blood, metabolic disorders, autoimmune diseases, and solid tumors (1-5). Of particular importance is the use of HSC transplantation for the treatment of hematological malignancies (6-8). In this setting, the beneficial graft-versus-leukemia (GVL) effects versus the detrimental GVHD effects are commonly intertwined (9). The beneficial effects of GVL appear to correlate directly with the severity of chronic GVHD and these two phenomena are difficult to study as independent phenomena in patients (10-12). A small animal model of human immunity that accurately mimics the clinical presentation of human GVHD would provide a model for the studies of GVL as well as GVHD and for testing the efficacy of human-specific therapeutics prior to their use in the clinic. Such a model system would also provide a tool for investigating the underlying mechanisms involved in the pathogenesis of human GVHD and to discover ways to dissociate GVHD from GVL effects.
1.1 Immunodeficient Mice Engrafted with Human Lymphohematopoietic Cells
The first description of engrafting immunodeficient mice with human PBMC was reported in 1988 (13). In those studies, CB17 mice harboring the Prkdcscid (scid) mutation were injected intraperitoneally with 10-100×106 human PBMC, and low levels of human PBMC engraftment were observed. However, instead of developing GVHD, these mice developed EBV-associated lymphomas (14). Most of the human T cells that did engraft became anergic (15, 16) and few engrafted mice developed GVHD (17). Over the ensuing two decades, genetically altered immunodeficient mice were found to support heightened engraftment with human lymphohematopoietic cells, with a major advance being the generation of NOD-scid mice (18). Additional hereditable modifications included the generation of NOD-scid mice genetically deficient in beta-2 microglobulin (β2mtm1Unc, abbreviated as β2mnull) (19). In the absence of β2m, natural killer (NK) cell numbers and activity are severely depressed due to the lack of MHC class I expression (19). This removed a major impediment to human lymphohematopoietic cell engraftment. Although NOD-scid β2mnull mice support higher levels of human PBMC engraftment than do CB17-scid or NOD-scid mice (19), development of symptoms of GVHD remained variable (20). In this and other model systems developed during this period, the low frequency and poor engraftment observed, and the high numbers of human PBMC required for engraftment and disease expression, precluded widespread use of these mice for studies of xeno-GVHD.
1.2 Immunodeficient Stocks of IL2rγnull Mice
A major breakthrough in modeling human immunity and human xeno-GVHD reactivity in immunodeficient mice followed the development by several research groups of immunodeficient mice bearing a targeted mutation in the gene encoding the IL2 receptor gamma chain (IL2rg), abbreviated as (IL2rγ) (21). The IL2 receptor gamma chain is important for high affinity signaling through the IL2, IL4, IL7, IL9, IL15, and IL21 cytokine receptors. Without these receptor signals, both adaptive and innate immunity are severely impaired (22). We and others are now building on the foundation of immunodeficientIL2rγnull mice that are available to knockout additional genes and to introduce new genes that will facilitate the engraftment of functional human immune systems. On the BALB/c strain background, this is being pursued using BALB/c embryonic stem cells to genetically manipulate the genome. For example, knocking in human genes into the BALB/c ES cells can rapidly introduce human genes of particular importance for the enhancement of human hematolymphopoietic cell engraftment without requiring 2 or more years of backcrossing the mutated genes made in B6/129 ES cells onto the BALB/c strain background. Development of NOD ES cells, currently underway in a number of laboratories, when available will provide an important tool for rapidly manipulating the NOD genome, facilitating the development of new stocks of NOD-scid IL2rγnull mice for human cell engraftment.
Using a Stock-H2d mouse strain bearing both the Rag2null and IL2rγnull targeted mutations, it was shown that there was improved engraftment of human PBMC as compared with NOD-scid mice. Unfortunately, even extensive preconditioning of the Stock-H2d-Rag2null IL2rγnull recipient with total body irradiation and macrophage depletion using chlodronate-containing liposomes did not lead to reproducible development of GVHD symptoms in all of the mice (23).
1.3 Engraftment of NOD-scid IL2rγnull Mice with Human PBMC
The independent development of NOD-scid mice bearing a targeted mutation in the IL2rγ gene was reported by two separate groups. The laboratory of Ito and colleagues developed a stock bearing a truncated IL2rγ (Il2rgtm1Sug, abbreviated as IL2rγtrunc) gene (24). We have developed a stock bearing a complete null IL2rγ mutation (Il2rgtm1Wjll, abbreviated as IL2rγnull) (25). We have recently described the parameters of human PBMC engraftment into unconditioned NOD-scid IL2rγnull mice (26). We observed that injection of as few as 1-5×106 human PBMC led to engraftment, and that all mice were successfully engrafted when injected with 10×106 or more human PBMC. At about 30-40 days after PBMC engraftment, we consistently observed that all mice were losing weight and developing symptoms of GVHD (26). Our observations here are consistent with the recent report of the gradual development of phenotypic changes resembling GVHD over the course of 4 to 8 weeks observed following injection of 10×106 human PBMC into unconditioned NOD-scid IL2rγnull mice (27). These data suggest that heightened engraftment of functional human PBMC in NOD-scid IL2rγnull mice, particularly that of CD8+ T cells, leads to the development of xenoreactivity, aggression against host tissues, and development of GVHD.
More recently, we have shown that the NOD-scid IL2rγnull mouse model supports higher levels of human hematolymphoid engraftment than does the CBy.Cg-Rag1tm1Mom Il2rgtm1wjl/Sz (abbreviated as BALB/c-Rag1null IL2rγnull) stock of mice (28). We have subsequently shown that NOD.129S7(B6)-Rag1tm1Mom Il2rgtm1Wjll (abbreviated as NOD-Rag1null IL2rγnull) mice also engraft with human PBMC at high levels that are comparable to those observed in NOD-scid IL2rγnull mice (29). These latter data document that the difference in human PBMC engraftment in NOD-scid IL2rγnull and BALB/c.129P2-Rag2tm1Mnz (abbreviated as BALB/c-Rag2null IL2rγnull) mice is not due to the effects of the scid vs. Rag1null or Rag2null genes but rather to other polymorphic background modifiers. One of these important background modifiers has been reported to be the Sirpa (also termed Shps-1 and Ptpns1) gene. The SIRP-α protein is a potent regulator of interactions between hematopoietic cells and the bone marrow microenvironment. The NOD SIRP-α protein is more similar to the human SIRP-α protein than that of the C57BL/6 SIRP-α protein, shows enhanced binding to the human CD47 ligand, and it's expression on mouse macrophages leads to better support of human hematopoiesis (30).
2.0 Role of Mouse MHC Class I and Class II in GVHD Following Engraftment of NOD-scid IL2rγnull Mice with Human PBMC
It has been suggested that mismatches at the human HLA class I loci are the most important predictor of human GVHD following hematopoietic stem cell transplantation (9). In humanized mice, there is contradictory evidence on the role of MHC class I and class II in the pathogenesis of GVHD. Human CD4 T cell clones derived from CB17-scid mice engrafted with PBMC exhibited reactivity against host MHC class II (15). However, the predominant human cell population engrafting in CB17-scid mice was the CD8 T cell population (31), which would more likely be driven to proliferate by mouse MHC class I rather than MHC class II. To re-examine the role of mouse MHC class I and II in the engraftment and development of xeno-GVHD following human PBMC engraftment, we developed two new stocks of NOD-scid IL2rγnull mice.
2.1 Development of NOD-scid IL2rγnull Mice That Are Also Deficient in Mouse MHC Class I or MHC Class II
The development of the NOD.Cg-B2mtm1Unc PrkdcscidIl2rgtm1Wjl/Sz (abbreviated as NOD-scid IL2rγnullβ2mnull) genetic stock was initiated by crossing NOD-scid/scid IL2rγnull females with NOD-scid/scid β2mnull males. The NOD-scid IL2rγnull/Y +/β2mnull F1 males were backcrossed to NOD-scid/scid IL2rγnull females. The NOD-scid/scid IL2rγnull +/β2mnull female offspring were mated with their NOD-scid/scid IL2rγnull/ Y +/β2mnull sibs to produce homozygous mice at each of the mutated genes. The homozygous NOD-scid IL2rγnull B2mnull offspring were then intercrossed to establish and maintain this strain.
The development of the NOD.Cg-PrkdcscidH2-Ab1tm1Gru Il2rgtm1Wjl/Sz (abbreviated as NOD-scid IL2rγnull Abnull) genetic stock was initiated by first crossing NOD-scid/scid IL2rγnull females with NOD-scid/scid Abnull males. The NOD-scid/scid IL2rγnull/Y +/Abnull F1 males were backcrossed to NOD-scid/scid IL2rγnull females. The NOD-scid/scid IL2rγnull +/Abnull female offspring were mated with their NOD-scid/scid IL2rγnull/ Y +/Abnull sibs to produce homozygous mice at each of the mutated genes. The homozygous NOD-scid IL2rγnull Abnull offspring were intercrossed to establish and maintain this strain. Both NOD and 129 strains of mice have a genetic deficiency in I-Eα expression. The crossing of the Abnull allele, made using 129 strain ES cells, onto the NOD strain background therefore results in a deficiency of both I-A and I-E expression and complete lack of MHC class II expression.
2.2 Phenotype of Cells Developing in NOD-scid IL2rγnull Mice Deficient in Mouse MHC Class I or MHC Class II
We have previously shown that NOD-scid IL2rγnull mice lack NK cells, mature T lymphocytes and mature B lymphocytes are excellent recipients of human PBMC (26). To determine if deletion of either the mouse MHC class I or class II altered the phenotype of the murine cell subsets in NOD-scid IL2rγnull mice, we performed flow cytometry analyses. As shown in Tables 1 and 2, and Figure 1, Panels A and B, there were no MHC class I positive cells in the bone marrow and spleens of NOD-scid IL2rγnull β2mnull mice. Similarly, there were no MHC class II positive cells in the bone marrow and spleens of NOD-scid IL2rγnull Abnull mice. We also confirmed the absence of mature mouse CD4 and CD8 T cells, mature cell-surface Ig-positive B cells, and LGL+/CD122+ NK cells in the spleens and bone marrow of NOD-scid IL2rγnull β2mnull and NOD-scid IL2rγnull Abnull mice. NOD-scid IL2rγnull β2mnull mice also exhibited decreased percentages of splenic Gr-1+/Mac1+ granulocytes, Gr-1-/Mac1+ macrophages, and Ter-119+ erythroid cells compared with NOD-scid IL2rγnull mice. There were also reduced percentages of Ter-119+ erythroid cells in the bone marrow of NOD-scid IL2rγnull β2mnull mice compared with NOD-scid IL2rγnull mice.
Table 1.
Flow cytometry analyses of spleen cells from NOD-scid IL2rgnul1 β2Mnull and NOD-scid IL2rgnul1 Abnul1 mice showing percentages of leukocyte populationsa
| Spleen Surface Marker | NOD +/+ (N=1) | NOD-scid IL2rgnul1 (N=5) | NOD-scid IL2rgnul1 p2M"ul1 (N=6) | NOD scid IL2rgnul1 Abnul1 (N=5) |
|---|---|---|---|---|
| CD3+/CD4+ | 26.1 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| CD3+/CD8+ | 10.6 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| B220+/IgK, light chain+ | 45.0 | 0.2±0.1 | 0.0±0.0 | 0.0±0.0 |
| B220+/IgK, light chain- | 1.3 | 3.1±0.7 | 0.9±0.2 | 1.6±0.5 |
| GR-1+/Mac-1+ | 6.3 | 17.3±1.0 | 10.9±2.0* | 21.1±0.9 |
| GR-1-/Mac-1+ | 5.1 | 9.6±0.9 | 5.9±0.4* | 11.0±1.2 |
| Ter 119+ | 2.8 | 45.4±2.6 | 25±1.8* | 44.3±1.7 |
| DX5+/CD122+ | 4.2 | 1.3±0.1 | 1.2±0.1 | 1.5±0.1 |
| LGL+/CD122+ | 0.7 | 0.1±0.0 | 0.3±0.1 | 0.1±0.0 |
| H-2Kd+ | 94.2 | 65.3±2.2 | 1.0±0.1** | 0.2+0.0** |
| H-2Db+ | 83.6 | 28.8±3.5 | N/A | 36.8±1.6 |
| I-Ag7+ | 50.9 | 3.6±0.4 | 6.4±0.0 | 0.0±0.0** |
Data are expressed as mean ± SE
p<0.05 NOD-scidIL2rgnul1 β2MTnul1 or NOD scidIL2rgnul1 Abnull mice versus NOD-scidIL2rgnull
>p<0.001 NOD-scidIL2rgnull β2Mnull or NOD scidIL2rgnull Abnul1 mice versus NOD-scidIL2rgnull
Table 2.
Flow cytometry analyses of bone marrow cells from NOD-scid IL2rgnul1 μ2Mnul1 and NOD-scid IL2rgnul1 Abnul1 mice showing percentages of leukocyte populationsa
| Bone Marrow Surface Marker | NOD +/+ (N=1) | NOD-scid IL2rgnull (N=5) | NOD-scid IL2rgnuU β2Muull (N=6) | NOD scid IL2rgnull Abnull (N=5) |
|---|---|---|---|---|
| CD3+/CD4+ | 0.5 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| CD3+/CD8+ | 0.5 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| B220+/IgK, light chain+ | 5.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 |
| B220+/IgK, light chain- | 14.9 | 1.6±0.1 | 0.8±0.0 | 1.3±0.2 |
| GR-1+/Mac-1+ | 48.8 | 57.9±0.3 | 67.7±1.4 | 52.1±2.8 |
| GR-1-/Mac-1+ | 6.9 | 9.6±0.4 | 5.9±0.4 | 9.2±0.3 |
| Ter 119+ | 10.4 | 14.7±0.5 | 6.5±0.7* | 18.3±1.0 |
| DX5+/CD122+ | 1.8 | 1.9±0.1 | 1.3±0.0 | 1.4±0.1 |
| LGL+/CD122+ | 0.41 | 0.2±0.0 | 0.1±0.0 | 0.2±0.0 |
| H-2Kd+ | 92.8 | 85.8±2.0 | 0.3±0.0** | 0.3±0.1** |
| H-2Db+ | 68 | 72.2±0.6 | N/A | 69.2±1.2 |
| I-Ag7+ | 12.6 | 2.9±0.2 | 4.3±0.6 | 0.3±0.0** |
Data are expressed as mean ± SE
p<0.05 NOD-scid IL2rgnul1(32Mfull or NOD scidIL2rgnull Abnull mice versus NOD-scid IL2rgnull
p<0.001 NOD-scidIL2rgnullji2Mnull or NOD scidIL2rgnull Abnull mice versus NOD-scidIL2rgnull
Fig. 1A and Fig 1B.
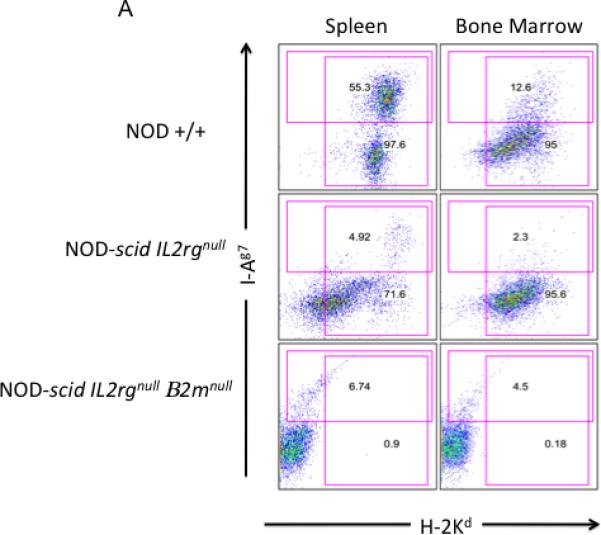
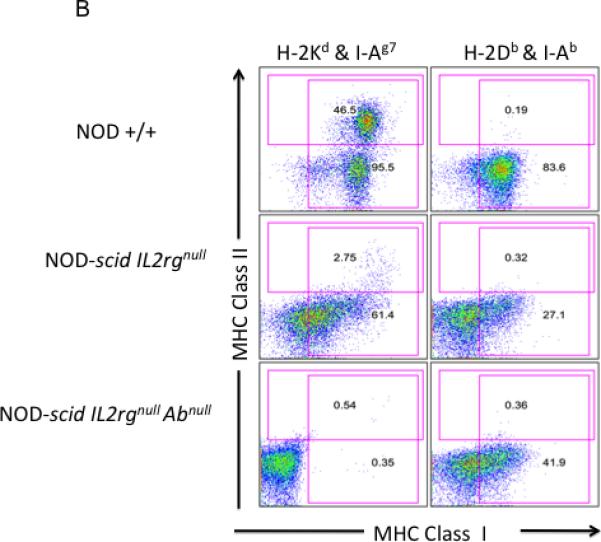
Flow cytometric analysis of MHC class I and II expression. Panel A. Analysis of MHC class I (H-2 Kd) and MHC class II (I-Ag7) in spleen and bone marrow cells. While nearly all NOD +/+ splenocytes and bone marrow cells express MHC class I (H-2 Kd), <1% of NOD-scid IL2rγnull β2mnull bone marrow cells or splenocytes are MHC class I positive. Approximately 50% of NOD +/+ splenocytes and 13% of NOD +/+ bone marrow cells express MHC class II. NOD-scid IL2rγnull as well as NOD-scid IL2rγnull β2mnull mice have reduced percentages of MHC class II positive cells, consistent with a lack of mature B cells. Panel B. Analysis of MHC class I (H-2 Kd, NOD-derived and H-2Db, C57BL/6-derived) and MHC class II (IAg7, NOD-derived and I-Ab, C57BL/6-derived) expression in splenic cells. Different sets of antibodies were used because genetic backcrossing of the MHC class II targeted mutation in 129 ES cells crossed onto the C57BL/6 J strain and subsequently crossed onto the NOD strain results in the crossing of the entire MHC region onto NOD. Thus, NOD MHC class II (I-A)null mice express the 129/C57BL/6 class I haplotype (H-2b) and fail to express either the NOD MHC class I (H-2 Kd) or the NOD MHC class II (I-Ag7) antigens. NOD +/+ mice express H-2 Kd and H-2Db MHC class I alleles. They also express I-Ag7 and do not express the IAb.allele. NOD-scid IL2rγnull mice express the H-2 Kd and H-2Db MHC class I alleles. Expression is duller because of the absence of MHC class I-bright lymphocytes. These mice have low numbers of I-Ag7 expressing cells and lack expression of I-Ab on their cells. NOD-scid IL2rγnullAbnull mice lack MHC class II expression and exhibit dull H-2Db expression with no expression of H-2 Kd.
2.3 Absence of Thymic Lymphomas in NOD-scid IL2rγnull β2mnull Mice
NOD-scid and NOD-scid β2mnull mice develop thymic lymphomas and die at an early age (19). NOD-scid mice have a median lifespan of 36 weeks (18) while in NOD-scid β2mnull mice the median lifespan is shortened to 24 weeks (19). In contrast, NOD-scid IL2rγnull mice do not develop thymic lymphomas (25). The absence of thymic lymphoma development suggests that signaling through IL2rγ receptor-dependent cytokines such as IL-2 or IL-7 is required for the thymic lymphomagenesis. Because of the increased incidence and accelerated kinetics of thymic lymphoma development in NOD-scid β2mnull mice, we investigated whether NOD-scid IL2rγnull β2mnull mice developed thymic lymphomas and die at an early age. We first examined the histology of the thymus from 10 week old NOD-scid IL2rγnull β2mnull mice and compared it with age-matched NOD-scid β2mnull mice. As shown in Figure 2A and B, the thymus of young adult NOD-scid IL2rγnull β2mnull mice is a hypoplastic, degenerate thymic rudiment with a cystic formation. In contrast, the thymus of age matched NOD-scid β2mnull mice display thymic lymphomas showing a highly proliferative thymus containing numerous mitotic cells. In addition, although NOD-scid β2mnull mice do not survive beyond 8 months of age due to thymic lymphomas, NOD-scid IL2rγnull β2mnull mice show an increased lifespan with no gross or histological evidence of thymic lymphomas at 8 months, with mice surviving past 14 months of age (unpublished observations). A cohort of NOD-scid IL2rγnull β2mnull retired breeders is being aged for evaluating thymic lymphoma development. Four of these mice euthanized at 12-18 months of age were found to have no histological evidence of thymic lymphoma.
Fig. 2.
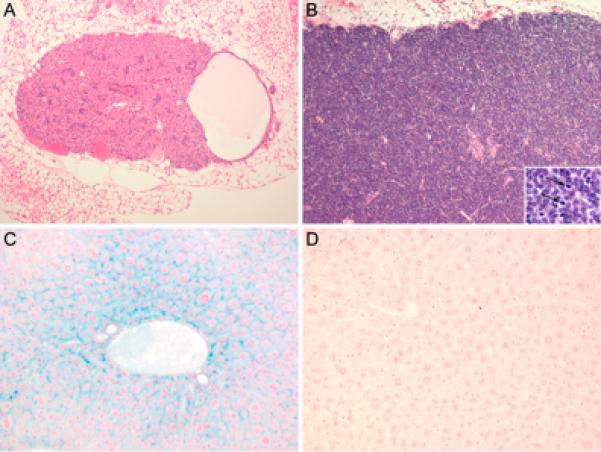
Histopathological sections of tissues from NOD-scid IL2rγnull β2mnull and control mice. Panel A. Thymic lobe from 10-week-old NOD-scid IL2rγnull β2mnull female. Thymus is composed of epithelial and stromal cells and is completely devoid of lymphocytes. Note the cyst lined by a single layer of occasionally ciliated epithelial cells present within the thymic parenchyma (branchial arch remnant). Panel B. Thymic lobe from 10-week-old NOD-scid β2mnull male. A thymic lymphoma is present. Thymic architecture is replaced by dense sheets of neoplastic lymphocytes. Note large atypical lymphocytes with abundant mitotic figures (insert). Panel C. Liver from 10-week-old NOD-scid IL2rγnull β2mnull male. Hepatocytes show intracytoplasmic deposition of iron. Panel D. Liver from 20-week-old NOD-scid IL2rγnull male. There are no detectable iron depositions (A and B, H&E; C and D, Gomori's iron) (A and B, × 100, insert × 400; C and D, × 200).
2.4 Development of Hemochromatosis in NOD-scid IL2rγnull β2mnull Mice
We have previously reported that the lack of β2-microglobulin led to iron accumulation in the liver of NOD-scid β2mnull mice (19), consistent with previous reports showing that β2-microglobulin is required for appropriate regulation of iron absorption (32, 33). To determine whether the addition of the IL2rγnull gene to NOD-scid β2mnull mice altered this phenotype, we stained livers from 10-week-old NOD-scid IL2rγnull β2mnull for the presence of iron. As shown in Figure 2C, we observed the accumulation of iron deposition in the liver. In contrast, there was no detectable iron accumulation in the liver of age-matched NOD-scid IL2rγnull mice (Figure 2D). These observations document that the accumulation of iron in the liver of β2-microglobulin knockout mice does not require common gamma chain-dependent receptor signaling.
2.5 Engraftment of NOD-scid IL2rγnull β2mnull or NOD-scid IL2rγnull Abnull Mice with Human PBMC
The next logical step in the development of this model system is to determine the proliferative response of human PBMC to antigen-presenting cells obtained from NOD-scid IL2rγnull deficient in MHC class I or class II. In preliminary studies, we have observed that the deficiency of MHC class I severely reduces the ability of mouse antigen-presenting cells to stimulate in vitro proliferation of human CD8 T cells and similarly, the deficiency of MHC class II reduces their ability to stimulate in vitro proliferation of human CD4 T cells. These data suggest that xeno-GVHD in immunodeficient mice may be dependent on both MHC class I and class II expression of the murine immunodeficient host. Furthermore, the in vitro proliferative data suggest that the development of GVHD in NOD-scid IL2rγnull mice will likely be mediated by both human CD4 and CD8 T cells targeting MHC class II or class I, respectively. If so, these MHC class I or class II deficient recipients may permit the ability to study the role of each cell subset in the absence of the confounding contribution of the other cell subset to the development of the disease. This hypothesis is currently being tested. This would make these excellent model systems to test the efficacy of therapeutics to modulate the activity of either cell subset in the absence of the functional contribution of the other cell subset to the disease process.
2.6 Human Immune Responses in PBMC Engrafted Mice
Previous attempts to measure immune function of human cells following PBMC engraftment of immunodeficient mice have been met with limited success (34). The lack of a vigorous primary immune response mediated by human PBMC engrafted into earlier humanized mouse models was associated with host murine NK cell activity and other innate immune function as well as an overwhelming xenogeneic GVHD response of the human T cells. The development of NOD-scid IL2rγnull mice provided an immunodeficient mouse lacking host NK cells, facilitating human PBMC engraftment. Although NOD-scid IL2rγnull mice support survival and improved function of engrafted human T cells, the human cells mount a severe xenogeneic GVHD following engraftment into these NK cell-deficient hosts. Our findings that removal of mouse MHC class I activity greatly reduces human in vitro T cell proliferative responses to murine cells should also lead to reduced xenogeneic GVHD following engraftment with human PBMC, an hypothesis we are currently testing. This may now allow immunization of PBMC engrafted NOD-scid IL2rγnull β2mnull mice with experimental vaccines to provide a model system for the assessment of the ability of these vaccines to elicit a protective response against infection with human pathogens in the absence of an overwhelming xeno-GVHD.
3.0 CONCLUSIONS
The use of immunodeficient mice for the in vivo study of human xeno-GVHD holds the promise of understanding the pathogenesis of human GVHD in vivo in a small animal model without putting patients at risk. This model will be critical for understanding the role of host MHC class I and class II in the pathogenesis of GVHD, as well as opening the possibility of setting up an allogeneic GVHD model system with the emergence of NOD-scid IL2rγnull mice deficient in MHC class I or II that also transgenically express human HLA molecules (35). Of particular importance will be the use of this model system for evaluating the efficacy of human-specific therapeutics on the pathogenesis of GVHD in vivo. This model will permit the in vivo study of human-specific drugs to test their ability to ameliorate or prevent the disease process without putting patients at risk. NOD-scid IL2rγnull mice have also been engrafted with blood obtained from patients with human acute myelogenous leukemia and chronic myelogenous leukemia as well as other hematopoietic tumors. This may provide “personalized” animal models of primary human tumors that can be used to screen drugs for their in vivo effectiveness on the tumor and tumor stem cells prior to their use in the patient. The combination of optimal immunodeficient recipients and the analyses of human immunity and xeno-GVHD reactions in vivo should provide insights into the mechanisms of human immunity and the pathogenesis of GVHD and other human diseases and their response to drugs in ways not possible in the clinic.
ACKNOWLEDGEMENT
We thank Jean Leif, Linda Paquin, Amy Cuthbert, Celia Hartigan, Amy Sands, Allison Ingalls, and Candy Knoll for their technical assistance. We also thank the participation of the Clinical Trials Unit at the University of Massachusetts Medical School. This work was supported by National Institutes of Health Grants AI46629, DK072473, CA34196, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520, RR-07068, the Beta Cell Biology Consortium, and grants from the Juvenile Diabetes Foundation, International, including a grant through the nPOD program. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Alderuccio F, Siatskas C, Chan J, Field J, Murphy K, Nasa Z, Toh BH. Haematopoietic stem cell gene therapy to treat autoimmune disease. Curr.Stem Cell Res.Ther. 2006;1:279–287. doi: 10.2174/157488806778226885. [DOI] [PubMed] [Google Scholar]
- 2.Passweg J, Tyndall A. Autologous stem cell transplantation in autoimmune diseases. Semin.Hematol. 2007;44:278–285. doi: 10.1053/j.seminhematol.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Ringden O, Le Blanc K. Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS. 2005;113:813–830. doi: 10.1111/j.1600-0463.2005.apm_336.x. [DOI] [PubMed] [Google Scholar]
- 4.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu.Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 5.Demirer T, Barkholt L, Blaise D, Pedrazzoli P, Aglietta M, Carella AM, Bay JO, Arpaci F, Rosti G, Gurman G, Niederwieser D, Bregni M. Transplantation of allogeneic hematopoietic stem cells: an emerging treatment modality for solid tumors. Nat.Clin.Pract.Oncol. 2008;5:256–267. doi: 10.1038/ncponc1104. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia M, Walters MC. Hematopoietic cell transplantation for thalassemia and sickle cell disease: past, present and future. Bone Marrow Transplant. 2008;41:109–117. doi: 10.1038/sj.bmt.1705943. [DOI] [PubMed] [Google Scholar]
- 7.Lebensburger J, Persons DA. Progress toward safe and effective gene therapy for beta-thalassemia and sickle cell disease. Curr.Opin.Drug Discov.Devel. 2008;11:225–232. [PubMed] [Google Scholar]
- 8.Pinto FO, Roberts I. Cord blood stem cell transplantation for haemoglobinopathies. Br.J.Haematol. 2008;141:309–324. doi: 10.1111/j.1365-2141.2008.07016.x. [DOI] [PubMed] [Google Scholar]
- 9.Kawase T, Matsuo K, Kashiwase K, Inoko H, Saji H, Ogawa S, Kato S, Sasazuki T, Kodera Y, Morishima Y. HLA mismatch combinations associated with decreased risk of relapse: Implications for molecular mechanism. Blood. 2008 doi: 10.1182/blood-2008-08-171934. On line publication, December, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Gale RP, Horowitz MM, Butturini A. Autotransplants in acute leukaemia. Br.J.Haematol. 1991;78:135–137. doi: 10.1111/j.1365-2141.1991.tb04401.x. [DOI] [PubMed] [Google Scholar]
- 11.Porter D, Levine JE. Graft-versus-host disease and graft-versus-leukemia after donor leukocyte infusion. Semin.Hematol. 2006;43:53–61. doi: 10.1053/j.seminhematol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Foss FM. The role of purine analogues in low-intensity regimens with allogeneic hematopoietic stem cell transplantation. Semin.Hematol. 2006;43:S35–S43. doi: 10.1053/j.seminhematol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 14.Mosier DE, Baird SM, Kirven MB. EBV-associated B-cell lymphomas following transfer of human peripheral blood lymphocytes to mice with severe combined immune deficiency. Curr.Top.Microbiol.Immunol. 1990;166:317–323. doi: 10.1007/978-3-642-75889-8_39. [DOI] [PubMed] [Google Scholar]
- 15.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J.Exp.Med. 1994;180:1817–1827. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tary-Lehmann M, Saxon A. Human mature T cells that are anergic in vivo prevail in SCID mice reconstituted with human peripheral blood. J.Exp.Med. 1992;175:503–516. doi: 10.1084/jem.175.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann-Fezer G, Gall C, Zengerle U, Kranz B, Thierfelder S. Immunohistology and immunocytology of human T-cell chimerism and graft-versus-host disease in SCID mice. Blood. 1993;81:3440–3448. [PubMed] [Google Scholar]
- 18.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, McKenna S, Mobraaten L, Rajan TV, Greiner DL, Leiter EH. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J.Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- 19.Christianson SW, Greiner DL, Hesselton RM, Leif JH, Wagar EJ, Schweitzer IB, Rajan TV, Gott B, Roopenian DC, Shultz LD. Enhanced human CD4+ T cell engraftment in β2-microglobulin-deficient NOD-scid mice. J.Immunol. 1997;158:3578–3586. [PubMed] [Google Scholar]
- 20.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, Bonyhadi ML, Berenson RJ, Prior JL, Piwnica-Worms D, Nolta JA, DiPersio JF. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp.Hematol. 2007;35:1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat.Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 22.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET, Paul WE, Katz SI, Love PE, Leonard WJ. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 23.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, Rijkers G, Martens AC, Ebeling SB. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 25.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rγnull mice engrafted with mobilized human hematopoietic stem cell. J.Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 26.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, Atkinson MA, Wasserfall C, Herold KC, Woodland RT, Schmidt MR, Woda BA, thompson m. j., Rossini AA, Greiner DL. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin.Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, Liu R, Balcarcel RR, Fisher N, Levine BL, Carroll RG, Warner N, Blazar BR, June CH, Riley JL. CD28 costimulation is essential for human T regulatory expansion and function. J.Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized mice for the study of type 1 diabetes and beta cell function. Ann.N.Y.Acad.Sci. 2008;1150:46–53. doi: 10.1196/annals.1447.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, Cuthbert A, Burzenski L, Gott B, Lyons B, Foreman O, Rossini AA, Greiner DL. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin.Exp.Immunol. 2008;154:270–284. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat.Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 31.Mosier DE. Adoptive transfer of human lymphoid cells to severely immunodeficient mice: models for normal human immune function, autoimmunity, lymphomagenesis, and AIDS. Adv.Immunol. 1991;50:303–325. doi: 10.1016/s0065-2776(08)60828-7. [DOI] [PubMed] [Google Scholar]
- 32.Rothenberg BE, Voland JR. beta2 knockout mice develop parenchymal iron overload: A putative role for class I genes of the major histocompatibility complex in iron metabolism. Proc.Nat'l.Acad.Sci. 1996;93:1529–1534. doi: 10.1073/pnas.93.4.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc.Nat'l.Acad.Sci.USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torbett BE, Picchio G, Mosier DE. hu-PBL-SCID mice: a model for human immune function, AIDS, and lymphomagenesis. Immunol.Rev. 1991;124:139–164. doi: 10.1111/j.1600-065x.1991.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 35.Banuelos SJ, Shultz LD, Greiner DL, Burzenski LM, Gott B, Lyons BL, Rossini AA, Appel MC. Rejection of human islets and human HLA A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1nullPrf1null mice. Clin.Immunol. 2004;112:273–283. doi: 10.1016/j.clim.2004.04.006. [DOI] [PubMed] [Google Scholar]


