Abstract
Purpose
To investigate the role of preoperative biometry for selecting initial contact lens power.
Methods
Patients randomized to receive contact lenses in the Infant Aphakia Treatment Study (IATS) were retrospectively analyzed. Inclusion criteria were availability of both a preoperative immersion axial length measurement and a 1-month postoperative refractive value. The target contact lens power for distance was determined using 1-month postoperative spherical equivalent refraction (after adjusting for a vertex distance) over the known contact lens power. We compared targeted contact lens power for distance with three other treatment techniques: (1) 30 D contact lens (32 D minus 2 D overcorrection for near vision based on IATS protocol); (2) regression-estimated contact lens power of 84.4 − 3.2 × axial length; and (3) IOL power calculated using the Sanders-Retzlaff-Kraff (SRK/T) regression formula with a modified A-constant (112.176). Prediction error (targeted minus estimated contact lens power) and its absolute values were calculated.
Results
A total of 34 eyes of 34 patients met inclusion criteria. Age at the time of cataract surgery was 2.4 ± 1.7 months. Follow-up refraction was performed at 31 ± 3 days after surgery. Target contact lens power for distance was 26.0 ± 4.5 D for the IATS cohort (which excluded infants with corneal diameter <9 mm). The mean prediction error was −4.0,−1.0, and −2.0 D and mean absolute prediction error was 4.4, 2.2, and 2.9 D, respectively, for 30 D contact lens, regression, and SRK/T-estimated power.
Conclusions
Preoperative biometry can be used to estimate contact lens power for distance if an accurate refraction cannot be obtained initially.
The 12-month postoperative outcome report from the Infant Aphakia Treatment Study (IATS) advised caution in considering intraocular lens (IOL) implantation in infants, given the high incidence of adverse events and the absence of improved short-term visual outcome as compared with contact lens usage.1 The problems with IOL implantation during infancy include difficulties in performing surgery, a relatively high level of surgical trauma, a possiblity of surgery for visual axis opacification, and difficulty in selecting an IOL power. For infants undergoing cataract surgery, many physicians prefer a surgical technique that includes primary posterior capsulectomy and anterior vitrectomy without IOL implantation.
When a primary IOL is not implanted, the resulting aphakia should be treated as early as possible to facilitate visual rehabilitation, either by means of aphakic glasses or contact lenses. Aphakic glasses are generally very heavy and difficult to wear for infants. In addition, they are less suitable for patients with unilateral aphakia. To treat unilateral infantile aphakia, most physicians prefer contact lenses—either a silicone elastomeric lens or a rigid, gas permeable (RGP) lens. For SilSoft contact lens (Bausch & Lomb, Rochester, NY), nearly all infants can be fitted with a 7.5 base curve. The contact lens power is more difficult to predict. Because it is difficult to obtain an accurate refraction immediately after surgery, a +32 D contact lens (the highest available power in the SilSoft super-plus series) tends to be preferred. In the IATS, if an accurate refraction could not be obtained initially, a +32 D contact lens was dispensed, and the lens power was subsequently refined at the earliest opportunity.2 The IATS design specifies a 2.0 D overcorrection to provide a near-point correction; a +32 D contact lens thus implies a +30 D correction for distance vision.
It was observed that the contact lens power often needed to be changed when a +32 D contact lens was initially prescribed (sometimes as early as a week after insertion of the contact lens). Our previous single-center study resulted in a model for selecting an initial contact lens power for distance based on preoperative axial length (AL).3 We projected that if a contact lens power of 32 D had been used, 22 of 50 eyes (44%) would have needed to have the contact lens power changed at the first postoperative refraction. Contact lens power for distance was predicted using the following regression formula: contact lens power = 84.4 − 3.2 × AL.3 (This was the power for distance vision; an age-appropriate addition for near vision should be provided as needed.) Contact lens power for distance was also estimated using an A-constant of 112.176 in the Sanders-Retzlaff-Kraff theoretic (SRK/T) IOL power calculation formula.
The purpose of the present study was to validate the regression formula and A-constant we developed from our previous single center study, using the IATS dataset. We sought to provide guidelines for the selection of an initial contact lens power for distance if retinoscopy over a diagnostic lens is not possible.
Methods
The Infant Aphakia Treatment Study is a multicenter, randomized, controlled clinical trial comparing IOL and contact lens treatments after cataract surgery performed in infants with unilateral congenital cataract at 1-7 months of age. The study design and clinical measures at enrollment have been previously reported.2 The study was approved by the institutional review boards of all the participating institutions and complied with the Health Insurance Portability and Accountability Act of 1996. Informed consent was obtained from parents or legal guardians of all the patients prior to randomization.
Patients randomized to the contact lens group in IATS were fitted with either a silicone elastomer (Silsoft Super Plus; Bausch & Lomb, Rochester, NY) or an RGP (X-Cel Laboratories, Duluth, GA) contact lens with + 2.0 D overcorrection to provide a near-point correction at 50 cm. Contact lens professionals were certified by written examination for each investigational site.4 If an accurate refraction could not be obtained initially, a 7.5 mm base curve/+32.0 D/11.3 mm diameter Silsoft Super Plus lens was dispensed, and the lens power and fit were subsequently refined at the earliest possible opportunity.2 Refractive error was determined using retinoscopy.
The present study analyzed patients randomized to receive contact lens treatment in the IATS. The following data were collected: age at the time of surgery, sex, ethnicity, axial length (and method of measurement), keratometry, first contact lens power prescribed (which included +2 D for near correction), and refraction at the 1-month postoperative visit. If refraction was performed over contact lens, the contact lens power used during refraction was noted. An eye was included if there was a valid preoperative axial length measurement using the immersion technique and the 1-month postoperative refractive value was available. We excluded outliers, defined as error was outside 2 standard deviations of average error.
The physician-estimated contact lens power for distance was calculated as the first prescribed contact lens power minus 2 D for near correction, based on the IATS protocol. The refractive value obtained 1 month postoperatively was converted into its spherical equivalent. The target contact lens power for distance was determined using this spherical equivalent refraction (adjusted for a vertex distance of 12 mm) over known contact lens power 1 month postoperatively. This target contact lens power for distance was compared with three other techniques for estimating contact lens power for distance: (1) 30 D contact lens (32 D minus 2 D overcorrection for near vision2; (2) regression-estimated contact lens power of 84.4 − 3.2 × AL3; and (3) SRK/T-estimated IOL power calculated using a modified A-constant (112.176).3 Prediction error was calculated as the targeted contact lens power for distance minus estimated contact lens power for distance using above three techniques. Absolute PE was calculated using absolute value of prediction error.
Results
A total of 114 patients were enrolled in the IATS, with 57 each randomized to the contact lens and the IOL treatment groups. For the present study, 34 patients were analyzed, of whom 16 were excluded because the A-scan was not performed using the immersion technique, 4 were excluded due to an invalid A-scan, 2 were excluded because the postoperative refractive value was not available at 1 month; and 1 was excluded as the patient was noted to be an outlier. Of the 34 included patients, 23 eyes (67.6%) were fitted with silicone elastomer contact lenses; 11 eyes (32.4%), with a RGP contact lens. Figure 1 shows how many eyes would have required which contact lens power (targeted contact lens power for distance +2 D for near correction). Figure 2 illustrates axial length versus targeted contact lens power for distance. The regresssion formula from IATS data revealed a contact lens power for distance of 70.4 − 2.5 × AL (R2 = 0.7488). Table 1 provides baseline patient charactersitics. The targeted contact lens power for distance based on the 1-month refraction was 26.0 ± 4.5 D. Table 2A gives the contact lens power estimated using a different technique, whereas Table 2B gives the prediction error and absolute prediction error.
Fig 1.
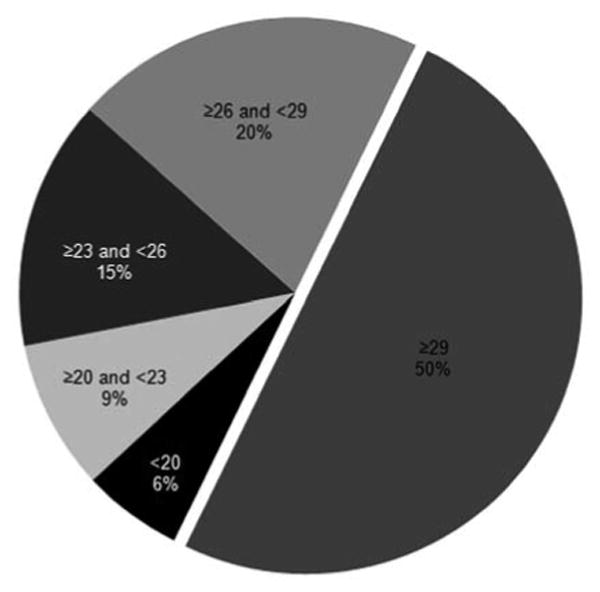
Pie chart illustrating how many eyes would have required which contact lens power (targeted contact lens power for distance +2 D for near correction).
Fig 2.
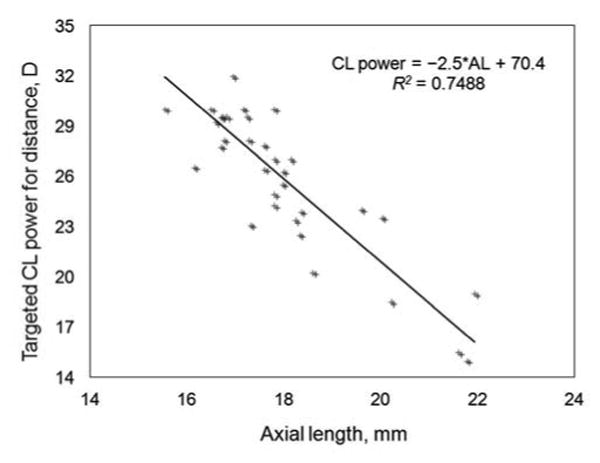
Scatterplot illustrating axial length versus targeted contact lens power.
Table 1. Baseline characteristics.
| Characteristics | Number |
|---|---|
| Age at surgery, months (range) | 2.4 ± 1.7 (1.0-6.6) |
| Sex | |
| Female | 17 |
| Male | 17 |
| Ethnicity | |
| African American | 2 |
| White | 30 |
| Asian | 2 |
| Preoperative axial length, mm (range) | 17.9 ±1.6 (15.6-21.9) |
| Preoperative keratometry,* D (range) | 46.0 ±3.1 (40.1-53.2) |
| Laterality | |
| Right eye | 21 |
| Left eye | 13 |
| Follow-up period, days (range) | 31 ± 3 (23-36) |
D, diopters.
Average of K1 and K2 in diopters.
Table 2A. Contact lens power.
| Contact lens power for distance | Dioptersa (range) |
|---|---|
| Targeted, based on 1 month refraction | 26.0 ± 4.5 (15.0-35.0) |
| Physician-estimated | 26.4 ± 4.9 (13.0-34.0) |
| Regression | 27.0 ± 5.1 (14.2-34.6) |
| SRK/T | 28.2 ± 5.1 (13.4-34.6) |
SRK/T, Sanders-Retzlaff-Kraff theoretic.
Appropriate correction for near vision should be added to this; the IATS used +2 D overcorrection for near.
Table 2B. Prediction error and absolute prediction error.
| Method | Prediction error, D | Absolute prediction error, D |
|---|---|---|
| +30 Da | −4.0 ± 4.5 | 4.4 ± 4.1 |
| Regression | −1.0 ± 2.5 | 2.2 ± 1.6 |
| SRK/T | −2.0 ± 2.5 | 2.9 ± 1.7 |
D, diopter; SRK/T, Sanders-Retzlaff-Kraff theoretic.
In the absence of cooperative refraction, +32 D CL would have been prescribed; 2 D was deducted for near per IATS protocol, and thus for distance the prescription would be 30 D.
Discussion
Trivedi and Wilson3 suggested powers for the initial postoperative infant aphakic contact lens when refraction is not possible based on the preoperative axial length. They noted that the initially prescribed contact lens frequently needed to be changed during the early postoperative period (days to weeks) because of high refraction over the contact lens, which ranged from −13 D to +11 D.3 As dictated by current practice and the IATS protocol, if a child was not cooperative for refraction and 32 D contact lens had been prescribed, almost half of the infants would have required a replacement contact lens (Fig 1). Inappropriate aphakic correction can be amblyogenic. In addition, the average cost of a silicone elastomer contact lens is US $175/unit. Replacement costs due to incorrect estimation of contact lens power can be decreased by using the results provided in this study. As seen in Figure 2, axial length shows a linear relationship with the targeted contact lens power for distance.
Martin and colleagues5 provided a regression analysis based on age (contact lens = 30.28 − 0.3554 × age in months; R2 = 0.96). Within the contact lens parameters available, the authors recommended a + 29 D contact lens for children undergoing surgery from 0 to 6 months of age. In the current series, the average targeted contact lens power was + 26 D, implying an average prescribed contact lens power of + 28 D (including 2 D correction for near); however, we do not recommend using a “one size fits all” strategy (Figure 1).
Trivedi and Wilson3 reported an average contact lens power of +29.6 D for distance as opposed to +26 D in the current series. It is important to note that the IATS excluded infants with <9 mm corneal diameter, thus excluding eyes with moderate or severe microphthalmia. Trivedi and Wilson, on the other hand, included all patients undergoing cataract surgery during infancy, which may explain the discrepancy in contact lens powers between the two studies. This can also explain minor difference in regression formulae. The present study shows that a change in axial length of 1 mm leads to a change of 2.5 D in contact lens power; the comparable value in Trivedi and Wilson's study was 3.2 D.
Aphakic infants are unable to accommodate and should be overcorrected to focus vision on a near viewing point. Various studies have reported additions of +4 D to +2 D.2 Considering an approximate overcorrection of +2 D and the availability of SilSoft contact lens powers, Trivedi and Wilson recommended a +32 D contact lens when the preoperative axial length is <17 mm; +29 D when it is 17–18.5 mm; +26 D when is 18.5–19.5 mm; +23 D for preoperative axial length of 19.5–20 mm (21 mm); and +20 D when it is 20–21 mm (20 D or less for >21 mm).3 These power suggestions are a starting point before performing an overrefraction using retinoscopy. When the contact lens power falls between available powers (SilSoft super-plus lenses are available in 3 D steps), the prescription should be based on age. For example, a child of 7 months requiring a contact lens of +30.5 D could use a +32 D contact lens because near vision is a priority. On the other hand, a child of 4 weeks might be prescribed +29 D contact lens because the eye will likely soon grow into that strength. Some physicians may prefer not to insert a contact lens at the close of surgery but delay until after clinical evaluation. De Brabander and colleagues5 reported insertion of contact lenses an average of 2.4 weeks after surgery.6
One of the limitations of the current study is that we used refraction at 1 month as an endpoint to calculate the targeted contact lens power. The eye may grow in 1 month, and thus the average required contact lens power might have been slightly higher; however, we did not take initially prescribed contact lens power as an endpoint because two groups were represented—those whose prescription was based on refraction and those in whom refraction was not possible. Thus taking physician-estimated or initially prescribed contact lens was not reasonable.
In conclusion, in infants who cannot be refracted, the preoperative axial length can be used as starting point for selecting contact lens power. We recommend using either a regression formula or modified A-constant to estimate the contact lens power for distance. Appropriate overcorrection to provide near-point correction should be added for infants. We hope that these guidelines will provide better aphakic correction than initial use of +32 D and decrease the need for changing the contact lens power due to high refraction over the initial +32 D contact lens.
Supplementary Material
Acknowledgments
Study was supported by National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by NIH Departmental Core Grant EY006360 and Research to Prevent Blindness, Inc, New York, New York.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambert SR, Buckley EG, et al. Infant Aphakia Treatment Study Group A randomized clinical trial comparing contact lens with intraocular lens correction of monocular aphakia during infancy: grating acuity and adverse events at age 1 year. Archives of ophthalmology. 2010;128:810–18. doi: 10.1001/archophthalmol.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128:21–7. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi RH, Wilson ME. Selection of an initial contact lens power for infantile cataract surgery without primary intraocular lens implantation. Ophthalmology. 2013;120:1973–6. doi: 10.1016/j.ophtha.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell B, Ward MA, Lynn M, et al. The infant aphakia treatment study contact lens experience: one-year outcomes. Eye & Contact Lens. 2012;38:234–9. doi: 10.1097/ICL.0b013e3182562dc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin NF, Kracher GP, Stark WJ, Maumenee AE. Extended-wear soft contact lenses for aphakic correction. Arch Ophthalmol. 1983;101:39–41. doi: 10.1001/archopht.1983.01040010041003. [DOI] [PubMed] [Google Scholar]
- 6.de Brabander J, Kok JH, Nuijts RM, et al. A practical approach to and long-term results of fitting silicone contact lenses in aphakic children after congenital cataract. CLAO J. 2002;28:31–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


